Abstract
A greenhouse experiment was conducted to investigate the changes of soil microbial activities and chemical properties under different water and nitrogen (N) supply conditions. A completely randomized design was subjected to three water regimes (80%, 40% and 20% water field capacity (FC)) and three N supply regimes (control, N0: 0 mg N kg−1 soil; low N supply, Nl: 92 mg N kg−1 soil; and high N supply, Nh: 184 mg N kg−1 soil) by potting with 2-month-old Sophora davidii seedlings. Water stress decreased the content of soil organic carbon (C), available N and phosphorus (P), the ratio of C/N, the ratio of C/P, as well as activities of soil invertase, urease and alkaline phosphatase, but not reduced microbial biomass C, N and P contents. Soil microbial and chemical characteristics also exhibited strong responses to N supply, and these responses were inconsistent among N supply levels. The contents of soil organic C and available P showed stronger positive responses to Nl than to Nh, while the available N content increased with increasing N supply. Additionally, Nl rather than the other two N treatments led to increased microbial biomass N and invertase activity under 20% FC treatment, even though the invertase activity increased in Nh treatment under 40% FC and 80% FC treatments. Nl treatment also increased the C/P ratio and alkaline phosphatase activity. These results suggest that water and N co-limited nutrient mineralization and microbial activity, and that these characteristics responded positively to Nl. Therefore, appropriate or low N supply is recommended to increase soil quality restrained by water stress, thereby facilitating S. davidii seedling establishment under water deficit conditions.
Introduction
In arid and semiarid ecosystems, the most serious effects of global climate change may be those that are related to increasing drought since water stress is already the principal constraint in plant growth (Bouwman et al., Citation2002; Acuña et al., Citation2010). A decline in total rainfall and/or soil water availability is expected over the next decades (IPCC, Citation2001). Nevertheless, soils often also suffer from nutrient deficiencies, especially nitrogen (N) and phosphorus (P) (Hooper & Johnson, Citation1999), and such deficiencies can be worsened by climate change. As a result, arid and semiarid land may be particularly sensitive to large-scale environmental changes because of the fragile and poor nature of their soils (Stursova et al., Citation2006; Reyes-Reyes et al., Citation2007). Accordingly, ecosystem managers must continually develop methods of improving poor soil quality and restoring degraded vegetation.
In recent years, soil characteristics such as microbiological and biochemical properties have been considered early and sensitive indicators of soil changes (de la Paz Jimenez et al., Citation2002; Saviozzi et al., Citation2002; Ros et al., Citation2003). Poor soil quality might result from soil water deficiency in arid and semi-arid environments (Stursova et al., Citation2006). Dry climatic conditions and deficiency of soil water availability often act as obstacles to soil microbial activity, resulting in decreased microbial biomass (Zhang & Zak, Citation1998; Pulleman & Tietema, Citation1999), inhibited microbial enzyme activity (Boissier & Fontvielle, Citation1995; Li & Sarah, Citation2003), low nutrient mineralization rates (Sardans & Peñuelas, Citation2004), and consequently in poor soil quality with low content of organic matter and available nutrients.
Fertilization increases the availability of limited nutrients and can stimulate plant productivity (Eckersten et al., Citation2010). The use of fertilizer might be a practical method of improving the quality of soil organic matter, thereby altering soil properties in infertile and dry environments (Singh et al., Citation2005; Zhong & Cai, Citation2007). However, the results of previous studies conducted to evaluate such uses of fertilizer have not always been consistent. For example, it has been reported that N fertilization increases microbial biomass (Zhang & Zak, Citation1998), enhances soil enzyme activity (Stursova et al., Citation2006), and improves carbon (C) sequestration (Sinsabaugh et al., Citation2005) and P mineralization (Saiya-Cork et al., Citation2002). In contrast, others have reported decreased soil microbial biomass in response to high rates of nitrogen fertilization (Thirukkumaran & Parkinson, Citation2000; Lee & Jose, Citation2003). These conflicting results might be induced, at least in part, by differences in the initial soil quality status, such as soil pH, organic matter and nutrient contents, and the N supply level. Moreover, soil ecological processes might be more sensitive to N supply under well-watered conditions than under water deficit (Hooper & Johnson, Citation1999; Stursova et al., Citation2006). However, the response is not clear, and very few studies have been conducted to elucidate the mechanism by which such responses occur.
The Minjiang River dry valley, which is one of the most fragile systems in the Yangtze River drainage area, is located at the eastern edge of the Qinghai-Tibet Plateau, China. The climate is characterized by low and irregular rainfall (around 494 mm annual precipitation), and high evapo-transpiration and temperatures with average values of 1019 mm and 11°C, respectively) (Liu et al., Citation1996; Bao et al., Citation1999). Water and nutrients (N and P) have been shown to be the two main factors limiting vegetation in the arid ecosystem (Wang et al., Citation2003).
A better understanding of soil chemical and microbiological characteristics in response to water and N supply is important to predict the responses of the soil processes to future environmental changes, and consequently to future vegetation practices. However, there is little information regarding these changes available for this semiarid system. Increased seedling growth and improved adaptation to arid environments were observed in response to a moderate N supply (Wu et al., Citation2008, Citation2009), but the soil processes were still unclear. Therefore, a greenhouse experiment was conducted using potted Sophora davidii seedling, which is the dominant species in this area. It is hypothesized that additional N supply could enhance the soil quality by improving soil C sequestration, N and P mineralization, as well as the microbial activity under dry conditions, thereby alleviating the negative effects of water deficits on soil processes. The objectives of this study were (1) to characterize the mechanisms involved in the control of water and N supply on the soil chemical and microbial processes, and (2) to determine the responses of soil microbial and chemical characteristics to N supply under different water conditions.
Materials and methods
Experimental design
This study was conducted in a greenhouse at the Maoxian Mountain Ecosystem Research Station, Chinese Academy of Science (experimental site, 103°53′58″ E, 31°41′07″ N, 1816 m a.s.l.), where day/night temperatures were 30/11°C and the relative humidity was kept between 45 and 85%. Precipitation was the natural water supply. Seeds of S. davidii were collected in September 2005 from Maoxian County, Sichuan, China (Wu et al., Citation2008, Citation2009). Surface soil (0–10 cm) from several sites in the arid Minjiang River valley was used as the growth substrate (). The soil was be classified as Aridosols. The collected soils were combined and thoroughly mixed. After 10 days, 7.5 kg of soil was placed into each of thirty-six 7.5 L plastic pots. Four seeds of approximately the same size were sown in each pot on 24 March 2006. These pots were well watered until thinning, to ensure sprout establishment. Shortly after emergence seedlings were thinned to one uniform plant per pot.
Table I. Physical and chemical characteristics of substrate soil.
Based on the investigated variations of soil water and N availability along the dry valley of the Minjiang River, the experiment was arranged in a completely random design with four replicates for three water supply (80%, 40% and 20% field water capacity (FC)) and three N supply (control, N0; low N supply, Nl; and high N supply, Nh) treatments. The water and N treatments were initiated on 28 June 2006 after the seedlings were established. Specifically, solutions (10 mL) containing 0, 1.5 and 3.0 g urea (46% N) corresponding to 0, 92 and 184 mg N kg−1 soil were applied to N0, Nl and Nh treatments at one time, respectively. These N supplies were equivalent amounts to around 0, 400 and 800 kg urea ha−1, and the normal N fertilization amounts were around 500 kg urea ha−1 in this area from local investigation. To avoid rapid N loss, the solution was applied at 5 cm beneath the soil surface. Evaporation from the soil surface was minimized by covering the pots with a 3 cm layer of quartz gravel. The transpired water loss was measured gravimetrically by weighing all pots every other day at 18:00. The amount of water lost from each pot was determined based on the difference in pot weight and that amount of distilled water was then added to the pot. Accordingly, the average soil volumetric water contents were maintained at 19.7±0.3%, 9.8±0.7%, and 4.9±1.1% for the 80%, 40% and 20% of FC water supply regimes, respectively. The experimental layout was surrounded with a single row of border plants to protect the experimental seedlings from external influences. All pots were rotated weekly to provide random distribution in the greenhouse. During the experimental period, increases in seedling weight were estimated based on regression of the relationship among seedling fresh weight (Y, g), plant height (X1, cm) and seedling basal diameter (X2, mm): Y = − 1.496 + 0.117 X1+0.998 X2, R2=0.848, p < 0.001 (Wu et al., Citation2008). The weight of the pot plus the seedling at field capacity was adjusted accordingly every 15 days.
Soil sampling
Soil was sampled on 9 October 2006 after removing the surface quartz gravel and the seedlings. The sampled soils were then put in polyethylene bags, placed in an insulated box and transported to the laboratory, where they were thoroughly mixed and sieved (2 mm mesh size). Visible plant materials and stones were removed by hand. About 100 g subsamples were allowed to air-dry and used for subsequent chemical analyses. The remaining soil was stored in the dark at 4°C for less than 2 weeks for enzymes, microbial biomass and available N content analyses.
Microbial biomass C, N and P analyses
The soil microbial biomass C, N and P (MBC, MBN and MBP) were determined using the fumigation–extraction method (Brookes et al., Citation1982, Citation1985; Vance et al., Citation1987). Briefly, 20.0 g (dry weight equivalent) of soil were either CHCl3 fumigated or non-fumigated, and then extracted with 0.5 mol L−1 K2SO4 for MBC and MBN, and 0.5 mol L−1 NaHCO3 for MBP. The extractable C, N and P were determined using the dichromate digestion, Kjeldahl and phosphorus molybdenum-blue colorimetry method, respectively. MBC, MBN and MBP were then calculated as the difference in extractable C, N or P between the fumigated and non-fumigated soil. To correct for incomplete extractability, conversion factors of Ec (0.38), E N (0.54) and E P (0.4) were used for MBC, MBN and MBP, respectively (Brookes et al., Citation1982, Citation1985).
Enzyme assays
The soil enzyme activities were determined as described by Guan (Citation1986) and Zhang et al. (Citation2005). To measure the invertase activity, 5 g of air-dried soils were incubated for 24 h at 37°C with 15 mL of 8% sucrose, 5 mL of phosphate buffer at pH 5.5 and 0.1 mL of toluene. The glucose released by invertase reacted with 3-5-dinitrosalicylic acid and 3-aminonitrosalicylic acid and then was measured based on the absorbance at 508 nm (UV-2450, Shimadzu Corporation, Kyoto, Japan). Results were expressed as mg glucose g−1 h−1.
To measure the urease activity, 5 g of air-dried soil was incubated for 24 h at 37 °C with 20 mL of citrate buffer at pH 6.7, 10 mL of 10% urea and 0.1 mL of toluene. The ammonium released by the urease was determined by indophenol-blue colorimetry. Results were expressed as mg NH3-N g−1 h−1.
To measure the proteinase activity, 5 g of air-dried soil was incubated for 24 h at 30 °C with 20 mL of 1% casein and 1 mL of toluene. Then 2 mL of 0.05 mol L−1 H2SO4 and 12 mL of 20% Na2SO4 were added. The aminoacetic acid released by the proteinase was reacted with 1 mL of 2% ninhydrin then measured at 500 nm (UV-2450, Shimadzu Corporation, Tokyo, Japan). Results were expressed as mg NH2-N g−1 h−1.
To measure the phosphatase activity, 5 g of air-dried soil was incubated for 2 h at 37 °C with 20 mL of disodium benzene phosphate dissolved in buffer (acetate buffer at pH 5 for acid phosphatase, citrate buffer at pH 7 for neutral phosphatase, and borate buffer at pH 9.4 for alkaline phosphatase) and 0.1 mL of toluene. The phenol released by phosphatase reacted with 0.25 mL of ammonium chloride–ammonium hydroxide buffer at pH 9.8, 0.5 mL of 4-aminophenazon and 0.5 mL of potassium ferricyanide, then measured at 510 nm (UV-2450, Shimadzu Corporation, Tokyo, Japan). Results were expressed as mg P2O5 g−1 h−1.
To measure the catalase activity, 2 g of air-dried soil were mixed with 40 mL of distilled water and 5 mL of 0.3% H2O2 by shaking for 20 min (shaking velocity was 150 n min−1), after which the filtrate was titrated with 0.025 mol L−1 KMnO4. The results were expressed as mL 0.025 mol L−1 KMnO4 g−1 h−1.
Chemical analyses
The soil pH was measured in 1:2.5 soil-H2O suspensions using a portable pH meter. Air-dried sampled soils were ground to pass through a 0.25-mm sieve for the determination of the organic C, total N, total P, and available P and N content as described by Lu (Citation1999). The soil organic C (SOC) content was determined using the dichromate oxidation-sulphateferrous titration method. The Kjeldahl and phosphorus molybdenum-blue colorimetry methods were used to determine the total N and total P content after digestion with 8 mL H2SO4 (ρ = 1.84 g cm−3) and 3 mL H2O2 solution, respectively. The available P content was extracted with 0.5 mol L−1 NaHCO3, and then determined by phosphorus molybdenum-blue colorimetry. Fresh soil was used to determine the NH4-N and NO3-N content. Indophenol-blue colorimetry and phenol disulphonic acid colorimetry methods were used to determine the NH4-N and NO3-N content after extraction with 2 mol L−1 KCl, respectively (Liu Citation1996).
All the analyses and assays were carried out in triplicate.
Statistical analysis
All variables were analysed using the Univariate process of the General Linear Model (GLM) with water stress and N supply regimes and their interaction as factors (n = 4). When significant differences were noted, the LSD multiple range test was used to determine where the differences existed. The relationships among variables were determined using the Pearson's correlations coefficient test at the 0.05 level. All of the statistical analyses were performed using SPSS software package (Standard released version 11.5 for Windows, SPSS Inc., IL. USA).
Results
Chemical properties
With the exception of the P content and pH, the other soil chemical properties showed obvious (p < 0.01) responses to water stress. In contrast, with the exception of the Kjeldahl N (p < 0.05), available N (p < 0.01) content and pH (p < 0.05), the soil chemical properties showed little response to N supply. The interaction between N supply and water stress showed significant (p < 0.01) effects on available N and P content (, III). Water stress decreased SOC, available N and P content, as well as the ratio of SOC to Kjeldahl N (C/N), and the C/P under the same N supply treatment. N supply slightly enhanced SOC, Kjeldahl N, available N and P content, and C/P under the same water condition, while SOC and available P content, and C/P showed stronger responses to Nl than Nh. Few apparent differences in total P content were observed among treatments.
Table II. Soil organic C (SOC), Kjeldahl N, total P, NH4-N, NO3-N and available P content (means±SD, n = 4) at different water and N supply regimes cultivated with Sophora davidii seedlings.
Microbial biomass C, N and P
As described in , water stress exerted few effects on MBC, MBN and MBP, but Nl treatment enhanced MBC under well-watered condition (80% FC), and enhanced MBN under the same water conditions. Nevertheless, the ratio of MBC/SOC, MBC/MBN and MBC/MBP showed significant (p < 0.01) responses to water stress. Higher MBC/MBN and MBC/MBP were observed under the well-watered condition (80% FC) when compared to the other two water treatments. Both N supply and its interaction with water stress had insignificant (p > 0.05) effects on MBC/MBN and MBC/MBP ().
Figure 1. Soil microbial biomass C (MBC), microbial biomass N (MBN) and microbial P (MBP) at different water (80%, 40% and 20% field water capacity (FC)) and N supply (control, N0; low N supply, Nl; and high N supply, Nh) regimes cultivated with Sophora davidii seedlings. Bars indicate SD (n = 4).
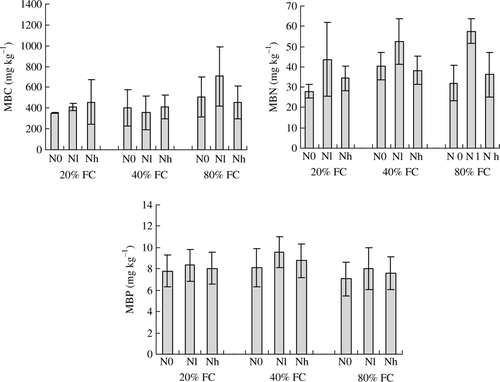
Table III. pH, the ratio of C to N (C/N), C to P (C/P), microbial biomass C to organic C (MBC/SOC), microbial biomass C to N (MBC/MBN), and microbial biomass C to P (MBC/MBP) of soils (means±SD) at different water and N supply regimes cultivated with Sophora davidii seedlings.
Enzyme activities
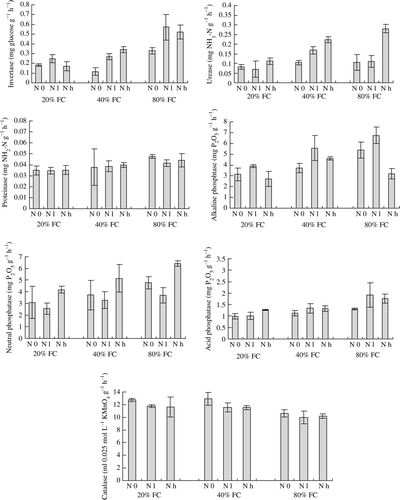
Water stress inhibited the invertase activity under the same N supply treatment (). Both Nl and Nh enhanced the invertase activity under relatively well-watered conditions (40% and 80% FC), but Nl only enhanced invertase activity under severe water stress condition (20% FC). Water stress inhibited the urease activity at Nh treatment and the alkaline phosphatase activity at N0 and Nl treatments. Moreover, Nl enhanced the alkaline phosphatase activity under the same water conditions. Both water and N supply showed few effects on the activities of protease, acid and neutral phosphatase, and catalase.
Figure 2. Soil invertase, urease, proteinase, alkaline phosphatase, neutral phosphatase, acid phosphatase and catalase activity at different water (80%, 40% and 20% field water capacity (FC)) and N supply (control, N0; low N supply, Nl; and high N supply, Nh) regimes cultivated with Sophora davidii seedlings. Bars indicate SD (n = 4).
Correlations
According to Pearson's correlationship statistics, the invertase activity was significantly (p < 0.05) correlated with the content of SOC, Kjeldahl N, total P, NH4-N, NO3-N and available P (). The urease activity was only statistically correlated with the content of Kjeldahl N (p < 0.05) and available N (p < 0.01). Among the three phosphatases, only the alkaline phosphatase was significantly (p < 0.01) correlated with total P and available P content. Additionally, MBC was significantly (p < 0.05) correlated with soil chemical properties, except for Kjeldahl N content.
Table IV. Pearson's correlation between soil chemical and microbiological characteristics.
Discussion
Changes in soil properties have often been considered early and sensitive responses to environmental changes (Saviozzi et al., Citation2002; Ros et al., Citation2003). In this study, water stress showed primary effects on soil chemical and microbial characteristics, suggesting that increasing drought plays important roles in soil ecological processes of this arid ecosystem. Additionally, the results indicated that both water stress and N deficit limited the soil chemical and microbial processes, although N supply alone cannot alter the diminishing tendency that is caused by water stress. Therefore, our results only partially support the hypothesis that N supply could alleviate the negative effects on soil processes caused by water deficit.
Soil nutrients are important determinants of dynamic and function of vegetation and are usually considered as the currency of ecological processes in an ecosystem (Gallardo & Covelo, Citation2005). Accordingly, soil chemical properties might play a dominant role in ecological processes since soil often acts as a primary bank of nutrients. In this study, water stress was not only associated with a dramatically reduced SOC, available N and P content, but also with reduced soil C/N and C/P. It is well-known that SOC is primarily derived from plant detritus, root exudates and microorganisms (Knops et al., Citation2002). The reduced soil C content observed in this study has primarily resulted from the inhibited productivity of plants (Wu et al., Citation2008) under water deficit conditions. Additionally, the low C/N and C/P might imply low nutrient mineralization rates under water deficit conditions, and contribute to a low available N and P content. Inhibited urease and alkaline phosphatase activity could also indicate low N and P mineralization rates under water stress conditions (). Sardans and Peñuelas (Citation2004) reported similar results and further suggested that the nutrient mineralization rates were strongly related to the soil water availability. These responses of soil chemical properties to water stress suggested that water deficit could decrease the soil fertility, and reduce the nutrient mineralization rate. However, the quality of soil organic matter (low C/N and C/P) was maintained under the water deficit condition, which was in agreement with the findings of Austin et al. (Citation2004). Conversely, the N supply appeared to improve the soil fertility, but to have limited effects on the organic matter quality, showing increased SOC, Kjeldahl N, available N and P content and C/P under the same water conditions. Interestingly, the content of SOC and available P and C/P showed stronger responses to Nl than Nh, which may have been due to the acidification caused by Nh (; Lee & Jose, Citation2003).
Soil microbial biomass is the active component of the soil organic matter pool, which is responsible for organic matter dynamics and thus nutrient availability to plants (Reyes-Reyes et al., Citation2007). It is also an important indicator of soil fertility and its measurement is often essential in soil ecological studies (Lin & Brookes, Citation1996; Insam, Citation2001). Our results agreed with the general theory that water deficit did not affect the microbial biomass (Zaman & Chang, Citation2004; Pascuala et al., Citation2007). However, water stress might decrease the soil C availability, and N and P use efficiency in microorganisms, showing declined MBC/SOC, MBC/MBN and MBC/MBP under water deficit conditions. Similar results have been reported by Yan et al. (Citation2003) and Xue et al. (Citation2006). Conversely, MBC under well-watered conditions and MBN under all three water conditions showed positive responses to Nl, which suggests that appropriate application of N could improve the microorganism activity in N-deficient habitats. This observation was consistent with the findings of Tu et al. (Citation2006).
Soil enzyme activities often indicate the potential for basic soil biochemical processes and cycling of important nutrients, such as N (urease and protease), P (phosphatase) and C (invertase) (Yang et al., Citation2005; Pascuala et al., Citation2007). Drier conditions often showed lower microbial enzyme activity (Li & Sarah, Citation2003; Sardans & Peñuelas, Citation2004). Similarity, significant impacts of water deficit on invertase, urease and alkaline phosphatase activities were observed in this study, indicating that these enzymes were quite sensitive to the low soil moisture content in this semiarid environment. Nevertheless, the invertase activity responds positively to N supply, which might be attributed to an increasing substrate (organic matter) since N supply increased plant productivity (Wu et al., Citation2008). The increased urea activity in the N supply treatment may be related to the increase of urease substrate in response to supplying urea (Xue et al., Citation2006; Chang et al., Citation2007). In addition, Nl induced an increase in alkaline phosphatase activity, implying that Nl might stimulate P mineralization. The catalase enzyme is involved in the biological processes of soil energy and nutrient transformation, and can facilitate decomposition of soil H2O2 that is harmful to plant growth (Zhang & Wang, Citation2006). We did not observe a significant effect of water stress and N supply on catalase activity, suggesting that catalase might be less sensitive to water deficit and N supply.
Finally, soil quality depends on a large number of chemical, physical, biological and biochemical properties, and its characterization requires the selection of properties most sensitive to changes in environment (de la Paz Jimenez et al., Citation2002). The present study implied that supplying N to soil had at least three beneficial effects on soil quality under water deficit conditions. First, soil chemical properties such as organic matter, nutrient and available nutrient content, might be the primary indicators of soil quality and fertility because of their direct role in plant growth. Supplying N increased the content of SOC, available N and P under water stress conditions, which represents a direct improvement in soil quality. Second, environmental changes often affect microbial activity initially and directly. There is also growing evidence that soil biological properties are impacted by environmental factors and may be potential indicators of ecological stress (Pascuala et al., Citation2007), management practices or climate changes (Kandeler et al., Citation1999). N supply increased invertase, urea and alkaline phosphatase activity, which were significantly related to the chemical properties according to Pearson's correlations under dry conditions. Third, plants and microorganisms often employ strong competition for limited resources such as N under infertile conditions (Hooper & Johnson, Citation1999). N supply could alleviate this competition and consequently facilitate soil quality. Moreover, our results demonstrated that N supply appears to accelerate P mineralization by increasing C/P and improving alkaline phosphatase activity under water stress conditions. However, it should be noted that excess N supply could inhibit microbial activity due to acidification. In addition, the general theory mentioned that N supply should have greater effects on ecological processes in wet environments than dry ones (Hooper & Johnson, Citation1999). In contradiction to this, the results here showed that the majority of soil chemical and microbial properties had similar responses to N supply under different water conditions. These findings imply that N might be the critical limiting factor in this semiarid ecosystem.
In conclusion, water stress not only decreased soil chemical fertility based on the content of SOC, available N and P, but also reduced microbial activity including activities of invertase, urease and alkaline phosphatase. Conversely, low N supply (Nl) increased the content of SOC, available N and P and improved the microbial activity under water stress conditions by enhancing the invertase and alkaline phosphatase activities. It was concluded that excess N supply such as Nh treatment resulted in negative effects on soil microbial activity under arid environments. Therefore, our short-term experiment clearly suggested that improving soil quality for the appropriate low N supply will result in better S. davidii seedling growth under water deficit conditions.
Acknowledgements
The authors are grateful to Maoxian Mountain Ecosystem Research Station of the Chinese Academy of Sciences for providing facilities and technical assistance. This study was funded by the State Key Basic Research and Development Plan of China (2005)CB422006) and Chinese Academy of Science Action-Plan for West Development (No: KZCX2-XB2-02). Authors acknowledged logical supports from The Key Laboratory of Mountain Ecological Restoration and Bioresource of the Chinese Academy of Sciences and The Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province.
References
- Acuña , H. , Inostroza , L. , Sánchez , M. P. and Tapia , G. 2010 . Drought-tolerant naturalized populations of Lotus tenuis for constrained environments . Acta Agriculturae Scandinavica, Section B – Plant Soil Science , 60 : 174 – 181 .
- Austin , A. T. , Yahdjian , L. , Stark , J. M. , Belnap , J. , Porporato , A. , Norton , U. , Ravetta , D. A. and Schaeffer , S. M. 2004 . Water pulses and biogeochemical cycles in arid and semiarid ecosystems . Oecologia , 141 : 221 – 235 .
- Bao , W. K. , Q. H. Chen , & K. M. Chen 1999 . Environment control techniques for vegetation restoration in dry valley of upper reaches of Minjiang River . Chinese Journal of Applied Ecology 10 , 542 – 544 . (in Chinese, with English abstract).
- Boissier , J. M. and Fontvielle , D. 1995 . Biological characteristics of forest soils and seepage waters during simulated rainfalls of high intensity . Soil Biology and Biochemistry , 27 : 139 – 145 .
- Bouwman , A. F. , Van Vuuren , D. P. , Derwent , R. G. and Posch , M. 2002 . A global analysis of acidification and eutrophication of terrestrial ecosystems . Water, Air and Soil Pollution , 141 : 349 – 382 .
- Brookes , P. C. , Landman , A. , Pruden , G. and Jenkinson , D. S. 1985 . Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil . Soil Biology and Biochemistry , 17 : 837 – 842 .
- Brookes , P. C. , Powlson , D. S. and Jenkinson , D. S. 1982 . Measurement of microbial biomass phosphorus in soil . Soil Biology and Biochemistry , 14 : 319 – 329 .
- Chang , E. H. , Chung , R. S. and Tsai , Y. H. 2007 . Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population . Soil Science and Plant Nutrition , 53 : 132 – 140 .
- de la Paz Jimenez , M. , de la Horra , A. M. , Pruzzo , L. R. and Palma , M. 2002 . Soil quality: a new index based on microbiological and biochemical parameters . Biology and Fertility of Soils , 35 : 302 – 306 .
- Eckersten , H. , Lundkvist , A. and Torssell , B. 2010 . Comparison of monocultures of perennial sow-thistle and spring barley in estimated shoot radiation-use and nitrogen-uptake efficiencies . Acta Agriculturae Scandinavica, Section B – Plant Soil Science , 60 : 126 – 135 .
- Gallardo , A. and Covelo , F. 2005 . Spatial pattern and scale of leaf N and P concentration in a Quercus robur population . Plant and Soil , 273 : 269 – 277 .
- Guan , S. Y. 1986 . Soil enzymes and its methodology . pp. 274 – 340 . Beijing : Chinese Agricultural Press (in Chinese) .
- Hooper , D. U. and Johnson , L. 1999 . Nitrogen limitation in dryland ecosystems: Responses to geographical and temporal variation in precipitation . Biogeochemistry , 46 : 247 – 293 .
- Insam , H. 2001 . Developments in soil microbiology since the mid-1960s . Geoderma , 100 : 389 – 402 .
- IPCC 2001 . Climate change 2001: The scientific basis . In J. T. Hougton , Y. Dung , D. J. Griggs , M. Noguer , P. J. Van der Linden , X. Dui , K. Maskell , and C. A. Johson CA (eds.) Contribution of Working Group I. Third assessment report of Intergovernmental Panel on Climate Change , Cambridge : Cambridge University Press .
- Kandeler , E. , Tscherko , D. and Spiegel , H. 1999 . Long-term monitoring of microbial biomass, N mineralization and enzyme activities of a chernozem under different tillage management . Biology and Fertility of Soils , 28 : 343 – 351 .
- Knops , J. M. H. , Bradley , K. L. and Wedin , D. A. 2002 . Mechanisms of plant species impacts on ecosystem nitrogen cycling . Ecology Letter , 5 : 454 – 466 .
- Lee , K. H. and Jose , S. 2003 . Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient . Forest Ecology and Management , 185 : 263 – 273 .
- Li , X. and Sarah , P. 2003 . Enzyme activities along a climate change transect in the Judean Desert . Catena , 53 : 349 – 363 .
- Lin , Q. and Brookes , P. C. 1996 . Comparison of methods to measure microbial biomass in unamended, ryegrass-amended and fumigated soils . Soil Biology and Biochemistry , 28 : 933 – 939 .
- Liu , G. S. 1996 Soil physical and chemical analysis and description of soil profiles , pp. 33 – 38 . Beijing : Chinese Standards Press (in Chinese) .
- Liu , Q. , W. K. Bao , & Y. K. Qiao 1996 . Studies on the interspecific relationships among dominant species of the semi-arid valley scrubs in Maoxiao on the upper reaches of the Mingjiang River . Chinese Journal of Applied and Environmental Biology , 2 36 – 42 (in Chinese, with English abstract) .
- Lu , R. K. Soil and agro-chemical analytical methods , pp. 296 – 338 . Beijing : Chinese Agricultural Science and Technology Press (in Chinese) .
- Pascuala , I. M. , Antolín , C. , Poloc , C. G. A. and Sánchez-Díaz , M. 2007 . Effect of water deficit on microbial characteristics in soil amended with sewage sludge or inorganic fertilizer under laboratory conditions . Bioresource Technology , 98 : 29 – 37 .
- Pulleman , M. and Tietema , A. 1999 . Microbial C and N transformations during drying and re-wetting of coniferous forest floor material . Soil Biology and Biochemistry , 31 : 275 – 285 .
- Reyes-Reyes , B. G. , Alcántara-Hernández , R. , Rodríguez , V. , Olalde-Portugal , V. and Dendooven , L. 2007 . Microbial biomass in a semi arid soil of the central highlands of Mexico cultivated with maize or under natural vegetation . European Journal of Soil Biology , 43 : 180 – 188 .
- Ros , M. , Hernández , M. T. and García , C. 2003 . Soil microbial activity after restoration of a semiarid soil by organic amendments . Soil Biology and Biochemistry , 35 : 463 – 469 .
- Saiya-Cork , K. R. , Sinsabaugh , R. L. and Zak , D. R. 2002 . The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil . Soil Biology and Biochemistry , 34 : 1309 – 1315 .
- Sardans , J. and Peñuelas , J. 2004 . Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest . Plant and Soil , 267 : 367 – 377 .
- Saviozzi , A. , Bufalino , P. , Levi-Minzi , R. and Riffaldi , R. 2002 . Biochemical activities in a degraded soil restored by two amendments . Biology and Fertility of Soils , 35 : 96 – 101 .
- Sinsabaugh , R. L. , Gallo , M. E. , Lauber , C. , Waldrop , M. and Zak , D. R. 2005 . Extracellular enzyme activities and soil carbon dynamics for northern hardwood forests receiving simulated nitrogen deposition . Biogeochemistry , 75 : 201 – 215 .
- Singh , S. P. , Bargali , K. , Joshi , A. and Chaudhry , S. 2005 . Nitrogen resorption in leaves of tree and shrub seedlings in response to increasing soil fertility . Current Science , 89 : 389 – 396 .
- Stursova , M. , Crenshaw , C. L. and Sinsabaugh , R. L. 2006 . Microbial responses to long-term N deposition in semiarid grassland . Microbial Ecology , 51 : 90 – 98 .
- Thirukkumaran , C. M. and Parkinson , D. 2000 . Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorus fertilizer . Soil Biology and Biochemistry , 32 : 59 – 66 .
- Tu , C. , Ristaino , J. B. and Hu , S. 2006 . Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching . Soil Biology and Biochemistry , 38 : 247 – 255 .
- Vance , E. D. , Brookes , P. C. and Jenkinson , D. S. 1987 . An extraction method for measuring soil microbial biomass C . Soil Biology and Biochemistry , 19 : 703 – 707 .
- Wang , C. , W. Bao , J. Chen , H. Sun , & J. Xie 2003 . Profile characteristics and nutrients of dry cinnamon soils in dry valley of the upper Minjiang River . Chinese Journal of Applied and Environmental Biology , 9 , 230 – 234 (in Chinese, with English abstract) .
- Wu , F. , Bao , W. , Li , F. and Wu , N. 2008 . Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings . Environmental and Experimental Botany , 63 : 248 – 255 .
- Wu , F. , Bao , W. , Zhou , Z. Q. and Wu , N. 2009 . Carbon accumulation, nitrogen and phosphorus use efficiency of Sophora davidii seedlings in response to N supply and water stress . Journal of Arid Environment , 73 : 1067 – 1073 .
- Xue , D. , Yao , H. and Huang , C. 2006 . Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils . Plant and Soil , 288 : 319 – 331 .
- Yan , T. , Yang , L. and Campbell , C. D. 2003 . Microbial biomass and metabolic quotient of soils under different land use in the three Gorges reservoir area . Geoderma , 115 : 129 – 138 .
- Yang , C. M. , Yang , L. Z. and Yan , T. M. 2005 . Chemical and microbiological parameters of paddy soil quality as affected by different nutrient and water regimes . Pedosphere , 15 : 369 – 378 .
- Zaman , M. and Chang , S. X. 2004 . Substrate type, temperature, and moisture content affect gross and net N mineralization and nitrification rates in agroforestry systems . Biology and Fertility of Soils , 39 : 269 – 279 .
- Zhang , Q. H. and Zak , J. C. 1998 . Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in West Texas . Soil Biology and Biochemistry , 30 : 39 – 45 .
- Zhang , Y. L. and Wang , Y. S. 2006 . Soil enzyme activities with greenhouse subsurface irrigation . Pedosphere , 16 : 512 – 518 .
- Zhang , Y. M. , Wu , N. , Zhou , G. Y. and Bao , W. K. 2005 . Changes in enzyme activities of spruce (Picea balfouriana) forest soil as related to burning in the eastern Qinghai-Tibetan Plateau . Applied Soil Ecology , 30 : 215 – 225 .
- Zhong , W. H. and Cai , Z. C. 2007 . Long-term effects of inorganic fertilizers on microbial biomass and community functional diversity in a paddy soil derived from quaternary red clay . Applied Soil Ecology , 36 : 84 – 91 .