Abstract
Soil nematode communities in greenhouses with different duration of continuous cropping were investigated from October 2007 to June 2008. Total nematode populations and trophic groups were observed. Fourteen families and 24 genera were identified; the genera Protorhabditis, Diploscapter, Meloidogyne and Helicotylenchus comprised 74.4% of the total population (from all tested samples). Plant-parasitic and bacterivorous nematodes were most abundant among the trophic groups. Populations of both trophic groups increased with increasing times of continuous cropping. The numbers of soil nematodes at different soil depths were significantly different (p <0.05). Shannon–Wiener index (H′) and Simpson index of diversity (D) were highest in 0-yr soil of all soils. Plant parasite index (PPI) and PPI/MI (maturity index) of soil nematodes increased with the increasing times of continuous cropping suggesting that continuous cropping resulted in gradual shift of plant-parasitic nematodes from k-strategists to r-strategists.
Introduction
Free-living nematodes are the most abundant metazoans in the soil, constitute one of the most numerically important components of the soil fauna and can significantly impact nutrient cycling and primary productivity in diverse ecosystems. Nematode populations can respond in predictable ways to ecosystem disturbance (Freckman and Ettema Citation1993), and are regarded as the most important secondary consumer (Mulder et al. Citation2005). Changing soil conditions have direct and indirect effects on soil nematode populations and community structure, which play an important role in indicating changes in the soil environment and the stability of soil ecological systems. Previous laboratory experiments and field studies have shown that nematodes that feed on bacteria and fungi play important roles in influencing the turnover of soil microbial biomass and the availability of plant nutrients (Bardgett et al. Citation1999). Nematode faunae in agroecosystems and their relationship with soil processes make them good bioindicators (Yeates and Bongers Citation1999). Nematodes vary in their sensitivity to pollutants and environmental disturbance (Bongers and Ferris Citation1999), and are considered to be biotic indicators of soil ecological function and biological diversity (Ekschmitt et al. Citation2001, Citation2003, Neher Citation2001).
The nematode diversity of plastic-film greenhouses offers many possibilities for use as a biological indicator of agricultural practices, soil characteristics and the degree of conservation of soils, especially in continuous cropping of the same soil. In recent years, plastic-film greenhouses have become very popular in Shandong, and tomato or cucumbers were planted continuously in the same soil, which are easily infected by root-knot nematode. The populations of root-knot nematode second-stage juveniles (J2) in comparison to other free-living soil nematodes in different continuous cropped soils were studied in our previous study; an adverse trend was found between the number of free-living nematodes and root knot nematode J2 (Wu and Shi Citation2011). However, nematode community structure in soil infected by root-knot nematodes under continuous cropping of tomato was not presented.
This study focused on the soil nematode community structure in greenhouses with different continuous cropping times. The object of this study was to (i) investigate the vertical distribution of soil nematodes in the soils in such greenhouses, (ii) identify changes in soil nematode trophic groups in soils with different continuous cropping times and (iii) evaluate nematode diversity in different continuous cropping time greenhouses on the basis of species-level taxonomy guild.
Materials and methods
Study site
The study was conducted at Zoujiazhuang Village (35°54′N, 117°11′E), Fangcun Town, Taian City, Shandong Province, China. All tested greenhouses were established in the same original field, a winter wheat–summer corn planting structure was applied before the greenhouse was built. There were identical soil texture and type (loam) and management methods (application of fertilizer, irrigation and application of agricultural chemicals), with different typical continuous cropping times which included non-continuous cropping (new greenhouse, winter wheat–summer corn system was used before, 0-yr) and 5-, 8-, 10- and 12-yr of continuous cropping, with the exception of the application of about 200 kg ha-1 organic manure in the 10-yr greenhouse every year. In each greenhouse, three 30 m2 sample micro-plots were selected randomly from which all soil samples were obtained. The plots were treated with 10% (m/v) cadusafos granules (developed and marketed by FMC Corporation) (60.0 kg ha-1) 15 d before seedling transplantation; tomato seedlings were transplanted on August 30, 2007, and the final harvest occurred on June 28, 2008. Fertilizer application and irrigation management were standard.
Samples collection
In order to monitor soil nematode community dynamic, soil sampling was performed eight times during the tomato growing season, at approximately 40-day intervals, i.e. at 38 (Oct. 6, 2007), 76 (Nov. 13, 2007), 114 (Dec. 21, 2007), 152 (Jan. 8, 2008), 190 (Mar. 6, 2008), 228 (Apr. 13, 2008), 266 (May 23, 2008), and 304 days after transplanting (June 28, 2008). Ten cores (diameter, 5 cm) were collected with a soil auger in a Z-pattern in each micro-plot, in the rhizosphere 5 cm from the nearest tomato plant. Samples were collected from three soil layers at depths of 0–10 cm, 10–20 cm and 20–30 cm, and samples from the same layer of the same plot were pooled, mixed, and divided into three sub-samples to represent each layer of the tested greenhouse.
Extraction and identification of soil nematodes
Nematodes were extracted from a 100 g (fresh weight) soil sample with a washing-sieving sugar flotation and centrifugation procedure and preserved in triethanolamine formalin (TAF) (Courtney et al. Citation1955). Soil moisture content was determined by oven drying. Nematodes were counted, identified and classified by using an optical stereoscope and microscope (Yin Citation1998, Xie Citation2000). Nematode populations were expressed as number per 100 g dry soil (DS) (Wu et al. Citation2008).
Trophic group and ecological indices
All nematodes extracted were classified into fungivores, bacterivores, plant parasites and predators/omnivores based on the classification of Yeates et al. (1993). Ecological indices were calculated as follows. Diversity (McSorley and Frederick Citation1996, Yeates and Bongers Citation1999): (1) Shannon–Wiener index ; (2) Simpson index
; and (3) Evenness index
, where p
i
is the proportion of taxon n
i
in the total nematode community n, s is the total number of species in the nematode community. (4) Species Richness: SR=(S-1)/ln N, where S is the total number of genus in the taxon and N is the number of individuals in the sample. (5) Maturity index (MI) for free-living nematodes and plant-parasite index (PPI) (Bongers Citation1990, Bongers and Bongers Citation1998).
,
, where v(i) is the c-p (colonizer-persister) (MI for non-plant parasites and PPI for plant parasites) value of taxon i, and f(i) is the frequency of that taxon in a sample. (6) Nematode channel ratio, NCR = B/(B + F) where B and F are the abundance of bacterial-feeding and fungal-feeding nematodes, respectively (Yeates Citation2003).
Statistical analysis
The value is the grand mean from the samples from different continuous cropping greenhouse soils during tomato growing season (3 replicates×8 times, 24 samples). Dominance was based on the proportion of individuals or trophic group in the total population of soil nematodes. A 2-way analysis of variance (ANOVA 9.0) was performed to determine significant differences of soil nematodes from different continuous cropping soils, and different layers; differences obtained at levels of p<0.05 were considered significant.
Results
Soil nematode community
Fourteen families and 26 genera were identified, including 11 bacterivores, two fungivores, nine plant parasites, and four predators/omnivores. The four trophic groups exhibited significant differences between soils from greenhouses with different continuous cropping times (). In 0-yr soil, fungivorous and predaceous/omnivorous nematodes had an abundance of 5.9% and 16.6%, which were 6.4 and 2.3 times, respectively, greater than those in 12-yr soil. Protorhabditis, Diploscapter, Meloidogyne and Helicotylenchus were the dominant groups, comprising 74.4% of the total nematode population. For the bacterivorous trophic group, in 0-yr soil, cp-2 group was more prevalent (42.4%) than cp-1 group (13.7%); in contrast, in the other soils, cp-1 group was more prevalent (cp-2 group, 6.0–9.6%; cp-1 group, 17.9–50.5%).
Table I. Trophic groups, families/genus, c-p value and relative abundance (%) of soil nematodes identified at 0–30 cm soil depth from different continuous cropping greenhouse soil in this study.
Nematode communities in soils with different continuous cropping times
Soil nematode communities differed among soils with different continuous cropping times. The relative abundance of bacterivorous nematodes in 0- and 10-yr soils was higher than those in other soils, 56.0% and 60.1% respectively, vs. 32.6%, 54.1% and 23.9% in 5-, 8- and 12-yr soils, respectively, and the abundance of different genera ranged from 0.1–21.5%, 0.0–16.3%, 0.0–37.1%, 0.0–27.3% and 0.0–9.6% in 0-, 5-, 8-, 10- and 12-yr soils, respectively. The abundance of fungivorous nematodes in 0-yr soil was the highest at 5.9%. Meloidogyne spp. and Helicotylenchus spp. were the dominant populations among the plant-parasitic nematodes, and showed differences between soils with different continuous cropping times. The abundance of Meloidogyne was high in the 8-, 10- and 12-yr soils; the relative abundance of the predator-omnivore group was highest in 0-yr soil of all soils.
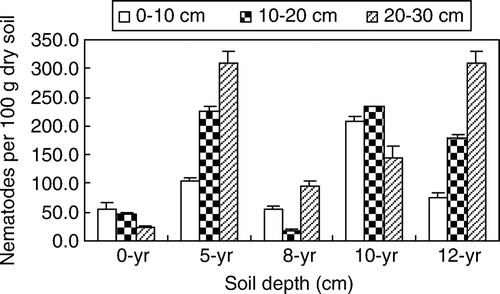
Vertical distribution of soil nematodes in soils with different continuous cropping times
Significant differences were found in the number of soil nematodes in different soils (, p <0.05). At depths of 0–10 cm and 10–20 cm, the same distributions were found in all soils. The abundance of soil nematodes at these two soil depths decreased in the order 10-yr soil > 5-yr soil > 12-yr soil > 0-yr soil > 8-yr soil. The maximum number of nematodes occurred in the 10-yr soil, with 209.3 and 235.3 individuals per 100 g dry soil at 0–10 cm and 10–20 cm depths, respectively, significantly higher than those in other soils (p <0.05). However, at 20–30 cm, the number of nematodes from different greenhouse soils showed the same pattern with 0–10 cm and 10–20 cm depths except that the nematode population from the 10-yr soil decreased. Therefore, it can be said that in 5- and 12-yr soils, the number of soil nematodes increased with soil depth (from 0 to 30 cm), whereas in 0-yr soil, the number of soil nematodes decreased with soil depth.
Soil nematode trophic groups in soils with different continuous cropping times
Plant parasites and bacterivores were the most abundant trophic groups in soils with different continuous cropping times; their relative abundance was 52.6% and 41.1%, respectively. The populations of predator-omnivores and fungivores were small; their relative abundance was 4.0% and 2.2%, respectively. Differences were found in the individual trophic groups among the soils with different continuous cropping times (p <0.05).
The mean number of bacterivorous nematodes at the 0–30 cm soil depth ranged from 10.1 to 150.1 individuals per 100 g dry soil (A). The number of bacterivorous nematodes in the 10-yr soil was higher than that in other soils at 0–10 and 10–20 cm, and the number in 5- and 12-yr soils increased with increasing soil depth. The relative abundance of bacterivorous nematodes in the 10-yr soil was higher, than that in other soils at 0–20 cm (p <0.05).
Figure 2. Four trophic groups nematodes in soils with different continuous-cropping time and depth. A, Bacterivores; B, Fungivores; C, Plant-parasites; D, Omnivore-predators. The value is a mean from 24 samples. Bar stands for standard deviation.
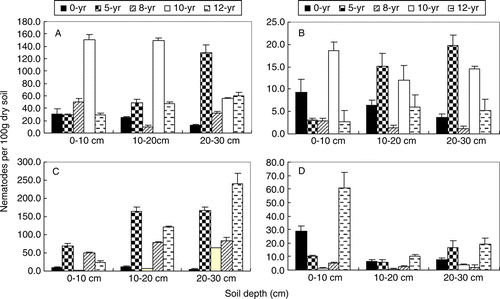
The abundance of fungivorous nematodes was low in the study during the tomato growing season (B). The mean number of fungivorous nematodes at 0–30 cm soil depths ranged from 1.1 to 19.8 individuals per 100 g dry soil. At 0–10 cm, the mean number in 10-yr soil was higher than in other soils (p <0.05). Fungivores in 0- and 8-yr soils decreased with increasing soil depth, and increased with soil depth in 12-yr soil.
Differences were found in populations of plant parasitic nematodes in different continuous cropping time soils and different soil depths (C, p<0.05). The mean value ranged from 2.7 to 241.0 individuals per 100 g dry soil (C). The number of plant parasites in 0-yr soil was the lowest, showing patterns similar to those of bacterivorous and fungivorous nematodes, and decreasing with soil depth. The abundance of plant-parasitic nematodes in 5-, 8-, 10- and 12-yr soils increased with soil depth. For predator-omnivores, the mean ranged from 0.6 to 60.9 individuals per 100 g dry soil at 0–30 cm soil depth (D). The mean number in 12-yr soil was significantly higher than those in other soils (p <0.05).
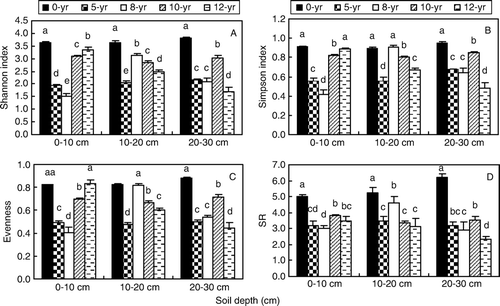
Diversity of soil nematodes in different continuous cropping soils
Shannon diversity index (H′), Simpson index (D), evenness (J) and SR of the different continuous cropping soils are presented in . In 0-yr soil, we found high values for H′, D, J and SR. H′, D, J and SR indices of soil nematodes were significantly different in different soils and at different soil depths (p <0.05). The minimum value of H′ was 1.5 in 8-yr soil at 0–10 cm soil depth, and the value in 12-yr soil decreased with increasing soil depth. The value of H′, D and J exhibited similar trends in different cropping times and soil depths. SR indices of soil nematodes in 0-yr soil were significantly higher than those in other soils (p<0.05), 5.0, 5.3 and 6.2 at 0–10 cm, 10–20 cm and 20–30 cm, respectively.
Characteristics of soil nematode functional groups
There were significant differences between MI indices of soils with different continuous cropping time at different depths (D, p<0.05). The MI index of 0-yr soil was significantly higher than that of other soils, except of the 12-yr soil at 0–10 cm depths, the highest value was 1.9 at 0–10 cm depths of 0-yr soil. The MI value decreased with increasing soil depth, except in the 5-yr soil; there was no significant difference between soil layers in 5-yr soil. PPI (B) indices of soil nematodes were significantly higher in continuous cropping soil than in 0-yr soil; PPI in 8-yr, 10-yr and 12-yr soils increased with soil depth, while the value of 0-yr samples was the lowest at all soil depths.
Figure 4. Maturity index (MI), plant parasite index (PPI), PPI/MI and Nematode channel ratio in soils with different continuous-cropping time and depth. A, Maturity index (MI), B: plant parasite index (PPI), C: PPI/MI, D: Nematode channel ratio (NCR) = B/(B + F) (B, Bacterivores; F, Fungivores) of soil nematodes in different continuous-cropping soil at 0–30 cm soil depth in the study. The value is a mean from 24 samples. Bar stands for standard deviation.
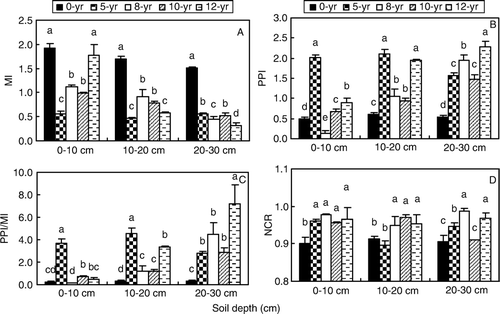
The PPI/MI value in 5-yr soil at 0–20 cm depth was significantly higher than that in other continuous cropping soils; the value in 0-yr soil was significantly lower at 0–30 cm depth than other greenhouse soils (p<0.05). The PPI/MI value in 12-yr soil at 20–30 cm depth was the highest (p<0.05), the value in 8-, 10- and 12-yr soils increased with the soil depth (0–30 cm). In the present study, nematode channel ratio (NCR) in all soils had a high value, greater than 0.90. Non-continuous cropping greenhouse soil (0-yr) had a significantly lower mean NCR (0.905) than other soils at 0–30 cm soil depth (p<0.05); NCR of 5-, 8-, 10- and 12-yr soils were 0.934, 0.971, 0.946 and 0.963, respectively (D).
Discussion
In this study, the number of soil nematodes and the abundance of trophic groups were different in soils with different continuous cropping times. Bacterivores were most abundant in 0- and 10-yr soils, and the least abundant in 12-yr soil. Fungivores and predator-omnivore nematodes were more abundant in 0-yr soil than in other soils. In contrast, plant-parasitic nematodes in 12-yr soil were most abundant of all soils. In the present study, soil nematodes showed different trends with increasing soil depth in different cropping time soils (nematode population decreased in 0-yr, increased in 5- and 8-yr with soil depth). From the results of vertical distribution of soil nematodes, continuous cropping has an effect on soil nematodes of deep soil, with an increase in nematode population in the 10–30 cm soil layer compared with non-continuous cropping soil.
Among the trophic groups in this study, plant parasites and bacterivores were more abundant, whereas predator-omnivores and fungivores were less abundant; these results were consistent with previous findings in agricultural soils (Wasilewska Citation1979). As per our investigation, in bacterial-feeding nematodes, 0-yr soil had a higher proportion of the cp-2 group than the cp-1 group, and the reverse trend was observed in soils with different continuous cropping times. These results are consistent with those of Yeates et al. (Citation2002), which report that the population of Cephalobus spp. (cp-2 group) was greater than that of Rhabditis spp. or Pristionchus spp. (cp-1 group) in undisturbed soils at various soil moisture tensions. The results suggested that soil nematode community structure can reflect the degree of human disturbance derived mainly from continuous cropping in greenhouses. Our study showed that the nematode community structure remained relatively stable in 0-yr soil during the tomato growing season, and the balance of nematode communities was disrupted with an increase in continuous cropping times.
The populations of Cephalobidae were the largest in previous studies (Yeates Citation1984, Korthals et al. Citation1996, Yeates et al. Citation2000). In this study, populations of Cephalobidae and Rhabditidae were dominant, and the plant parasites Helicotylenchus and Meloidogyne gradually became dominant with increasing continuous cropping time in plastic film greenhouses. Eucephalobus and Aporcelaimus were the dominant groups in 0-yr soil. Meloidogyne was the most abundant in 12-yr soil. The ecological structures of soil nematode communities were broken, because of the repeated disturbances such as those caused by the continuous planting of the same crops and use of the same management methods. This phenomenon was confirmed from the evidence that the adverse trend is found in populations of free-living nematodes and second stage juveniles (J2) of root-knot nematode with increasing of continuous cropping time (Wu and Shi Citation2011). Plant-parasitic nematode, r-strategists population increases with increasing continuous cropping time, and the root-knot nematode gradually becomes dominant and seriously damages host plants in all soils with an exception in 10-yr soil. This suggests that continuous cropping resulted in a gradual shift of plant-parasitic nematodes from K-strategists to r-strategists. The low Meloidogyne spp. population in 10-yr soil can be explained in that organic matter amendments to soil might increase antagonistic microorganisms or produce a nematicidal substance to control root knot nematode (Rodríguez-Kábana et al. Citation1987; Oka and Yermiyahu Citation2002).
H' and SR can be used to assess nematode diversity in continuous cropping systems. Our results showed that H′ decreased due to continuous cropping, H′ and SR the nematode fauna were all greatest in the 0-yr soil. The decrease in diversity index H′ of nematode fauna with increasing of continuous cropping time reflects not only continuous physical disturbance, but also increases in specific plant-feeding nematodes associated with crops. The nematode MI, based on the species composition of the nematode community, was proposed as a gauge of the condition of the soil ecosystem, reflecting the degree of disturbance in the soil ecosystem (Bongers Citation1990); MI would decrease after disturbance and subsequently increase with recovery and succession (Ettema and Bongers Citation1993). We found that 0-yr soil has a higher MI value and lower PPI than other soils (p<0.05), which suggested that the soil was less disturbed than other soils with more years of tomato cropping. The higher PPI value in continuous cropping soil was due to the more abundant Meloidogyne spp. population, as a result of the long-term presence of the same host plant providing a food source to the nematodes. Nematode channel ratio in this study was 0.90–0.99, higher than what has been previously reported in other studies. In Nebraska, United States, continuous corn resulted in an NCR of 0.554 (Neher and Olson Citation1999), while in the Netherlands, integrated plots had an NCR of 0.966 and conventional plots had an NCR of 0.736 (Bouwman and Zwart Citation1994).
Bongers et al. (Citation1997) demonstrated that under certain conditions, the PPI and MI behave in opposition, and suggested that the ratio of the MI and PPI is a sensitive monitor of agro-ecosystems. The PPI/MI of the natural environment is lower than that of a disturbed environment. In this study, the MI of soil nematodes in 0-yr soil was maximized and decreased with increasing continuous cropping times in greenhouses.
In conclusion, soil nematode biodiversity decreased, root-knot nematode population increased, PPI and PPI/MI of soil nematodes increased with the increasing of duration of continuous tomato cropping under this greenhouse condition, suggesting that the degree of soil disturbance increased with continuous cropping time in greenhouses, and the community structure of soil nematodes was altered by continuous cropping tomato.
Acknowledgements
This study was funded by Special Fund for Agro-scientific Research in the Public Interest (200903040), The Technology Program of the Higher Education Institutions of Shandong Province (J11LC23).
References
- Bardgett , R. D. , Cook , R. , Yeates , G. W. and Denton , C. S. 1999 . The influence of nematodes on below-ground processes in grassland ecosystems . Plant and Soil , 212 : 23 – 33 .
- Bongers , T. 1990 . The maturity index: An ecological measure of environment disturbance based on nematode specie composition . Oecologia , 83 : 14 – 19 .
- Bongers , T. and Bongers , M. 1998 . Functional diversity of nematodes . Applied Soil Ecology , 10 : 239 – 251 .
- Bongers , T. and Ferris , H. 1999 . Nematode community structure as a bioindicator in environmental monitoring . Trends in Ecology and Evolution , 14 : 224 – 228 .
- Bongers , T. , Van Der Meulen , H. and Korthals , G. 1997 . Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions . Applied Soil Ecology , 6 : 195 – 199 .
- Bouwman , L. A. and Zwart , K. B. 1994 . The ecology of bacterivorous protozoans and nematodes in arable soil . Agriculture Ecosystems and Environment , 51 : 145 – 160 .
- Courtney , W. D. , Polley , D. and Miller , V. L. 1955 . TAF, an improved fixative in 15 nematode technique . Plant Disease Reporter , 39 : 570 – 571 .
- Ekschmitt , K. , Bakonyi , G. , Bongers , M. , Bongers , T. , Boström , S. , Dogan , H. , Harrison , A. , Nagy , P. , O'Donnell , A. G. , Papatheodorou , E. M. , Sohlenius , B. , Stamou , G. P. and Wolters , V. 2001 . Nematode community structure as indicator of soil functioning in European grassland . European Journal of Soil Biology , 37 : 263 – 268 .
- Ekschmitt , K. , Stierhof , T. , Dauber , J. , Kreimes , K. and Wolters , V. 2003 . On the quality of soil biodiversity indicators: abiotic parameters as predictor of soil faunal richness at different spatial scales . Agriculture Ecosystems and Environment , 98 : 273 – 283 .
- Ettema , C. H. and Bongers , T. 1993 . Characterization of nematode community colonization and succession in disturbed soil using the Maturity Index . Biology and Fertility of Soils , 16 : 79 – 85 .
- Freckman , D. W. and Ettema , C. H. 1993 . Assessing nematode communities in agroecosystems of varying human intervention . Agriculture Ecosystems and Environment , 45 : 239 – 261 .
- Korthals , G. W. , Alexiev , A. D. , Lexmond , T. M. , Kammenga , J. E. and Bongers , T. 1996 . Long-term effects of copper and pH on the nematode community in an agroecosystem . Environmental Toxicology and Chemistry , 15 : 979 – 985 .
- McSorley , R. and Frederick , J. J. 1996 . Nematode community structure in rows and between rows of a soybean field . Fundamental and Applied Nematology , 19 : 251 – 261 .
- Mulder , C. , Schouten , A. J. , Hund-Rinke , K. and Breure , A. M. 2005 . The use of nematodes in ecological soil classification and assessment concepts . Ecotoxicology and Environmental Safety , 62 : 278 – 289 .
- Neher , D. A. 2001 . Role of nematodes in soil health and their use as indicators . Journal of Nematology , 33 : 161 – 168 .
- Neher , D. A. and Olson , R. K. 1999 . Nematode communities in soils of four farm cropping management systems . Pedobiologia , 43 : 430 – 438 .
- Oka , Y. and Yermiyahu , U. 2002 . Suppressive effects of composts against the root-knot nematode Meloidogyne javanica on tomato . Nematology , 4 : 891 – 898 .
- Rodríguez-Kábana , R. , Morgan-Jones , G. and Chet , I. 1987 . Biological control of nematodes: Soil amendments and microbial antagonists . Plant and Soil , 100 : 237 – 247 .
- Wasilewska , L. 1979 . The structure and function of soil nematode communities in natural ecosystems and agrocenoses . Polish Ecological Studies , 5 : 97 – 145 .
- Wu , H. Y. and Shi , L. B. 2011 . Effects of continuous cropping duration on population dynamics of second-stage juvenile Meloidogyne spp. and free-living soil nematodes . African Journal of Agricultural Research , 6 : 307 – 312 .
- Wu , H. Y. , Li , X. X. , Shi , L. B. , Wang , Z. H. and Ma , F. Y. 2008 . Distribution of nematodes in wetland soils with different distance from the Bohai sea . Plant Soil and Environment , 54 : 359 – 366 .
- Xie , H. 2000 Plant nematodes taxonomy Hefei, China: Science Technology Press of Anhui. 206 pp [In Chinese].
- Yeates , G. W. 1984 . Variation in soil nematode diversity under pasture with soil and year . Soil Biology Biochemistry , 16 : 95 – 102 .
- Yeates , G. W. 2003 . Nematodes as soil indicators: functional and biodiversity aspects . Biology and Fertility of Soils , 37 : 199 – 210 .
- Yeates , G. W. , Dando , J. and Shepherd , T. G. 2002 . Pressure plate studies to determine how moisture affects access of bacterial-feeding nematodes to food in soil . European Journal of Soil Science , 53 : 355 – 365 .
- Yeates , G. W. , Hawke , M. F. and Rijkse , W. C. 2000 . Changes in soil fauna and soil conditions under Pinus radiata agroforestry regimes during a 25-year tree rotation . Biology and Fertility of Soils , 31 : 391 – 406 .
- Yeates , G. W. and Bongers , T. 1999 . Nematode diversity in agroecosystems . Agriculture Ecosystems and Environment , 74 : 113 – 135 .
- Yeates , G. W. , Bongers , T. , de Goede , R. G. M. , Freckman , D. W. and Georgieva , S. S. 1993 . Feeding habits in soil nematode families and genera: an outline for ecologists . Journal of Nematology , 25 : 315 – 331 .
- Yin , W. Y. 1998 Soil animal retrieval illustrated handbook of China Beijing: Science Press. 756 pp [In Chinese].