Abstract
A study was conducted investigating the possible utilization of mycorrhiza to enhance yield and quality of tomatoes grown in a soilless culture system using sawdust or coir as growing media. The experiment was carried out in temperature-controlled and non-temperature-controlled (NTC) tunnels. Fertigation was applied at three levels (100%, 75%, and 50%) of the recommended nutrient concentration. Mycoroot™, containing four arbuscular mycorrhizal species (Glomus etunicatum, Paraglomus occultum, Glomus clarum, and Glomus mosseae), was applied at seeding, as well as transplanting. Growing tomatoes under reduced nutrient supply reduced the total soluble solids in the juice of the fruits, but improved total and marketable yield, as well as the number of marketable fruits. This effect was more substantial in the temperature-controlled tunnel than in the NTC tunnel. Fruit firmness and leaf chlorophyll concentrations were significantly higher in plants grown in the temperature-controlled tunnel. Growing tomatoes in sawdust improved the leaf Mn and Ca concentration over that of tomato plants grown in coir. Mycorrhiza colonization did not have a beneficial effect on tomato yield and quality. Further studies, including different media, nutrient composition, and concentration need to be carried out to investigate the possible effect of AMF failing to improve yield, despite AMF root colonization, and to reveal the cause of the poor beneficial effect of AMF on tomato plants grown under soilless culture.
Introduction
Arbuscular mycorrhizal fungi (AMF) are able to form mutualistic relationships with 80% of all terrestrial plants, including most agricultural, particularly horticultural crops, as well as certain forestry species (Pozo & Azcon-Aguilar Citation2007). Such interactions result in the transfer of carbon (sugars) from the host plant to the fungi while the fungi improve the uptake of water and nutrients by the root system (Tahat et al. Citation2008). The intra-radical colonization of plant roots by AMF results in the formation of arbuscules, specialized fungal structures (for the exchange of nutrients with the host plants), and vesicles (storage organelles), which can significantly enhance the absorbing capacity of the root for water and nutrients (Kaya et al. Citation2003). Many improvements can be achieved by this AMF–host plant interaction, such as better plant establishment and growth, enhanced water and nutrient uptake, and improved resistance to biotic and abiotic stresses (Davies et al. Citation1992; Smith & Read Citation1997; Muok & Ishii Citation2006; Sawers et al. Citation2008), ultimately leading to increased growth and yield. The resultant improved productivity of AMF-inoculated plants has been attributed to enhanced acquisition of nutrients of low mobility, such as P, Zn, and Cu (Lambert et al. Citation1979; Ortas et al. Citation1996; Liu et al. Citation2002; Kaya et al. Citation2003; Ryan & Angus Citation2003). The transport and absorption of such nutrients in soils low in P, Ca, and Mg (Liu et al. Citation2002) result in increased root and shoot biomass, as well as enhanced yield.
Previous studies have shown that the mycorrhizal colonization of tomato plants had beneficial effects on plant growth (Al-Karaki et al. Citation2001) and can cause yield increases in field-grown crops. Host plants benefit from AMF through the enhanced production of growth-regulating substances, increased photosynthesis, and improved osmotic adjustment under water and salinity stress (Al-Karaki Citation2006). However, Mueller et al. (Citation2009) did not observe beneficial effects of AMF on growth and nutrient uptake when plants were grown in peat or sand.
Plant cultivation under soilless conditions suppresses the AMF–plant root association (Linderman & Davis Citation2003), possibly due to high rates of fertilizers applied to plants grown under this system. Mycorrhiza colonization is also influenced by the growing medium (Corkidi et al. Citation2004); certain soilless media, like redwood shavings, and certain barks contain high concentrations of phenols which have an inhibitory effect on mycorrhizal colonization (Biermann & Linderman Citation1983; Graham & Timmer Citation1984; Johnson & Hummel Citation1986). Mycorrhiza formation has been successful when soil was added to the soilless medium (Linderman & Davis Citation2003). Alternatively, the use of slow release fertilizers (Coltman et al. Citation1988) or reduced phosphorus fertilization (Caron & Parent Citation1988, Peters & Habte Citation2001) also allows the establishment of AMF in the medium. The beneficial effects of AMF on crops grown in soil have been reported; however, there is limited research on the benefits of mycorrhiza for plants grown under soilless conditions (Al-Karaki Citation2006, Dasgan et al. Citation2008, Abak et al. Citation2010).
In southern Africa, as well as in many other countries in tropical/subtropical climates the potential for high productivity of tomatoes exists, due to the high solar radiation received. However, constraints such as excessive heat, especially during the summer season, can reduce the productivity. Under such conditions, farmers in South Africa tend to produce tomatoes under protection in greenhouses or tunnels that rely on natural ventilation (Maboko et al. Citation2012). However, during the hot summer season in non-temperature-controlled (NTC) tunnels, there is a tendency towards poor plant growth, low yield, and poor quality (Maboko et al. Citation2012).
Due to the beneficial effects of mycorrhiza on plant growth, particularly under environmental stress, this study was carried out to investigate the effects of mycorrhiza, growing media, and strength of supplied nutrient concentration on tomatoes grown in temperature-controlled (TC) and NTC tunnels.
Materials and methods
Application of treatments
Experiments were conducted in NTC and TC tunnels at the Agricultural Research Council-Roodeplaat Vegetable and Ornamental Plant Institute (ARC-Roodeplaat VOPI), Roodeplaat, South Africa (25°59′S; 28°35′E, altitude 1200 m a.s.l.) from October 2010 to February 2011.
Five-week-old fresh-market tomato seedlings (cultivar ‘FA593,’ Sakata seed, Southern Africa, Pty. Ltd) were transplanted into 10 L plastic bags containing sawdust or coir as a growing medium. The growing media were washed thoroughly with tap water (three times) before filling the bags and moistened again before the transplanting of seedlings. Arbuscular mycorrhiza fungi were applied at seeding and transplanting, as early inoculation results in the rapid spread of mycorrhiza to new roots during germination (Ikiz et al. Citation2009), contributing to higher yields following transplantation to the field (Stewart et al. Citation2005, Douds et al. Citation2008). Mycoroot™, containing four arbuscular mycorrhiza species (Glomus etunicatum, Paraglomus occultum, Glomus clarum, and Glomus mossea), was applied at seeding and transplanting. Mycoroot™ was applied at a rate of 1 g L−1 Hygromix® (commercial seedling growth medium, Hygrotech) and it was thoroughly mixed before seeding. One teaspoon (7 g) of Mycoroot™ granules (1 g Mycoroot™ contains approximately 100 propagules, with a minimum of 10 spores per gram) was applied to the planting holes at the time of transplanting. The root system of the seedling was placed on top of the Mycoroot™ granules and covered with growing medium.
The composition and chemical concentration of fertilizers used for tomato production were: Hygroponic® (comprising N (68 mg/kg), P (42 mg/kg), K (208 mg/kg), Mg (30 mg/kg), S (64 mg/kg), Fe (1.254 mg/kg), Cu (0.022 mg/kg), Zn (0.149 mg/kg), Mn (0.299 mg/kg), B (0.373 mg/kg), and Mo (0.037 mg/kg)); calcium nitrate [Ca(NO3)2] (comprising N (117 mg/kg) and Ca (166 mg/kg)); and potassium nitrate (KNO3) (comprising K (38.6 mg/kg) and N (13.8 mg/kg)). Plants were also subjected to three fertigation treatments, i.e., 100%, 75%, and 50% of the recommended nutrient concentration (). The different fertilizer regimes were applied to tomato plants grown in a TC tunnel equipped with two fans and a pad (1.1 kW fans, 1300 mm diameter) cooling system, and a NTC tunnel which relied on natural ventilation by means of a flap and door system that could be opened on each side. Tunnels (10 m width×30 m length) were covered with a 200 µm light-diffusive plastic (Evadek green tint). The floor of the plastic tunnel was covered with 200 µm white plastic. Plants were planted at a density of 2.5 plants m−2, with eight data plants in each replicate per treatment. The treatments consisted of three fertigation, two growing media, and two mycorrhiza treatments in each tunnel. Plants were irrigated, one dripper per plant (discharge rate of 35 mL min−1) at two hourly intervals, seven times a day. The irrigation volume was gradually increased as the plants enlarged to ensure that 10–15% of the applied water leached out to reduce salt build-up in the growing medium (total daily irrigation during the growing season ranged from 735 to 2205 mL per plant equivalent to three to nine minutes, respectively) (Maboko et al. Citation2012). The physical properties of sawdust and coir used in this study were % moisture, pH, bulk density (g mL−1), water-holding capacity (%), and air porosity (%) which was 8.39 and 13.30, 6.30 and 6.60, 0.059 and 0.0619, 51.5 and 71.7, and 24.69 and 6.93, respectively. The chemical properties of sawdust and coir (mg L−1) was, respectively, NO3– (0.3 and 1.0), NO2– (0.2 and <0.6), Cl– (30.1 and 154.6), SO4 − (24.4 and 489.0), PO4 − (5.9 and 12.5), Na+ (5.1 and 109.5), Ca2 + (13.5 and 9.1), Mg2 + (5.8 and 13.7), Fe+ (1.60 and 1.45), Mn2 + (0.69 and 0.10), and Cu2 + (0.13 and 0.05). The electrical conductivity of sawdust and coir was 0.23 and 1.41 dS m−1, respectively.
Table I. Amount of fertilizer applied (g L−1) for fertigation treatments at different growth stages of the tomato plant.
The pH of the nutrient solution was maintained within a range of 5.8–6.1. Maximum, minimum, and mean monthly ambient temperatures for the experimental sites during the experimental period were recorded using data-loggers (Tinyview, Gemini data loggers (UK) Ltd), which were placed at a height of 1.5 m and covered with a Stevenson-type screen ACS-500.
Plants were trained to a single stem by twisting trellis twine around the main stem and fixing it to a stay wire 2 m above the ground surface to support the plant. Side branches were removed weekly to maintain a single stem system. When the plants had reached the horizontal wire at 2 m, the growing point was removed to stop further plant growth.
Mycorrhiza colonization
At the end of the experiment, two plants per replicate per treatment were used to determine the percentage of AMF colonization. Roots were rinsed carefully with tap water, with root clearing and staining procedures performed according to Koske and Gemma (Citation1989). Colonization by AMF was examined microscopically to determine the percentage of root segments containing arbuscules and vesicles using the gridline intercept method (Giovannetti & Mosse Citation1980).
Plant growth and fruit yield measurements
Fruit were harvested weekly at the breaker stage in mid-summer from December to February. Yield data were collected from six plants per treatment, and the performance of the treatments evaluated using total yield, marketable and unmarketable yield, as well as physiological and pathological disorders as parameters. Fruit were regarded as unmarketable when they exhibited cracking, zippering, rotting, blossom-end rot, rain-check, cat-face, or fell into the extra small size category (less than 40 mm fruit diameter) (Maboko et al. Citation2011). Fruit firmness was measured using an Effegi-type Bishop FT 327 firmness tester with an 11.3 mm diameter plunger. Six ripe fruits of larger size (60–70 mm diameter) per treatment and replicate were collected, and readings were taken at four areas in the equatorial region of the fruit. The percentage of total soluble solids (oBrix) and the electrical conductivity (EC) of the tomato juice were determined in five fruit per replicate and treatment obtained from the fifth truss. Fruit were placed in a blender and the resultant puree filtered through cheese cloth, to determine oBrix and EC of the tomato juice using a pocket refractometer PAL-1 (ATAGO®) and an EC meter, respectively.
Leaf analysis
The leaf chlorophyll concentration was measured at the leaf tip of the fourth leaf from the growing point. Four plants were selected per replicate per treatment to determine the leaf N, P, K, Ca, Mg, Mn, Zn, and B concentrations in the fourth leaf from the growing point. Tomato leaves were oven-dried at 70°C for 48 h and, subsequently, ground using a mill with a 1 mm sieve. Nitrogen was determined on dry-milled material using a Carlo Erba NA 1500 C/N/S Analyzer. An aliquot of the digest solution was used for the ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry) for the determination of Ca, Mg, P, K, Zn, Mn, and B. All nutrient analyses were expressed on a dry mass basis.
Statistical procedures
A complete randomized block design was used for each of the two tunnel facilities (TC and NTC tunnels). A 3×2×2 factorial design was used, with three factors (fertigation treatment, i.e., 100%, 75%, or 50% nutrient concentration, growing media, i.e., sawdust or coir, and mycorrhiza, i.e., AMF applied or no AMF applied) randomly replicated within each of the four block replicates.
The data obtained from the two tunnels were tested for homogeneity of variances using Levene's test. In cases where the variability in the observations of the two tunnel facilities were of comparable magnitude, an analysis of the two tunnels observations together could be validly carried out. In cases where there was strong evidence against homogeneity, a weighted analysis of the two tunnel facilities’ observations together was carried out using the inverse of the pooled variances of each tunnel as weight (John & Quenonille Citation1977). The Shapiro–Wilk test was performed to test for normality (Shapiro & Wilk Citation1965). Student's t-Least Significant Differences were calculated at the 5% level to compare treatment means of significant effects. All data analyses were carried out using GenStat® version 11.1 (Payne et al. Citation2008).
Results
In cases where there were no significant interaction effects among the treatments, only the main factors were discussed.
Percentage of AMF root colonization
There was no significant interaction between nutrient concentration, growing medium, and tunnel facilities on root colonization (). There was a tendency towards increased root colonization in the TC tunnel, as compared with the NTC tunnel, although not significant. Similarly, neither the growing medium nor the nutrient concentration had a significant effect on root colonization.
Table II. Effect of tunnel facility, nutrient concentration, and growing medium on AMF root colonization in tomatoes.
Effects of growing medium
Analysis of physical and chemical properties of sawdust and coir indicated a better water-holding capacity and higher salt concentration of coir compared with sawdust. The colonization of tomato roots by AMF was not affected by the growing medium; in both media, three-quarters of the roots were colonized by AMF (). The chlorophyll concentration of leaves increased in plants grown in sawdust as compared with coir, although only significantly 84 DAT (). Plants grown in coir produced firmer fruit than those grown in sawdust (). There was a tendency towards an increase in marketable yield, number of marketable fruit, and total yield of plants grown in coir compared with sawdust (). Neither °Brix nor EC of the tomato juice were significantly influenced by the growing medium (). Sawdust improved the Mn and Ca leaf concentration compared with coir (). Other elements were not significantly affected by the growing medium, although Zn and B concentrations showed a tendency towards an increase with sawdust as medium.
Table III. Effects of tunnel facility, nutrient concentration, growing medium, and arbuscular mycorrhiza on tomato leaf chlorophyll concentration (SPAD) of the fourth youngest, fully developed leaf, and fruit firmness.
Table IV. Effects of growing medium and mycorrhiza on tomato yield and quality.
Table V. Effects of growing media on tomato leaf nutrient concentration (% DM basis).
Effects of mycorrhiza
Mycorrhiza treatment did neither affect leaf chlorophyll concentration (), nor yield, nor quality of hydroponically grown tomatoes significantly (); neither was the concentration of selected mineral nutrients in leaf tissues altered by the AMF inoculation (). Moreover, neither °Brix nor EC of tomato juice were significantly affected by AMF (); however, there was a tendency towards higher leaf chlorophyll and leaf mineral concentrations in plants inoculated with AMF compared with non-inoculated plants.
Table VI. Effects of tunnel facilities, nutrient concentration, and mycorrhiza on nutrient concentration of tomato leaf tissues (% on dry weight basis).
Effects of nutrient concentration
The nutrient concentration did not have a significant influence on AMF root colonization; however, there was a tendency towards a decrease in AMF root colonization with an increase in nutrient concentration (). The leaf chlorophyll concentration was highest 70 DAT in plants fertigated at 100%, compared with fertigation at 50% and 75% of the recommended nutrient concentration (). Fruit firmness was not significantly affected by nutrient concentration (). Plants fertigated with the recommended nutrient concentration had a significantly higher N, K, and Mn leaf concentration than plants fertigated with 50% or 75% of the recommended nutrient solution ().
Effects of tunnel facility
The tunnel facilities, i.e., TC and NTC tunnel, did not influence AMF root colonization significantly (). Temperature differences were observed between the two tunnel facilities, with higher maximum temperatures in the NTC tunnel than the TC tunnel (). Plants in the TC tunnel contained higher leaf chlorophyll concentrations, as well as firmer fruit than plants grown in the NTC tunnel (). However, in the NTC tunnel, the tomato leaves had higher Mg, Ca, and Zn concentration than plants grown in the TC tunnel ().
Table VII. Maximum, minimum, and mean monthly ambient temperature for the experimental sites during the experimental period.
Interaction effects of tunnel facilities and fertigation
Independent of fertigation level, leaf chlorophyll concentrations were highest 70 DAT in the TC tunnel, followed by the 100% nutrient concentration in the NTC tunnel (). Tomato leaves of plants in the TC tunnel had a higher chlorophyll concentration than those in the NTC tunnel. Surprisingly, the chlorophyll concentration was not affected by the applied nutrient concentration in the TC tunnel, while in the NTC tunnel, leaf chlorophyll concentration increased when plants were supplied with 100% compared with 50% or 75% of the recommended nutrient concentration.
Figure 1. Interaction effects of tunnel facilities and fertigation on tomato leaf chlorophyll concentration at 70 days after transplanting. NTC, non-temperature-controlled tunnel; TC, temperature-controlled tunnel; 50%, 75%, and 100%, percentage of nutrient concentration; LSD, least significant difference; values marked with the same letter are not significantly different (p > 0.05).
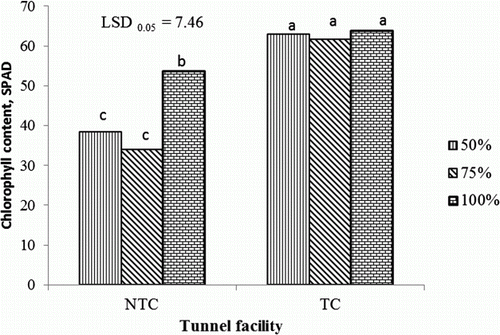
The highest total yield, marketable yield, and number of marketable fruits were obtained from plants fertigated at 50% and 75% of the recommended nutrient concentration in the TC tunnel (). Unmarketable yield was significantly lower at all fertigation treatments in the TC tunnel, whereas plants in the NTC tunnel, fertigated at 50% and 75% of the recommended nutrient concentration, had the highest unmarketable yield ().
Table VIII. Interaction effects of tunnel facility and nutrient concentration on tomato yield and quality.
Plants fertigated at 100% of the recommended nutrient concentration in the TC tunnel, as well as plants fertigated at 75% and 100% of the recommended nutrient concentration in the NTC tunnel, had the highest oBrix, compared with other treatments ()); NTC fruit showed a tendency toward higher oBrix than TC ones.
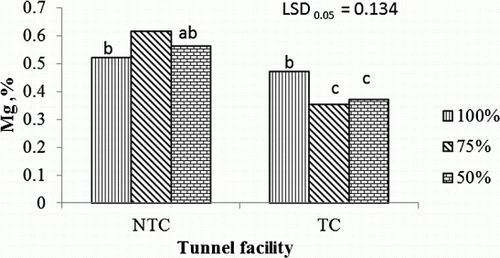
The Ca leaf concentration was significantly higher in leaves of tomato plants fertigated at 50% and 75% of the recommended nutrient concentration and grown in the NTC tunnel than leaves from all plants in the TC tunnel (). The 75% and 100% fertigation treatments resulted in the highest Ca leaf concentration in the NTC and TC tunnel facilities, respectively. The NTC tunnel also significantly increased the Mg concentration when plants were supplied with 50% and 75% of the recommended nutrient concentration, while no such difference was found when plants were supplied with the recommended nutrient concentration of 100% ().
Figure 2. Interaction effects of fertigation and tunnel facility on Ca concentration of tomato leaf tissues.
NTC, non-temperature-controlled tunnel; TC, temperature-controlled tunnel; 50%, 75%, and 100%, percentage of nutrient concentration; LSD, least significant difference; values marked with the same letter are not significantly different (p > 0.05).
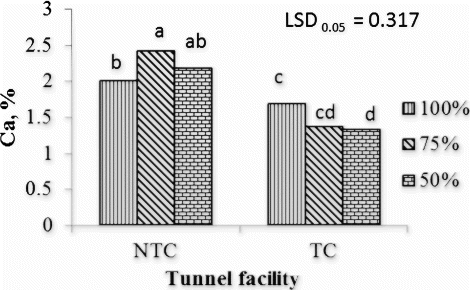
Figure 3. Interaction effects of fertigation and tunnel facility on Mg concentration of tomato leaf tissues.
NTC, non-temperature-controlled tunnel; TC, temperature-controlled tunnel; 50%, 75%, and 100%, percentage of nutrient concentration; LSD, least significant difference; values marked with the same letter are not significantly different (p>0.05).
Interaction effects of growing media and mycorrhiza, and growing media, fertigation, and tunnel facilities
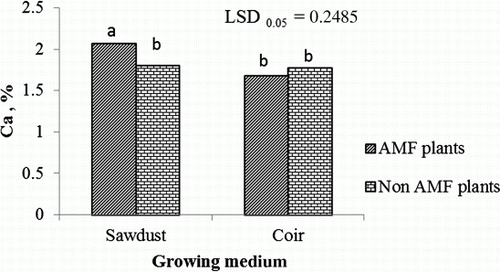
Plants grown in sawdust and inoculated with AMF showed a significant increase in leaf Ca concentration in contrast to plants grown in coir ().
Figure 4. Interaction effects of growing medium and mycorrhiza on leaf Ca concentration of tomatoes.
AMF plants, plants inoculated with arbuscular mycorrhiza; Non-AMF plants, plants without arbuscular mycorrhiza inoculation. LSD, least significant difference; values marked with the same letter are not significantly different (p>0.05).
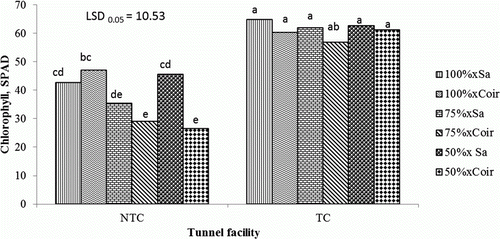
Generally, leaf chlorophyll concentration was significantly higher in plants grown in sawdust and coir at all nutrient concentrations in the TC tunnel, compared with plants grown in the NTC tunnel, with the lowest leaf chlorophyll concentration recorded for plants grown in sawdust, and fertigated at 50% and 75% of the recommended nutrient concentration ().
Figure 5. Interaction effects of growing media, fertigation and tunnel facility on leaf chlorophyll concentration of tomato at 84 days after transplanting.
TC, temperature-controlled tunnel; NTC, non-temperature-controlled tunnel; Sa, sawdust; 50%, 75%, 100% percentage of nutrient concentration; LSD, least significant difference; values marked with the same letter are not significantly different (p>0.05).
Discussion
Organic growing media are commonly colonized by fungi (Koohakan et al. Citation2004); however, in this study, AMF colonization in coir and sawdust was at 78.2% and 77.7%, respectively. Despite this relatively high AMF root colonization of tomato plants () compared with reports by Abak et al. (Citation2010), Ikiz et al. (Citation2009), Dasgan et al. (Citation2008), and Al-Karaki et al. (Citation2001), a significant improvement of tomato yield could not be detected (). Previous investigations in soilless growing medium (perlite) on various plant species, such as muskmelon (Abak et al. Citation2010) and pepper (Ikiz et al. Citation2009), have shown that AMF colonization can increase plant growth and yield; however, in our study, mycorrhiza colonization did neither have a significant influence on tomato yield nor quality. This effect might be aligned to the choice of growing medium as organic growing media (sawdust and coir) might release phenolics, lignin, and other organic compounds, thereby reducing mycorrhizal development that could have otherwise improved yield and quality of tomatoes under soilless conditions.
The higher water-holding capacity of coir compared with sawdust might have contributed to firmer fruit and the tendency towards increased tomato yield in the former medium by allowing uninterrupted water uptake resulting in more turgid cells (Jones & Corlett Citation1992). The reduced Mn and Ca concentrations of leaf tissues of plants grown in coir () might be explained by the high Na, S, K, and Cl concentrations of the medium, possibly suppressing the uptake of other nutrients, particularly Ca through cationic competition (). This finding supports observations by Adams and Ho (Citation1995) that high soil salinity can result in reduced Ca uptake into tomato fruit. Inoculation with AMF only increased the leaf Ca concentration of plants grown in sawdust, but not of plants grown in coir (). Several reports indicate that in soils with low mineral content, AMF colonization improves acquisition of low mobile nutrients, such as P, Zn, and Cu (Lambert et al. Citation1979; Ortas et al. Citation1996; Liu et al. Citation2002; Kaya et al. Citation2003; Ryan & Angus Citation2003), as well as improving transport and absorption of P, Ca, and Mg (Liu et al. Citation2002). Our study also found a tendency towards higher leaf mineral concentrations in AMF-inoculated plants, compared with non-AMF-inoculated plants; however, AMF did not enhance the uptake of any of the analyzed nutrients into leaf tissue, except for higher Ca, resulting in a higher Ca leaf concentration of tomato plants grown in sawdust ().
Surprisingly, fertilizers with the recommended nutrient concentration (100%) resulted in a lower total and marketable yield than using the reduced nutrient concentrations (50% and 75% of recommended fertilizer amount); this indicates that either the recommended nutrient concentration is excessive or the EC of the nutrient solution was too high for optimal root development. In future studies, the EC of the 10% drainage water should be measured to confirm such assumption. The colonization of tomato roots with AMF was effective; however, the rate was not significantly affected by the nutrient concentration, indicating that inoculating tomato plants with AMF cannot be used as a tool to reduce the quantity of fertilizer application. The highest marketable and total yield was obtained in plants fertigated with 50% or 75% of the recommended nutrient concentration; however, the oBrix of the tomato juice was significantly higher in fruit from plants cultivated at 100% of the recommended nutrient concentration. Seemingly, the higher TSS of fruit from plants grown in the recommended nutrient concentration compromised yield, an indication that the recommended nutrient concentration is not optimal. The high nutrient concentration was also reported by another author to reduce yield (Adams Citation1991). The 100% nutrient concentration might have restricted water transportation to fruits and thus increased oBrix (Adams Citation1991; Cornish Citation1992; Lin & Glass Citation1999).
Growing conditions in the TC tunnel resulted in a higher leaf chlorophyll concentration of tomato plants, compared with those in the NTC tunnel. The higher chlorophyll concentration in plants grown in the TC tunnel () indicates that plants were able to photosynthesize more effectively than those in the NTC tunnel, and, thereby, supply assimilates for fruit development and plant growth contributing to the higher total and marketable yield obtained in the TC tunnel.
The reduced fruit firmness and the lower chlorophyll concentration in the NTC tunnel could be due to extremely high temperatures (45.4–51.9°C) in the NTC tunnel, compared with the TC tunnel (32.7–39.1°C) (). Such reduction in fruit firmness and leaf chlorophyll concentration underline the importance of the high maintenance required in protected cultivation when aiming at producing high-quality fruit, as the high temperatures in the TC tunnel were due to the unusual failure of electricity and malfunctioning of the wet walls. Poor fruit firmness could also be the consequence of the processes that involve biochemical changes in cell wall structure, resulting in flesh softness due to high temperature. Such effects caused by high air temperatures have been reported as mainly associated with the reduction of the photosynthetic activity (Ciu et al. Citation2006).
The poor yield in the NTC tunnel could be explained by the prevailing high air temperature, which can increase unmarketable yield and reduce fruit set (Maboko et al. Citation2012). This is in accordance with Peet et al. (Citation1997) who reported decreased fruit number and fruit weight per plant as well as a decrease in seed number per tomato fruit at an air temperature of 29°C, compared to an air temperature of 25°C. In tomatoes, temperatures above 25°C cause nonlinear yield reductions (Peet et al. Citation1997). Similarly, Sato et al. (Citation2000) and Saeed et al. (Citation2007) reported that impairment of pollen and anther development by high temperatures contribute to a poor fruit set in tomatoes, while high ambient air temperature in NTC tunnels seems to have no significant influence on AMF root colonization. AMF root colonization is reported to improve plant resistance to biotic and abiotic stresses. Zhu et al. (Citation2010) reported that inoculation of maize roots with AMF protects plants against high temperature stress (40°C) by improving photosynthesis and plant water status. It was, therefore, expected that mycorrhiza colonization would improve yield in the NTC tunnel due to avoiding heat stress and improving leaf chlorophyll concentration, water status, and nutrient uptake. Although there was high AMF colonization in this study, this did not improve yield under NTC conditions, possibly due to the high ambient temperature or the choice of growing media not appropriate to AMF colonization. Relying on natural ventilation to reduce the heat load inside the tunnel was seemingly insufficient to gain benefits from AMF inoculation; however, the leaf mineral concentration in the NTC tunnel was higher than in the TC tunnel.
Data presented indicate that TC tunnels can significantly increase total as well as marketable yield over that what can be achieved in NTC tunnels; AMF root colonization was not able to establish significant influence on tomato yield and quality. Further studies, including different media, nutrient composition, and concentration need to be carried out to investigate the possible effects of AMF failing to improve yield, despite successful AMF root colonization, and to reveal the cause of the poor beneficial effects of AMF on tomato plants grown under soilless conditions.
Acknowledgments
The authors acknowledge the financial support from Agricultural Research Council-Vegetable and Ornamental Plant Institute (ARC-VOPI). The assistance by Ms Maphefo Wendy Sekgota from the ARC-PPRI in determining mycorrhiza colonization and Ms Liesl Moorey from the ARC-Biometry Unit in advising on methods of statistical analysis are gratefully acknowledged.
References
- Abak , K , Dasgan , HY , Rehber , Y and Ortas , I . 2010 . Effect of vesicular arbuscular mycorrhizas on plant growth of soilless grown muskmelon . Acta Hortic. , 871 : 301 – 306 .
- Adams , P. 1991 . Effects of increasing the salinity of the nutrient concentration with major nutrients or sodium chloride on the yield, quality and composition of tomato grown in rockwool . J Hortic Sci. , 66 : 201 – 207 .
- Adams , P and Ho , LC. 1995 . Uptake and distribution of nutrients in relation to tomato fruit quality . Acta Hortic. , 412 : 374 – 385 .
- Al-Karaki , GN. 2006 . Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water . Sci Hortic. , 109 : 1 – 7 . doi: 10.1016/j.scienta.2006.02.019
- Al-Karaki , GN , Hammad , R and Rusan , M. 2001 . Response of two cultivars differeng in salt tolerance to inoculation with mycorrhizal fungi under salt stress . Mycorrhiza. , 11 : 43 – 47 . doi: 10.1007/s005720000055
- Biermann , B and Linderman , RG. 1983 . Effect of container plant growth medium and fertilizer phosphorus on establishment and host growth response to vesicular- arbuscular mycorrhizal fungi . J Am Soc Hortic Sci. , 108 : 962 – 971 .
- Caron , M and Parent , S. 1988 . Definition of a peat-lite medium for the use of vesicular arbuscular mycorrhizae in horticulture . Acta Hortic. , 221 : 289 – 294 .
- Ciu , L , Li , J , Fan , Y , Xu , S and Zhang , Z. 2006 . High temperature effects on photosynthesis, PII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility . Bot Stud. , 47 : 61 – 69 .
- Coltman , RR , Waterer , DR and Huang , RS. 1988 . A simple method for production of Glomus aggregatum inoculum using controlled-release fertilizer . Hort Science. , 23 : 213 – 215 .
- Corkidi , L , Allen , EB , Merhaut , D , Allen , MF , Downer , J , Bohn , J and Evans , M. 2004 . Assessing infectivity of commercial mycorrhiza inoculants in plant nursery conditions . J Environ Hortic. , 22 : 149 – 154 .
- Cornish , PS. 1992 . Use of high electrical conductivity of nutrients solution to improve the quality of salad tomatoes grown in hydroponic culture . Aust J Exp Agric. , 32 : 513 – 520 . doi: 10.1071/EA9920513
- Dasgan , HY , Kusvuran , S and Ortas , I. 2008 . Response of soilless growth tomato plant arbuscular mycorrhizal fungal (Glomus faciculatum) colonization in recycling and open system . Afr J Biotechnol. , 7 : 3606 – 3613 .
- Davies , FY , Potter , JR Jr and Linderman , RG. 1992 . Mycorrhiza and repeated drought exposure affect drought resistance and extraradical hyphae development of pepper plants independent of plant size and nutrient content . J Plant Physiol. , 139 : 289 – 294 . doi: 10.1016/S0176-1617(11)80339-1
- Douds , DD , Nagahashi , G , Reider , C and Hepperly , RR. 2008 . Choosing a mixture ratio for the on-farm production of AM fungus inoculum in mixtures of compost and vermiculite . Compost Sci Util. , 16 : 52 – 60 .
- Giovannetti , M and Mosse , B. 1980 . An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots . New Phytol. , 84 : 489 – 500 . doi: 10.1111/j.1469-8137.1980.tb04556.x
- Graham , JH and Timmer , LW. 1984 . Vesicular-arbuscular mycorrhizal development and growth response of rough lemon in soil and soilless media: effect of phosphorus source . J Am Soc Hortic Sci. , 109 : 118 – 121 .
- Ikiz , O , Abak , K , Dasgan , HY and Ortas , I. 2009 . Effects of mycorrhizal inoculation in soilless culture on pepper plant growth . Acta Hortic. , 807 : 533 – 540 .
- John , JA and Quenouille , MH. 1977 . Experiments: design and analysis. London and High Wycombe: Charles Griffin . Chapter , 13 : 232 – 248 .
- Johnson , CR and Hummel , RL. 1986 . Influence of media on endomycorrhizal infection and growth response of Severinia buxifolia . Plant Soil. , 93 : 35 – 42 . doi: 10.1007/BF02377143
- Jones , HG and Corlett , JE. 1992 . Current topics in drought physiology . J Agric Sci. , 119 : 291 – 296 . doi: 10.1017/S0021859600012144
- Kaya , C , Higgs , D , Kirnak , H and Tas , I. 2003 . Mycorrhizal colonization improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well- watered and water-stressed conditions . Plant Soil. , 253 : 287 – 292 . doi: 10.1023/A:1024843419670
- Koohakan , P , Ikeda , H , Jeanaksorn , T , Tojo , M , Kusakari , SI , Okada , K and Sato , S. 2004 . Evaluation of the indigenous microorganisms in soil-less culture: occurrence and quantitative characteristics in the different growing systems . Sci Hortic. , 101 : 179 – 188 . doi: 10.1016/j.scienta.2003.09.012
- Koske , RW and Gemma , JN. 1989 . A modified procedure for staining roots to detect VA mycorrhizas . Mycol Res. , 92 : 486 – 505 . doi: 10.1016/S0953-7562(89)80195-9
- Lambert , DH , Baker , DE and Cole , H Jr. 1979 . The role of mycorrhizae in the interactions of phosphorus with zinc, copper and other elements . Soil Sci Soc Am J. , 43 : 976 – 980 . doi: 10.2136/sssaj1979.03615995004300050033x
- Lin , WC and Glass , ADM. 1999 . The effects of NaCl addition and macronutrients concentration on fruit quality and flavor volatiles of greenhouse tomatoes . Acta Hortic. , 481 : 487 – 491 .
- Linderman , RG and Davis , EA. 2003 . Soil amendment with different peatmesses affects mycorrhizae of onion . HortTechnology. , 1 : 285 – 289 .
- Liu , A , Hamel , C , Elmi , A , Costa , C , Ma , B and Smith , DL. 2002 . Concentrations of K, Ca and Mg in maize colonized by arbuscular mycorrhizal fungi under field conditions . Can J Soil Sci. , 82 : 271 – 278 . doi: 10.4141/S01-022
- Maboko , MM , Du Plooy , CP and Bertling , I. 2011 . Comparative performance of tomato cultivars cultivated in two hydroponic production systems . S Afr J Plant Soil. , 28 : 97 – 102 .
- Maboko , MM , Du Plooy , CP and Bertling , I. 2012 . Comparative performance of tomato cultivars in temperature and non temperature controlled plastic tunnel . Acta Hortic. , 927 : 405 – 411 .
- Mueller , A , Franken , P and Schwarz , D. 2009 . Nutrient uptake and fruit quality of tomato colonized with Mmycorrhizal fungus Glomus mosseae (BEG) under deficient supply of nitrogen and phosphorus . Acta Hortic. , 807 : 383 – 388 .
- Muok , BO and Ishii , T. 2006 . Effect of arbuscular mycorrhizal fungi on tree growth and nutrient uptake of Sclerocarya birrea under water stress, salt stress and flooding . J Japan Soc Hort Sci. , 75 : 26 – 31 . doi: 10.2503/jjshs.75.26
- Ortas , I , Harries , PJ and Rowell , DI. 1996 . Enhanced uptake of phosphorus by mycorrhizal sorghum plants as influenced by form of nitrogen . Plant Soil. , 184 : 255 – 264 . doi: 10.1007/BF00010454
- Payne RW , Murray DA , Harding SA , Baird DB , Soutar DM. 2008 . GenStat for Windows® (11th Edition) Introduction . Hemel Hempstead , , UK : VSN International .
- Peet , MM , Willits , DH and Gardner , RG. 1997 . Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress . J Exp Bot. , 48 : 101 – 111 . doi: 10.1093/jxb/48.1.101
- Peters , SM and Habte , M. 2001 . Optimizing solution P concentration in a peat-based medium for producing mycorrhizal seedlings in containers . Arid Land Res Manag. , 15 : 359 – 370 . doi: 10.1080/153249801753127642
- Pozo , MJ and Azcon-Aguilar , C. 2007 . Unraveling mycorrhiza-induced resistance . Curr Opin Plant Biol. , 10 : 393 – 398 . doi: 10.1016/j.pbi.2007.05.004
- Ryan , MH and Angus , JF. 2003 . Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn uptake but no increase in P-uptake or yield . Plant Soil. , 250 : 225 – 239 . doi: 10.1023/A:1022839930134
- Saeed , A , Hayat , K , Khan , AL and Iqbal , S. 2007 . Heat tolerance studies in tomato (Lycopersicon esculentum Mill.) . Int J Agric Biol. , 9 : 649 – 652 .
- Sato , S , Peet , MM and Thomas , JF. 2000 . Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress . Plant Cell Environ. , 23 : 719 – 726 . doi: 10.1046/j.1365-3040.2000.00589.x
- Sawers , RJH , Gutjahr , C and Paszkowski , U. 2008 . Cereal mycorrhiza: an ancient symbiosis in modern agriculture . Trends Plant Sci. , 13 : 93 – 97 . doi: 10.1016/j.tplants.2007.11.006
- Shapiro , SS and Wilk , MB. 1965 . An analysis of variance test for normality (complete samples) . Biometrika. , 52 : 591 – 611 .
- Smith , SE and Read , DJ. 1997 . Mycorrhizal symbiosis , San Diego , CA : Academic Press .
- Stewart , LI , Hamel , C , Hogue , R and Moutoglis , P. 2005 . Arbuscular mycorrhizal inoculated strawberry plant responses in a high soil phosphorus rotation crop system . Mycorrhiza. , 15 : 612 – 619 . doi: 10.1007/s00572-005-0003-z
- Tahat , MM , Kamaruzaman , S , Radziah , O , Kadir , J and Masdek , HN. 2008 . Response of (Lycopersicum esculentum Mill.) to different arbuscular mycorrhizal fungi species . Asian J Plant Sci. , 7 : 479 – 484 . doi: 10.3923/ajps.2008.479.484
- Zhu , X , Song , F and Xu , H. 2010 . Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidative enzyme activity of maize plants under temperature stress . Mycorrhiza. , 20 : 325 – 332 . doi: 10.1007/s00572-009-0285-7