Abstract
A comparative study was conducted on the toxicity of Cd to alkaline phosphatase activity (ALP) and dehydrogenase activity (DHA) in 18 top soils with contrasting soil properties representative of 14 major soil types in China. Soil pH and carbonate content, soil organic matter, and cation exchange capacity (CEC) largely affected the Cd toxicity on two enzyme activities; with the soil pH having only minor effect on the median ecological dose values based on total Cd concentrations (ED50 T). The values of ED50 T/ED50 W (based on water-soluble Cd content) of alkaline phosphatase and dehydrogenase were strongly influenced by pH and CEC contents, which explained up to 71% of the variation for alkaline phosphatase, 82% of the variation for dehydrogenase, and also were significantly correlated with the parameter KF derived from Freundlich adsorption isotherms. This study suggests that the values of ED50 T/ED50 W could be useful to evaluate the buffer capacity of soils which protects soil enzymes from harmful effects of heavy metal.
Introduction
Anthropogenic activities have increased the concentration of heavy metals in the environment, producing toxic effects on agro-ecosystems and natural ecosystems, which is one of the major environmental problems in many parts of the world. Also, there has been a growing public concern about the potential accumulation of trace elements in agricultural soils in China owing to the rapid industrialization and urbanization and increasing reliance on chemicals in the last two decades (Luo et al. Citation2009). It has been estimated that contaminated soils result in a loss of more than 1.0 × 107 tons of food supplies in China annually (Wei & Chen Citation2001). Increasing inputs of Cd to agricultural soil in China as well as many other World's areas is of particular concern, and it is expected that the Cd concentration in soils will exceed the current environmental standard (0.3 mg kg−1 for soil with pH 6.5–7.5) [State Environmental Protection Administration of China (SEPAC) Citation2006] in the next 50 years at the current Cd input rate of 0.004 mg kg−1 yr−1 (Luo et al. Citation2009). Cadmium has no known biological function and poses serious health risk due to plant absorption and spreading in the food chain through animal grazing and a direct threat to humans via uptake into rice (Ros et al. Citation2009). Cd is one of the most studied heavy metals in terms of its toxicity to biota and their beneficial processes in soil (Vig et al. Citation2003; Giller et al. Citation2009; Karaca et al. Citation2010).
Determination of the effects of Cd on soil biological activity often yields conflicting results. Bååth (Citation1989) summarized data from both laboratory and field studies in terms of the highest metal concentration in the soil where no effect (HNOEC) was found and the lowest metal concentration where an effect (LOEC) was observed and concluded that for Cd the HNOEC and LOEC values differed from 100 to 1000 folds among the various soils. Giller et al. (Citation1998) reported the highest amount of metal added that did not produce an effect (HNOED) and the lowest amount added that resulted in a decrease (LOAED) in the rate of base respiration for Cd in laboratory studies also varied between 100 and 1000 folds.
The ED50 values for Cd reported in the vast literature on the topic lie in a wide concentration range. For example, ED50 values in the range 90–5555 mg kg−1 were reported for dehydrogenase activity (DHA) (Welp Citation1999; Moreno et al. Citation2001; Gao, Zhou, Mao, Zhi, Shi Citation2010; Gao, Zhou, Mao, Zhi, Zhang, et al. Citation2010), from 30 to 7240 mg kg−1 for urease activity (Doelman & Haanstra Citation1986; Welp Citation1999; Moreno et al. Citation2001, Citation2003), from 14 to 9870 mg kg−1 for acid phosphatase activity (Doelman & Haanstra Citation1989; Moreno et al. Citation2003; Renella et al. Citation2003; Gao, Zhou, Mao, Zhi, Shi Citation2010; Gao, Zhou, Mao, Zhi, Zhang, et al. Citation2010), from 4 to 5485 mg kg−1 for alkaline phosphatase activity (ALP) (Doelman & Haanstra Citation1989; Renella et al. Citation2003), from 357 to 4975 mg kg−1 for β-glucosidase activity (Moreno et al. Citation2003), from 43 to 8677 mg kg−1 for N-a-benzoyl-L-argininamide protease activity (Moreno et al. Citation2003), from 121 to 3192 mg kg−1 for arylsulfatase activity (Haanstra & Doelman Citation1991), from 77 to 33,333 mg kg−1 for adenosine triphosphate (ATP; Moreno et al. Citation2001), and from 85 to 1270 mg kg−1 for Fe (III) reduction (Welp & Brümmer Citation1997).
It is important to underline that different ED50 values are obtained by using different dose response models, as discussed by Speir et al. (Citation1995, Citation1999). While the inhibition of soil enzyme activity should be related to the soluble meal fraction (e.g. water soluble, diethylenetriaminepentaacetic acid-extractable), valid fittings of soil enzyme activity in dose response models have only been obtained by plotting the soil enzyme activity in function of total heavy metal concentrations independently on the used model (Moreno et al. Citation2001; Renella et al. Citation2003). This may be explained by the fact that heavy metal speciation in soil depends on several soil properties such as soil organic matter (SOM) content, cation exchange capacity (CEC), soil pH value, metal ionic properties, and time. For example, it has been reported that Cu and Cr toxicity to soil microorganisms increased with increasing of SOM and CEC and decreased with increasing of soil pH values (D'Ascoli et al. Citation2006), and similar results were reported by Welp and Brümmer (Citation1997) on the toxicity thresholds for Ni and Hg in various German soils. Cd solubility in soil is mainly controlled by soil pH and sorption onto clay particles (McBride Citation1989) and by carbonate content and quality (Renella et al. Citation2004). However, an in depth analysis of the responses of soil enzyme activity to Cd solubility in dose response experiments has not been published so far.
The objective of the present study was to describe the response of soil enzyme activity to Cd solubility in various Chinese soils using dose response experiments. The importance of main soil properties in modeling of enzyme responses to Cd is also discussed.
Materials and methods
Soil samples and handling
A total of 18 surface (0–15 cm depth) soils representative of the major soil types in China were collected from agricultural soils of 18 provinces in China (). These soils cover a wide range in soil properties expected to affect the bioavailability and toxicity of Cd in soils. The soils were air dried and sieved (mesh size, 1 mm) prior to use.
Table 1. Selected soil properties for the 18 soil samples tested.
Soil organic matter (OM) was determined by the K2Cr2O7–H2SO4 oxidation method. Soil calcium carbonate (CaCO3) was determined from pressure increases after addition of HCl to the soil in closed containers. Soil texture was measured by pipette method. CEC was analyzed by 1 mol l−1 ammonium acetate exchange method. Soil pH was measured in 1:5 soil:water (Lu Citation1999). Total Cd was analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS; X-7, Thermo-Elemental, USA) after digestion with a mixture of hydrofluoric (HF) and perchloric (HClO4) acid by microwave digestion system with 15 min.
Experimental design
Air-dried soils were spiked with an aqueous stock solution of CdSO4 to result in final Cd concentrations of 0, 0.6, 5, 25, 50, 100, 200, 300, and 500 mg kg−1 soil (soil:CdSO4 solution ratio, 1:1). The Cd solution was added dropwise to moisten the whole soil sample. After 30 min of equilibration, the enzyme activities in the soils were measured according to Acosta-Martínez and Tabatabai (Citation2001).
Soil analyses
ALP activity was determined using 5 g air-dried soil mixed with 5 ml solution of CdSO4, after 30 min mixed with five drops of toluene, 10 ml of disodium phenyl phosphate solution, and 10 ml of Borax sodium hydroxide buffer (pH 9.4). The suspensions were stationarily incubated for 24 h at 37 °C. The samples were then filtered and the filtrate was colored with 0.25 ammonia–ammonium chloride buffer at pH 9.6, 0.5 ml of 2% 4-aminoantipyrine, and 8% potassium ferrocyanide, the phenol released was determined colorimetrically in a spectrophotometer at 510 nm. Results were expressed as µg phenol·g−1 soil·h−1(Guan et al. Citation1987).
Soil DHA was assayed according to Burns (Citation1978). Briefly, 3 g soil sample was mixed with 3 ml solution of CdSO4, after 30 min, added to 0.03 g calcium carbonate, was mixed with 0.5 ml of 3% (w/v) solution of triphenyl tetrazolium chloride as the substrate and 1.25 ml of distilled water. The soil slurries were mixed thoroughly and incubated at 37 °C for 24 h. The triphenyl formazan (TPF) produced by the DHA was extracted with methanol and the activity were expressed as µg TPF TPF·g−1 soil·h−1. All results are the means of three replicates.
Analysis of Cd solubility: the water-soluble Cd was determined on 5 g air-dried soil mixed with 5 ml of Cd aqueous solution into 50-ml centrifuge tubes. The soil suspensions were mixed for 30 min, and then added with 20 ml of distilled water and shaken end-over-end for 2 h. After centrifugation (15 min, 4000 × g) the supernatant was filtered through membrane filter paper and stabilized with HNO3. The supernatant was decanted into clean test tubes and kept at 4 °C until Cd concentration measurement. The Cd concentrations were measured by atomic absorption spectrophotometer and Inductively Coupled Plasma Mass Spectrometry (ICP-MS; X-7, Thermo-Elemental, USA).
Data modeling
A Michaelis Menten kinetic approach was used to model inhibition of the biological properties by Cd. The mathematical models are as follows:
Meanwhile, the logarithm of the added concentrations as independent variables was described using a logistic response model (Doelman & Haanstra Citation1986). This relationship is expressed using the following equation:
Mean results of the triplicate measurements at each inhibitor concentration were used as the raw data for the models. Statistical analyses were performed using SPSS 18.0 and Origin 8.5 software.
Results
Soil physico-chemical properties and Cd solubility
Soil pH ranged from 4.90 at Hunan to 8.80 at Inner Mongolia, with six of the soils having a pH below 6.5 (). The OM content of the soils varied a great deal (8.57–47.7 g kg−1) and the average of OM content was 22.0 g kg−1. The maximum OM was 4.8 % at Jiangsu. Nine out of 18 soils (pH > 7) had free CaCO3 (the mean of 28.56 g kg−1). Clay content ranged from 6.7% to 46% and CEC ranged from 8.12 to 31.1 cmol kg−1. Background metal concentrations for Cd ranged from 2.22 to 167 µg kg−1.
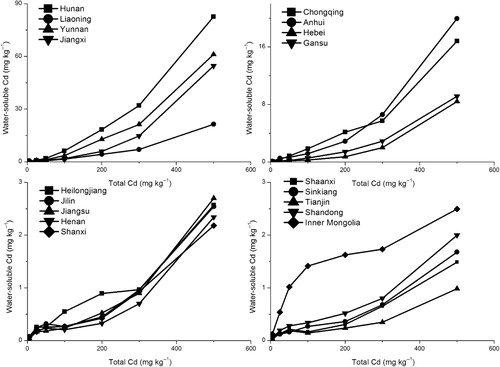
The water-soluble Cd concentrations in soils increased with an increase in total Cd of contaminated soil in all samples (). Except Heilongjiang, water-soluble Cd was higher in acid soils (pH<6.5) than other soils. In these soils water extracted a large percentage of the added Cd, which ranged from 3.36% (16.8 mg kg−1) for Chongqing soil to 16.5% (82.6 mg kg−1) for Hunan soil contaminated with Cd at 500 mg kg−1. The other neutral and alkaline soils and Heilongjiang had low water-soluble Cd content and the values were less than 0.6%, except Hebei and Gansu soil samples. Water-soluble Cd in 500 mg kg−1 treatments were negative related to soil pH and CEC content, and the correlation coefficients were −0.73 (n = 18, p < 0.01) and −0.51 (n = 18, p < 0.05).
Effect of Cd on soil ALP and DHA
Toxicity data were expressed using two different measures of metal content in soil (i.e. total and water soluble) as a direct comparison between the different fractions and to improve Cd toxicity predictions across sites. The ED50 values determined in function of total and water-soluble Cd fractions are shown in . Models 2 and 3 described well ALP and DHA activities, respectively, with a coefficient of determination (R2) higher than 0.8, suggesting a high degree of fitting with these models.
Table 2. ED50 values of alkaline phosphatase activity (ALP) and dehydrogenase activity (DHA) in different soils.
A large variance of ED50 values were found among soils irrespective of the metal fraction used. ED50 values for the effects of Cd (water soluble) on ALP varied from 0.17 to 1.93 mg kg−1 and for DHA ranged from 0.2 to 13.5 mg kg−1. Using total concentrations of Cd in soil, ED50 values varied from 6 to 699 mg kg−1 for ALP and from 45 to 468 mg kg−1 for DHA. Soil solution-based ED50 values for ALP varied approximately 10-fold less than total-based ED50 values across a variety of soils. But the variation of soil solution-based toxicity values for DHA was 6-fold larger than the corresponding variation of values based on total metal concentrations.
The water extractable Cd-based ED50 values showed an increase with increasing OM content for ALP (r = 0.538, p < 0.05), whereas for DHA the ED50 values decreased with an increase in soil solution pH (r = −0.533, p < 0.05). When expressed on a total metal concentration, there was a strong relationship between ED50 value of ALP and OM and CEC content (ED50/OM, r = 0.748, P < 0.001; ED50/CEC, r = 0.763, p < 0.001); the ED50 value of DHA was also strongly correlated with CEC content (r = 0.600, p < 0.001). No remarkable correlation was found between water extractable Cd-based and total Cd-based ED50 values of DHA, while there was a strong relationship for ALP (r = 0.675, p < 0.001).
The ratios of total to water solution-based ED50 of two enzymes were remarkably correlated with soil pH (ALP: ED50 T/ED50 W, r = 0.583, p < 0.05; DHA: ED50 T/ED50 W, r = 0.742, p < 0.001) and CEC (ALP: ED50 T/ED50 W, r = 0.617, p < 0.001; DHA: ED50 T/ED50 W, r = 0.531, p < 0.05).
Correlation between soil properties and ED50 values
To a large extent, Cd toxicity thresholds can be explained by soil properties (all data except pH were log-transformed). Results of multiple linear regressions (stepwise forward) for ED50 and soil properties are presented in .
Table 3. Multiple linear regressions between Cd toxicity threshold values and soil properties.
For ALP, soil OM and CEC were the most influential factors affecting Cd toxicity across soils. OM was the best predictor for water-soluble Cd toxicity thresholds, which explains up to 25% of the variation. The CEC content of the soil was the best predictor for the toxicity thresholds based on total Cd, which explains 50% of the variation in ED50 values. The ED50 values of DHA could be modeled by regression models using pH and OM. In contrast to ALP, soil pH explained to a large extent (50%) the variation in DHA ED50 values based on water-soluble Cd. The OM generally explained most of the variation (up to 41%) in toxicity thresholds of added Cd among soils.
Ratios of toxicity thresholds based on total to water-soluble Cd were explained by soil properties (). The pH and the CEC content of the soil were the best predictors for the ratio of toxicity thresholds for ALP and DHA assays (up to 71% of the variation for ALP, and 82% of the variation for DHA).
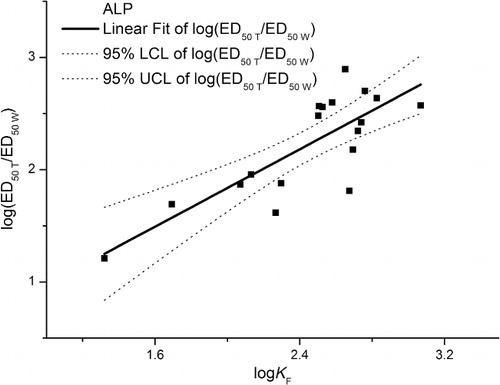
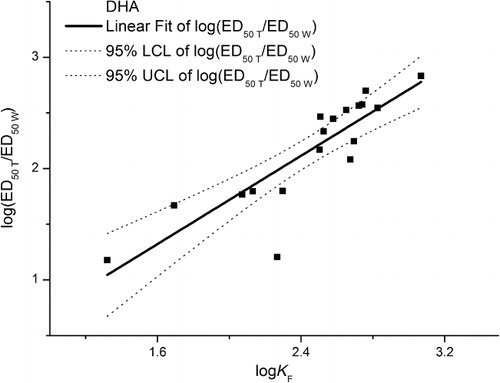
In order to find the reason for why the ratios of toxicity thresholds had significant correlation with soil properties than toxicity thresholds based on total or water-soluble Cd, we did further work on sorption mechanisms. Sorption isotherms helps to better understand the relationship between the sorbed and soluble fraction of Cd. Wang (Citation2012) reported that the partitioning of Cd between the solid and the solution phase can be fitted by the Freundlich equation in these soils (R2 > 0.92). The relationship between the ratios of total to water solution-based ED50 values of ALP and DHA and the Freundlich sorption constants KF (in logs) of the metals tested is shown in and . Values of ED50 T/ED50 W (in logs) ranged from 1 to 3 and increasing logKF should increase the ratios of toxicity thresholds. The variations of the ratios of toxicity thresholds using logKF are described by the following equations:
Discussion
The water-soluble Cd was greater in acidic soils than in neutral and alkaline soils (). In the Heilongjiang soil which had a pH value of 6.5 and higher values of SOM and clay contents and CEC, the water-soluble Cd was 32 times lower than in other soils (). The water-soluble Cd fraction in soil can be considered to be the forms capable of inhibiting the soil enzyme activity (Vig et al. Citation2003). The water-soluble Cd fraction accounted for 1.7% and 1.8% of total Cd for Hebei and Gansu soils, respectively, which could be due to their low CEC and clay contents. Moreno et al. (Citation2001) reported that the water-soluble Cd content was higher in sandy acidic soils than in clay alkaline soils. The low Cd water solubility in the Hebei and Gansu soils could be also due to their carbonate contents. Rapid Cd insolublization has been reported (Renella et al. Citation2004) and changes in the Cd water-soluble fractions depending on the soil management have been reported (Vig et al. Citation2003).
The water-extracted fractions of Cd are generally very low in field soils. Thus, some smelter soils had very low water extractable Cd with a mean value of only 0.13% of the soil total Cd (Ivask et al. Citation2010) and only about 0.1% of Cd was extracted from some other polluted soils (Jopony & Young Citation1994). François et al. (Citation2004) reported on average 0.5% water extractability of Cd while Naidu et al. (Citation1994) showed that Cd was barely extracted from soils with water in soils at pH above 6.5. By comparison, it is shown that the concentration of soil solution Cd in freshly contaminated alkaline soils were lower than acid soils, even being at the same level of field studies. Precipitation of Cd as carbonates and hydroxides could occur in alkaline soil due to the presence of carbonates and alkalinity.
The effect of heavy metals on different soil enzymatic activities has been well studied. The mode of action of heavy metals varies from one enzyme to another, and the effects on enzymes also depend on the heavy metal itself (Moreno et al. Citation2003). The ED50 T values obtained for ALP and DHA in this study were comparable to those previously reported (Welp & Brümmer Citation1997; Welp Citation1999; Moreno et al. Citation2001). Renella et al. (Citation2003) observed that in the acidic soil the ALP was more sensitive to Cd than the acid phosphatase activity whereas in the alkaline soils the opposite behavior was observed, and suggested that a stronger inhibition of phosphatase activity by Cd metals at alkaline than acidic pH values could be due to higher reactivity of deprotonated amino acids. The stronger enzyme activity inhibition by Cd under sub-acidic and alkaline conditions could be also due to the formation of hydroxo–metal complexes, with the monovalent Cd(OH)+ being the predominant Cd species interacting with enzymes and soil microorganisms.
Both the ED50 T and ED50 W values increased with increasing of SOM and the ED50 T values increased with CEC. It is well known that the reaction of metal ions with organic ligands influences their solubility and bonding capacity in soil (Welp & Brümmer Citation1997). Differences in ED50 values in soils with different sorptive capacity have been reported (Speir et al. Citation1995). Oorts et al. (Citation2006) reported that total Cu toxicity thresholds on nitrification potential, glucose-induced respiration, and C-mineralization of plant residues increased with increasing SOM content and CEC, and Ni toxicity thresholds increased with increasing CEC and clay content for the same soil microbial endpoints. For substrate-induced nitrification in 12 Australian field trials, soil pH was the most influential factor affecting Cu and Zn toxicity across soils, explaining 73% and 55% of the variation in EC50 values, respectively (Broos et al. Citation2007). Welp and Brümmer (Citation1997) reported that the EC10 values for Cd increased with increasing concentration of dissolved organic C. However, the above mentioned microbial activities are mainly intracellular and therefore more similar to the soil DHA than to the ALP. In dose response soil toxicity experiments, the soil properties generally explain less than 50% of the variation of the toxicity thresholds based on the measurement of total or water-soluble metal concentrations, this due to the fact that too large difference of soil properties in present study.
In spite of the fact that ED50 of ALP and DHA had different variances (wide concentration range), the values of ED50 T/ED50 W of the two enzymes exhibited same properties. The stronger a metal is sorbed by the soil, the bigger is the difference between the total and water solution-based ED50. The sorption and solubility data fitted with the Freundlich sorption constants KF (log scale) and were remarkably correlated with the ED50 T/ED50 W ratio values of both ALP and DHA, confirming that soil sorptive capacity is a suitable predictor Cd toxicity thresholds for enzymatic and microbial activity in soil (Welp Citation1999). In this sense we propose that the ED50 T/ED50 W ratio values can be used to evaluate the mitigation capacity of soils toward metal inhibition of soil enzymes activity. Relationships such as those developed in this study will permit China environmental regulation to move from single-value national soil quality guidelines to soil-specific quality guidelines and permit soil-specific risk assessments to be undertaken.
Funding
This work was supported by the National Hi-tech Research and Development Program of China [grant number 2012AA101402] and the Fundamental Research Funds for Scientific Research Innovation Key Projects for the Northwest A&F University [grant number ZD2013012] and the Special Fund for Agro-scientific Research in the Public Interest [grant number 200903015].
Additional information
Funding
References
- Acosta-Martínez V, Tabatabai MA. 2001. Arylamidase activity in soils: effect of trace elements and relationships to soil properties and activities of amidohydrolases. Soil Biol Biochem. 33:17–23. 10.1016/S0038-0717(00)00109-7
- Bååth E. 1989. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 47:335–379. 10.1007/BF00279331
- Broos K, Warne MSJ, Heemsbergen DA, Stevens D, Barnes MB, Correll RL, McLaughlin MJ. 2007. Soil factors controlling the toxicity of copper and zinc to microbial processes in Australian soils. Environ Toxicol Chem. 26:583–590. 10.1897/06-302R.1
- Burns RG. 1978. Soil enzyme. London: Academic Press.
- D'Ascoli R, Rao MA, Adamo P, Renella G, Landi L, Rutigliano FA, Terribile F, Gianfreda L. 2006. Impact of river overflowing on trace element contamination of volcanic soils in south Italy: part II. Soil biological and biochemical properties in relation to trace element speciation. Environ Pollut. 144:317–326. 10.1016/j.envpol.2005.11.017
- Doelman P, Haanstra L. 1986. Short- and long-term effects of heavy metals on urease activity in soils. Biol Fertil Soils. 2:213–218. 10.1007/BF00260846
- Doelman P, Haanstra L. 1989. Short- and long-term effects of heavy metals on phosphatase activity in soils: an ecological dose-response model approach. Biol Fertil Soils. 8:235–241.
- François M, Dubourguier H-C, Li D, Douay F. 2004. Prediction of heavy metal solubility in agricultural topsoils around two smelters by the physico-chemical parameters of the soils. Aquat Sci. 66:78–85. 10.1007/s00027-003-0688-z
- Gao Y, Zhou P, Mao L, Zhi Y, Shi W. 2010. Assessment of effects of heavy metals combined pollution on soil enzyme activities and microbial community structure: modified ecological dose-response model and PCR-RAPD. Environ Earth Sci. 60:603–612. 10.1007/s12665-009-0200-8
- Gao Y, Zhou P, Mao L, Zhi Y, Zhang C, Shi W. 2010. Effects of plant species coexistence on soil enzyme activities and soil microbial community structure under Cd and Pb combined pollution. J Environ Sci. 22:1040–1048. 10.1016/S1001-0742(09)60215-1
- Giller K, Witter E, McGrath S. 1998. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem. 30:1389–1414. 10.1016/S0038-0717(97)00270-8
- Giller K, Witter E, McGrath S. 2009. Heavy metals and soil microbes. Soil Biol Biochem. 41:2031–2037. 10.1016/j.soilbio.2009.04.026
- Guan SY, Zhang DS, Zhang ZM. 1987. Soil enzyme and their research methods. Beijing: China Agricultural Science and Technology Press.
- Haanstra L, Doelman P. 1991. An ecological dose-response model approach to short- and long-term effects of heavy metals on arylsulphatase activity in soil. Biol Fertil Soils. 11:18–23. 10.1007/BF00335828
- Ivask A, Dubourguier HC, Põllumaa L, Kahru A. 2010. Bioavailability of Cd in 110 polluted topsoils to recombinant bioluminescent sensor bacteria: effect of soil particulate matter. J Soils Sediments. 11:231–237. 10.1007/s11368-010-0292-5
- Jopony M, Young SD. 1994. The solid solution equilibria of lead and cadmium in polluted soils. Eur J Soil Sci. 45:59–70.
- Karaca A, Cetin S, Turgay O, Kizilkaya R. 2010. Effects of heavy metals on soil enzyme activities. In: Sherameti I, Varma A, editors. Soil heavy metals soil biology. Berlin: Springer; p. 237–262.
- Lu RK. 1999. Analytical methods of soil and agricultural chemistry. Beijing: China Agricultural Science and Technology Press.
- Luo L, Ma YB, Zhang SZ, Wei DP, Zhu YG. 2009. An inventory of trace element inputs to agricultural soils in China. J Environ Manage. 90:2524–2530. 10.1016/j.jenvman.2009.01.011
- McBride MB. 1989. Reactions controlling heavy metal solubility in soils. In: Stewart BA, editor. Advances in soil science. New York, NY: Springer; p. 1–56.
- Moreno J, Garcia C, Hearnandez T. 2003. Toxic effect of cadmium and nickel on soil enzymes and the influence of adding sewage sludge. Eur J Soil Sci. 54:377–38610.1046/j.1365-2389.2003.00533.x
- Moreno J, Garcia C, Landi L, Falchini L. 2001. The ecological dose value (ED50) for assessing Cd toxicity on ATP content and dehydrogenase and urease activities of soil. Soil Biol Biochem. 33:483–489. 10.1016/S0038-0717(00)00189-9
- Naidu R, Bolan NS, Kookana RS, Tiller KG. 1994. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur J Soil Sci. 45:419–429.
- Oorts K, Ghesquiere U, Swinnen K, Smolders E. 2006. Soil properties affecting the toxicity of CuCl2 and NiCl2 for soil microbial processes in freshly spiked soils. Environ Toxicol Chem. 25:836–844. 10.1897/04-672R.1
- Renella G, Adamo P, Bianco MR, Landi L, Violante P, Nannipieri P. 2004. Availability and speciation of cadmium added to a calcareous soil under various managements. Eur J Soil Sci. 55:123–133.
- Renella G, Ortigoza ALR, Landi L, Nannipieri P. 2003. Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50). Soil Biol Biochem. 35:1203–1210. 10.1016/S0038-0717(03)00181-0
- Ros M, Pascual JA, Moreno JL, Hernandez MT, Garcia C. 2009. Evaluation of microbial community activity, abundance and structure in a semiarid soil under cadmium pollution at laboratory level. Water Air Soil Pollut. 203:229–242. 10.1007/s11270-009-0006-z
- Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar LNC. 1995. A simple kinetic approach to derive the ecological dose value, ED50, for the assessment of Cr(VI) toxicity to soil biological properties. Soil Biol Biochem. 27:801–810. 10.1016/0038-0717(94)00231-O
- Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar LNC. 1999. Simple kinetic approach to determine the toxicity of AS[V] to soil biological properties. Soil Biol Biochem. 31:705–713. 10.1016/S0038-0717(98)00169-2
- State Environmental Protection Administration of China (SEPAC). 2006. Farmland environmental quality evaluation standards for edible agricultural products (HJ 332-2006). Beijing: China Environment Press.
- Vig K, Megharaj M, Sethunathan N, Naidu R. 2003. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res. 8:121–135. 10.1016/S1093-0191(02)00135-1
- Wang JG. 2012. Adsorption–desorption characteristics of cadmium in typical agricultural soils in China. Yangling: Northwest A&F University.
- Wei CY, Chen TB. 2001. Hyperaccumulators and phytoremediation of heavy metal contaminated soil: a review of studies in China and abroad. Acta Ecol Sin. 21:1196–1203.
- Welp G. 1999. Inhibitory effects of the total and water-soluble concentrations of nine different metals on the dehydrogenase activity of a loess soil. Biol Fertil Soils. 30:132–139. 10.1007/s003740050599
- Welp G, Brümmer GW. 1997. Microbial toxicity of Cd and Hg in different soils related to total and water-soluble contents. Ecotoxicol Environ Safety. 38:200–204. 10.1006/eesa.1997.1577