Abstract
The accumulation of phenolic acids in soil is one of the main problems associated with continuous cropping of peanut. Although laccases secreted by fungi can efficiently transform phenolic acids, there are few reports on the use of these enzymes to bioremediate continuous cropping soil. Food waste and wheat straw are waste products; however, they could be used productively as resources for laccase production by the endophytic fungus Phomopsis liquidambari B3. We cultured Phomopsis liquidambari B3 in medium containing food waste as the main nitrogen source and wheat straw as the main carbon source. In order to study the effects of fermentation liquid on phenolic acid degradation, rhizosphere soil microbial communities and peanut seedling growth, the fermentation product, which had high laccase activity, was added to continuously cropped soil of peanut. The concentration of 4-hydroxybenzoic acid, vanillic acid, and coumaric acid in soil had decreased by 57.4, 52.5, and 49.4%, respectively, compared with no-treatment control during 28 days. Analysis of denaturing gradient gel electrophoresis profiles showed that the bacterial and fungal community structures in rhizosphere soil were affected by changes in the phenolic acids concentration. The biomass of peanut plants and the number of root nodules were increased 68.3% and 5.9-fold, respectively. These results showed that the laccase product reduced the accumulation of phenolic acids in soil, the decrease in phenolic acids concentration and the increase in certain dominant microorganisms promoted peanut seedling growth and nodulation. This technology provides a new strategy for bioremediation of continuous cropping soil, while simultaneously reducing waste and protecting the environment.
Introduction
The accumulation of phenolic acids in soil is one of the main problems associated with continuous cropping of peanut. Li PD et al. (Citation2010) found that phenolic acids not only inhibited the growth of peanut seedlings directly but also promoted the infectious activity of soil-borne plant pathogens, increasing the incidence of diseases. For example, Zhou and Wu (20Citation12) found that p-coumaric acid increased the density of Fusarium oxysporum populations in soil. Koschorreck et al. (Citation2008) and Wang et al. (Citation2004) demonstrated that laccase could catalyze the dissociative transformation of phenolic acids into dimers, which are less toxic to plants and less readily absorbed, or into oligomers, which can crosslink with other compounds. Leonowicz et al. (Citation1984) reported that laccase could degrade phenolic acids into different products, including dimers and benzoquinone, under different pH conditions. Although laccases secreted by fungi can efficiently transform phenolic acids, there are few reports on the use of these enzymes to bioremediate continuous cropping soil.
Food wastes with a high moisture content and high organic content are a major component of municipal waste streams (Li R et al. Citation2010). If food wastes are not treated properly, then the rotten odor and residues become severe environmental hazards and directly affect human health (Li et al. Citation2011). Wheat straw is a major organic waste in China, most wheat straw is burned in the field directly after harvest (Chang et al. Citation2012). The burning of such a massive amount of biomass results in severe air pollution (Talebnia et al. Citation2010). Air-borne pollutants readily spread to urban areas, leading to severe haze events (Wang et al. Citation2011). However, food waste and wheat straw are two of the most promising materials for laccase production. Rosales et al. (Citation2002) reported the successful use of food waste as a substrate for laccase production by the white-rot fungus Trametes hirsuta in solid state conditions. The advantages of this process were that it was economical, there was an adequate supply of nutrients to the microbe, and it used waste materials, which helped to address pollution problems. Similarly, Sharma and Arora (Citation2010) demonstrated that solid state fermentation of wheat straw with Phlebia floridensis was an efficient approach to produce laccase and improve the digestibility of wheat straw. Previous studies indicated that the endophytic fungus Phomopsis liquidambari B3 (hereafter, B3) can produce laccase and degrade lignin (Dai, Chen, et al. Citation2010; Dai, Tian, et al. Citation2010; Chen et al. Citation2013). When B3 was applied to continuous cropping soil, it increased the growth and yield of peanut, and mitigated some other problems arising from continuous cropping (Wang et al. Citation2012). Under natural conditions, B3 can initiate saprophytic growth when detached from the host. Therefore, it has great potential for use in environmental restoration of agricultural ecosystems. Previous studies have shown that it can improve the soil microbial community structure, increase soil enzyme activity, increase microorganism biomass carbon and nitrogen, reduce the quantity of pathogenic bacteria, and increase the resistance and yield of crops (Dai et al. Citation2009; Dai, Xie et al. Citation2010; Dai et al. Citation2013; Wang et al. Citation2012). Chen et al. (Citation2011) demonstrated that in vitro, B3 strains can rapidly and thoroughly degrade high concentrations of the phenolic acid, 4-hydroxybenzoic acid. It is worth noting that the ecological functions of B3 are related to its ability to secrete laccase. However, in field conditions, laccase production is limited because of the shorter survival time of B3 in soil. This also limits the extent to which B3 can restore soil quality and promote plant growth. Wang et al. (Citation2014) cloned a novel laccase LACB3 from B3 and applied it to long-term cropping soil. This treatment resulted in a 12% increase in peanut biomass, and 40%, 21%, and 27% decreases in the concentrations of 4-hydroxybenzoic acid, vanillic acid, and coumaric acid, respectively, in soil. Therefore, there are better effects, in terms of optimizing the soil microenvironment and promoting plant growth, if the laccase is added to soil directly, rather than adding B3 itself.
To reduce the environmental load of waste materials and maximize the use of available resources, we used a mixture of food waste and wheat straw as the substrate for B3 to produce laccase. The fermentation product with high laccase activity was added to soil of continuously cropped peanut. Although food waste has a high salt content (Chen T et al. Citation2012), B3 is a salt-resistant strain that can grow under conditions of high osmotic pressure (Dai, Chen et al. Citation2010). Wheat straw, as an auxiliary material, can supply the carbon source and induce laccase production. Therefore, this approach is feasible for laccase production. We hypothesized that when applied to continuous cropping soil, the laccase produced from food waste and wheat straw would reduce the concentration of phenolic acids, promote the nutrient cycle, and positively affect the soil microecological environment, thereby improving the growth of peanut. Ultimately, this treatment may significantly improve the peanut agronomic index. This approach is both an economical and a practical measure for restoration of agricultural ecosystems. It offers solutions for both rural and urban pollution problems, that is, it reduces the amount of waste material that needs to be disposed of by burning or dumping, and it also produces a valuable product that can be used to bioremediate continuous cropping soil.
Materials and methods
Microorganism
We used the endophytic fungus P. liquidambari B3 in these experiments. This strain, which was isolated from the inner bark of Bischofia polycarpa (Chen et al. Citation2011), is maintained at the Jiangsu Key Laboratory for Microbes and Functional Genomics, Nanjing Normal University. The fungus was stored at 4°C on potato dextrose agar (200 g/L potato extract, 20 g/L glucose, and 20 g/L agar) and was activated before use. To activate the fungus, it was inoculated into potato dextrose broth (200 g/L potato extract and 20 g/L glucose) and cultured for 48 h at 28°C with a rotation speed of 180 rpm. To evaluate the dry weight, the mycelia from 10 mL of culture broth was washed with sterile distilled water twice and then dried in an oven at 80°C to a constant weight. A total of 3.30 g (equal to 0.33 g dry weight) of fungal mycelia was collected and washed twice with sterile distilled water, and then diluted with distilled water to a final volume of 200 mL. The solution was used as seed liquid of B3.
Materials
The food waste used in this research was collected from North District Canteen of Xianlin Campus, Nanjing Normal University. Food waste in the recycling bin was mixed thoroughly before sampling. The samples were mixed manually and then homogenized using a pulp refiner. The homogenate was freeze-dried and then ground. This allowed long-term storage of the food waste before use in experiments. The wheat straw was collected from farmland in a suburb of Nanjing. The straw was broken into pieces less than 1-cm long using a universal high-speed smashing machine. The main components of food waste and wheat straw used in this study are shown in .
Table 1. Compositional analysis of food waste and wheat straw.
We used the peanut (Arachis hypogaea L.) cultivar “Quanhua 7” in these experiments. The long-term cropping soil used in this study was red soil that had developed from red clay, which was sampled from Ecological Experimental Station of Red Soil, Chinese Academy of Sciences, Yingtan, Jiangxi Province, China. The chemical properties of the soil were as follows: organic matter content, 15.64 g/kg; total nitrogen, 0.70 g/kg; total phosphorus, 0.59 g/kg; available potassium, 228.07 mg/kg; and pH 5.8.
Acetonitrile (high-performance liquid chromatography [HPLC] grade) was purchased from the Honeywell Burdick & Jackson Company (Morristown, NJ, USA). We purchased HPLC-grade methanol and acetic acid from Hanbon Sci. Co. Ltd. (Jiangsu, China).
Experimental design
Food waste and wheat straw were used as the nitrogen source and the carbon source, respectively, in the fermentation medium. The fermentation medium was prepared according to the results of previous research, which are as follows: (per L) 0.5 g K2HPO4, 0.5 g MgSO4·7H2O, 0.25 g KCl, 0.01 g FeSO4, 0.002 g CuSO4·5H2O, 2.03 g food waste, 0.49 g straw, 1.05 g sucrose, and 1.11 g NaNO3. The fermentation conditions, which were established in a previous study, were as follows: inoculation volume, 6.24% (v/v); culture time, 185.92 h; rotation speed, 176.92 rpm; culture temperature, 26.7°C; and initial pH, 4.37. All of the fermentation experiments were performed in 5-L fermenter containing 3-L liquid medium. The fermenter and medium were autoclaved at 121°C for 20 min before use.
The effects of the laccase fermentation product on peanut growth were evaluated using a pot experiment. The pots were kept in a growth chamber at 28°C with humidity of 65%. The pots were 15 cm in diameter and 13 cm high. The soil was dried naturally and then screened through a 1-mm mesh sieve. Each pot contained approximately 1.5 kg soil. Three peanut seeds were sown in each pot. The treatments which were applied 200 mL in each pot were as follows: CK: soil with no additions; A: B3 only; B: fermentation medium containing food waste and wheat straw, un-inoculated with B3; C: fermentation broth containing food waste and wheat straw, inoculated with B3 (laccase activity 607.2 ± 4.7 U/mL). Each treatment had three independent replicates. Previous studies showed that P. liquidambari can survive for 30 days in the soil environment outside of its host plant (Chen et al. Citation2010). Therefore, we used a 28-day cultivation period. Samples of rhizosphere soil were collected at 0, 3, 7, 14, 21, and 28 days. The soil samples were freeze-dried and then screened through a 1-mm mesh sieve. These samples were used for the determination of phenolic acids and for polymerase chain reaction (PCR)-denaturing gradient gel electrophoresis (DGGE) analysis.
Calculation of peanut plant agronomic index
Peanut seedlings were harvested from pots on the final day (day 28). The plants were washed, cleaned, and then plant height, root length, and the number of root nodules were determined. The seedlings were dried to constant weight and then weighed to determine total weight.
Determination of phenolic acids in soil
Phenolic acids were detected by HPLC according to the method of Dai et al. (Citation2013), with modifications. A 5-g sample of oven-dry soil was added to a centrifuge tube, then 25 mL 1 M NaOH was added, and the mixture was left overnight. Then, the mixture was agitated for 30 min and centrifuged at 10,000 g for 10 min. The supernatant was adjusted to pH 2.5 with 12 M hydrochloric acid, and then left for 2 h to allow humic acid to precipitate. The precipitate was removed by centrifugation. The supernatant was filtered through a 0.22-μm cellulose acetate membrane before analysis by HPLC. We used an Agilent 1100 HPLC system, equipped with an ODS-C18 (4.6 × 150 mm) column. The mobile phase consisted of acetonitrile and 1.3% acetic [17:83 (v:v)]. The flow rate was 1 mL/min, the detection wavelength was 260 nm, and the column temperature and injection volume were 35°C and 20 μL, respectively. Qualitative analysis of phenolic acids was based on comparison of the retention time with those of authentic standards. The amounts of phenolic acids were calculated from the peak areas on the chromatogram. All analyses were conducted in triplicate and the average value for each parameter was calculated.
DNA extraction, PCR amplification, and DGGE analysis
Total DNA was extracted from soil using an UltraClean Soil DNA Isolation kit (Mo BIO Laboratories, Solana Beach, CA, USA). The PCR conditions used to amplify products from soil DNA are shown in . For the DGGE analysis, bacterial 16S ribosomal RNA (rRNA) was amplified using the universal forward primer 338F-GC clamp and the reverse primer 518R. Fungal 18S rRNA was amplified using the forward primer Fung-GC clamp and the reverse primer NS1. The PCR-DGGE primers and procedures are summarized in . The DGGE was carried out using a CBS-DGGE electrophoresis system (CBS Scientific Co., Del Mar, CA, USA). The concentration of polyacrylamide in the gel and the denaturing gradient conditions are shown in . The electrophoresis buffer was 0.5 × TAE. We mixed samples of PCR products obtained from triplicate amplifications. The loading quantity of samples was 500 ng. Electrophoresis was conducted for 12 h at 60°C and 100 V. Gels were stained with ethidium bromide for 20 min. Images of gels were acquired using the UVP gel image analysis system.
Table 2. PCR-DGGE primers and conditions.
Data analysis
We used SPSS 17.0 and Excel 2010 for statistical analyses. Significant differences among groups were determined by Duncan’s test (p = 0.05). DGGE fingerprint profiles were analyzed using Gelcompar II software. For the cluster analysis, we used the unweighted pair group method with arithmetic average method. We used Primer 5 to calculate the following indicators of community biodiversity: (1) Shannon’s index, H′ = − Σ(ni /N) ln (ni /N), where ni is the peak area, and N is the sum of all peak areas in the densitometric curve and (2) S, the number of bands in each lane.
Results and discussion
Phenolic acids concentrations in soil
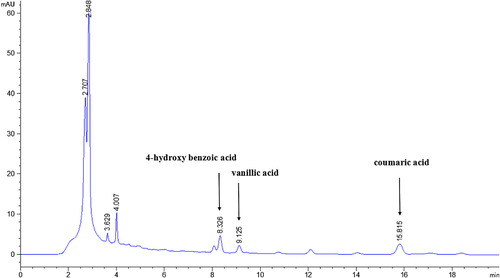
In this study, the main phenolic acids detected in the peanut continuous cropping soil were 4-hydroxybenzoic acid, vanillic acid, and coumaric acid. In the HPLC analysis, the retention times of 4-hydroxybenzoic acid, vanillic acid, and coumaric acid were 8.326, 9.125, and 15.815 min, respectively (). The effects of the different treatments on the concentration of 4-hydroxybenzoic acid in soil are shown in . Treatments A, B, and C resulted in efficient decomposition of 4-hydroxybenzoic acid; treatment C had the strongest effect. In treatment C, the concentration of 4-hydroxybenzoic acid in soil had decreased by 0%, 23.7%, 38.9%, 30.2%, 30.2%, and 57.4%, compared with that in CK, at 0, 3, 7, 14, 21, and 28 days, respectively. The effects of the different treatments on the vanillic acid concentration in soil are shown in . The concentration of vanillic acid in soil had decreased by 0%, 50.4%, 41.3%, 34.5%, 28.0%, and 52.5%, compared with that in CK, at 0, 3, 7, 14, 21, and 28 days, respectively. The changes in coumaric acid concentrations in soil are shown in . The concentration of coumaric acid in soil had decreased by 0%, 18.0%, 28.0%, 29.8%, 40.0%, and 49.4%, compared with that in CK, at 0, 3, 7, 14, 21, and 28 days, respectively. Together, these results indicated that treatment C was the most effective for decomposition of phenolic acids in soil. The rates of decomposition were higher than those reported by Wang et al. (Citation2014) after application of the cloned laccase LACB3.
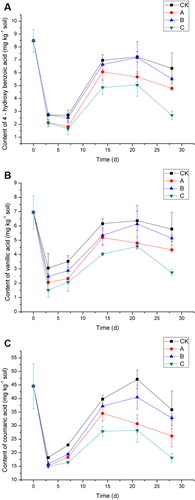
As shown in , the concentrations of 4-hydroxybenzoic acid, vanillic acid, and coumaric acid in soil decreased significantly from days 0 to 3. However, their concentrations increased significantly after day 7, and then decreased from days 21 to 28. There are many possible explanations for the fluctuations in phenolic acids concentrations. Phenolic acids are released into the external environment via secretion from plant roots (Seal et al. Citation2004) and leachates from plant leaves (Inderjit & Nilsen Citation2003). In peanut, nodulation can induce the secretion of phenolic acids from roots, thus increasing the phenolic acids concentration in the soil. The results of some previous studies have suggested that phenolic acids, at appropriate concentrations and under certain conditions, can serve as carbon and energy sources for soil microbes (Kefeli et al. Citation2003). For example, in vitro, B3 can use 4-hydroxybenzoic acid as the sole carbon and energy source, showing a maximum degradation rate of up to 99% (Chen et al. Citation2011). However, the concentration of phenolic acids available to microbes may differ at different stages of plant growth, because the amount of phenolic acids secreted by plants varies according to the growth stage (Zhou & Wu Citation2012).
DGGE analysis of microbial communities in rhizosphere soil
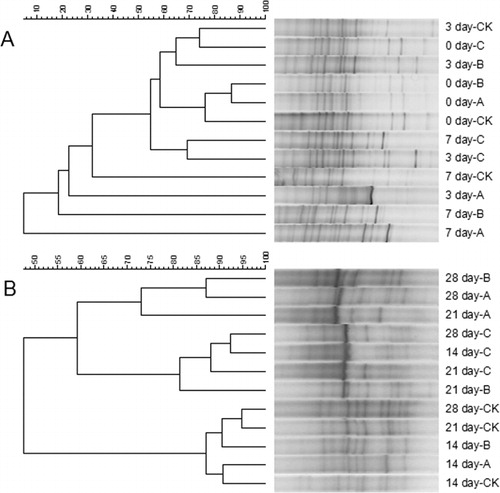
The effects of the different treatments on microbial communities in rhizosphere soil were evaluated by DGGE analysis. On day 0, the soil bacterial communities were quite similar in all of the treatments (). The CK on day 7 was grouped with CK on day 3. Treatment C on day 7 was grouped with treatment C on day 3. The changes in soil bacterial communities over the following 3 weeks are shown in . Treatment C on day 28 was grouped with C on day 14, and then grouped with C on day 21. This result showed that the community structures were similar in treatment C on days 14, 21, and 28. There were differences between C and the other three treatments. The brightness of some bands in C was greater than those in CK, A, and B, probably because of an increase in the abundance of certain dominant soil microbes. This result indicated that treatment C affected the bacterial communities in rhizosphere soil. Specifically, the bacterial population structure showed an enrichment of a dominant community that consisted mainly of beneficial microorganisms.
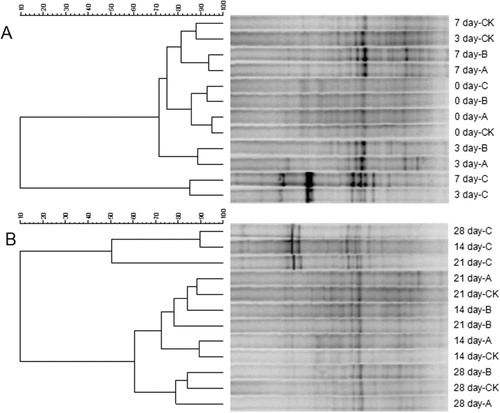
The effects of the different treatments on rhizosphere soil fungal communities are shown in . As shown in , treatments CK, A, and B on day 0 were grouped together, and there was another grouping of C on day 0 and CK and B on day 3. Treatment C on day 3 and C on day 7 formed a group. As shown in , CK on day 21 and CK on day 28 were grouped together; CK, A, and B on day 14 showed similar community structures; C on days 14 and 28 formed a group, and then grouped with C on day 21. These analyses showed that in treatment C, the community structure on days 14, 21, and 28 was markedly different from that on days 0, 3, and 7. Therefore, treatment C changed the fungal community structure in soil. There were larger differences in fungal communities than in bacterial communities, suggesting that the fungal and bacterial communities in rhizosphere soil responded differently to phenolic acids concentrations. The bacterial communities were more stable than fungal communities. These results were consistent with trends in changes in phenolic acids concentrations determined by HPLC.
The number of bands (S) and Shannon’s index (H′) of rhizosphere soil bacteria and fungi are shown in . In terms of the diversity of the bacterial community (as reflected by H′), the four treatments could be ranked from highest to lowest as follows: A > CK > B > C. For fungi, the trend in H′ differed between the early and late stages of the experiment. On days 0, 3, and 7, the treatments could be ranked, in terms of H′ values, from highest to lowest as follows: C > CK > B > A. On days 14, 21, and 28, the ranking was as follows: CK > B > A > C. The H′ values obtained for these treatments indicated that the community structure changed over time. The H′ values do not represent the true diversity of the community structure in the soil; instead, they are a useful indicator to compare changes in the community structure of dominant microbes. The S and H′ values in treatment C were lower than their corresponding values in other groups. This result indicated that in treatment C, some dominant microbes that may have beneficial effects on the soil were continuously enriched, and outcompeted other more vulnerable groups; therefore, the H′ value was lower in treatment C than in other treatments. This is consistent with the findings of Chen LH et al. (Citation2012), who reported that the abundance of certain microbial groups changed as a result of competition. Our findings were also consistent with the results of Zhou et al. (Citation2012b), who reported that addition of phenolic compounds stimulated some soil microbes, but inhibited others.
Table 3. Genotypic diversity of soil bacteria and fungi by DGGE analysis.
The main reason for the changes in soil microflora after addition of the laccase product is probably because the concentration of phenolic acids in the soil decreased. Qu et al. (Citation2008), Wu et al. (Citation2009), and Zhou et al. (2Citation012b) reported that phenolic acids tend to promote the growth of microorganisms at low concentrations, and inhibit their growth at high concentrations. This is consistent with the trends observed in the present study, that is, the phenolic acids concentration decreased and the amount of microorganisms increased after applying the laccase product. At present, the mechanism by which phenolic acids affect soil microorganisms is not clear. Some researchers have proposed that high concentrations of phenolic acids can inhibit certain microbial functions, for example, the production of gas and volatile fatty acids, and reduce the microbial consumption of growth medium (Murray et al. Citation1996). However, at low concentrations, small molecular weight phenolic acids can provide an additional carbon source to promote the growth of some indigenous microorganisms (Souto et al. Citation2000).
Agronomic index of peanut seedlings
We measured plant height, root length, total dry weight, and number of root nodules as indices to evaluate the agronomic characteristics of peanut seedlings. As shown in , after 28 days, seedling growth was better in treatment C than in CK, A, and B. There was no significant difference between CK and B in terms of plant height, root length, total dry weight, and number of root nodules. Seedlings in treatment A showed better growth than those in CK and B. This is probably because B3 itself can degrade phenolic acids, and it can change the soil microflora, resulting in conditions that are conducive to degradation of toxic substances. This can alleviate some of the problems related to continuous cropping soil. However, in terms of seedling growth, treatment A was significantly inferior to C, because laccase production would have been limited in the soil environment in treatment A. Shoot elongation was 90.1% greater in treatment C than in CK (), and root elongation was 30.6% greater in treatment C than in CK (). The frequency of root nodulation was 68.3% higher in treatment C than in CK (), and the biomass of seedlings was 5.9-fold greater in treatment C than in CK (). Together, these results showed that treatment C significantly promoted peanut seedling growth and nodulation. The promoting effects of treatment C were significantly superior to those of the cloned laccase LACB3, as reported by Wang et al. (Citation2014).
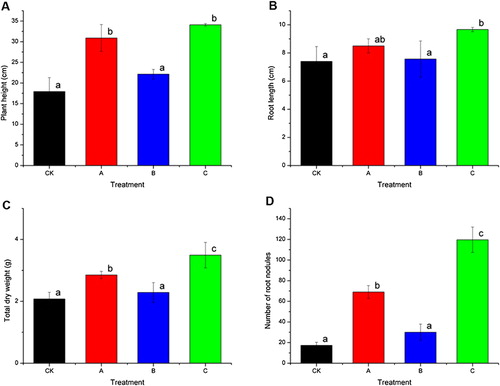
When soil is degraded by continuous cropping, the growth and nodulation of peanut seedlings are decreased. Continuous cropping soil shows lower enzyme activity, imbalanced microflora, and increased levels of toxic compounds such as phenolic acids (Zhou & Wu Citation2012; Zhou et al. Citation2012a, Citation2012b). In this study, the biomass of peanut plants and the number of root nodules were significantly increased in treatment C (fermentation broth with laccase activity), compared with their respective values in the other treatments. The promotion of peanut seedling growth after application of treatment C is likely because of improvements in the soil microenvironment. Such improvements include a reduction in the concentration of phenolic acids. Therefore, this treatment directly reduces the autotoxicity of allelochemicals and results in changes in the rhizospheric microbial communities. Such changes can modify the functional roles of the microbial community, and consequently, affect plant growth (Zhou et. al Citation2012a). Further research should focus on obtaining direct evidence that the changes in rhizosphere soil microbial communities after degradation of phenolic acids are responsible for the improved performance of peanut seedlings.
This rapid liquid fermentation method has the potential for broad applications, on both a small scale and an industrial scale. Compared with solid fermentation, liquid fermentation is easier to up-scale, and much faster. These characteristics are very important when considering disposal of food waste and wheat straw that may be geographically distant from each other. We cultured the B3 endophyte using a fermentation broth that included food waste and wheat straw, taking laccase activity as the marker for maturity. We applied the fermentation product containing laccase to soil, along with appropriate controls, and measured various ecological parameters in the soil. We designed these experiments to include controls that would show that the ecological functions were not caused by B3 itself (treatment A), or to components of the growth medium (treatment B). Our results showed that the high-efficiency liquid fermented substrate (treatment C) with high laccase activity resulted in the degradation of phenolic acids, changes in rhizospheric microbial communities, and increased plant growth. To our knowledge, there have been no reports on application of laccase to ameliorate problems associated with continuous cropping. This study describes a creative approach to use food waste and wheat straw to produce laccase, which can be used to improve the health of continuously cropped peanut soil. These experiments confirm that the technology is feasible. It has great potential for application in diverse situations because it simultaneously addresses two important issues; environmental pollution from the disposal of food waste and wheat straw, and soil degradation resulting from continuous cropping.
Acknowledgment
We thank the North District Canteen of Nanjing Normal University Xianlin Campus for their support.
Funding
This work was funded by the National Natural Science Foundation of China [grant number 31370507], the Ph.D. Programs Foundation of Ministry of Education of China [grant number 20133207110001], and the Major Natural Science Research Programs of Jiangsu Higher Education Institutions of China [grant number 13KJA180003].
Additional information
Funding
References
- Chang C, Xu G, Jiang X. 2012. Production of ethyl levulinate by direct conversion of wheat straw in ethanol media. Bioresour Technol. 121:93–99. 10.1016/j.biortech.2012.06.105
- Chen LH, Huang XQ, Zhang FG, Zhao DK, Yang XM, Shen QR. 2012. Application of Trichoderma harzianum SQR-T037 bio-organic fertiliser significantly controls Fusarium wilt and affects the microbial communities of continuously cropped soil of cucumber. J Sci Food Agric. 92:2465–2470. 10.1002/jsfa.5653
- Chen T, Jin Y, Liu F, Meng X, Li H, Nie Y. 2012. Effect of hydrothermal treatment on the levels of selected indigenous microbes in food waste. J Environ Manage. 106:17–21. 10.1016/j.jenvman.2012.03.045
- Chen Y, Dai CC, Wang XX, Zhang B, Ju Q. 2010. Effects on endophytic fungus (Phomopsis SP.) on decomposition of plant (Atractylodes lancea (Thunb) DC) litters and activity of degrading enzymes in soil. Acta Pedologica Sinica. 47:537–544.
- Chen Y, Peng Y, Dai CC, Ju Q. 2011. Biodegradation of 4-hydroxybenzoic acid by Phomopsis liquidambari. Appl Soil Ecol. 51:102–110. 10.1016/j.apsoil.2011.09.004
- Chen Y, Xie XG, Ren, CG, Dai CC. 2013. Degradation of N-heterocyclic indole by a novel endophytic fungus Phomopsis liquidambari. Bioresource Technol. 129:568–574. 10.1016/j.biortech.2012.11.100
- Dai CC, Chen Y, Tian L, Shi Y. 2010. Correlation between invasion by endophytic fungus Phomopsis sp. and enzyme production. Afr J Agric Res. 5:1324–1330.
- Dai CC, Chen Y, Wang XX, Li PD. 2013. Effects of intercropping of peanut with the medicinal plant Atractylodes lancea on soil microecology and peanut yield in subtropical China. Agrofor Syst. 87:417–426. 10.1007/s10457-012-9563-z
- Dai CC, Tian L, Zhao Y, Chen Y, Xie H. 2010. Degradation of phenanthrene by the endophytic fungus Ceratobasidum stevensii found in Bischofia polycarpa. Biodegradation. 21:245–255. 10.1007/s10532-009-9297-4
- Dai CC, Xie H, Wang XX, Li PD, Zhang TL, Li YL, Tian X. 2009. Intercropping peanut with traditional Chinese medicinal plants improves soil microcosm environment and peanut production in subtropical China. Afr J Biotechnol. 8:3739–3746.
- Dai CC, Xie H, Wang XX, Li PD, Li YL, Zhang TL, 2010. The effects of intercropping with medicinal plants and addition of endophytic fungi on soil microflora and peanut yield, Acta Ecologica Sinica. 30:2105–2111.
- Inderjit HH, Nilsen ET. 2003. Bioassays and field studies for allelopathy in terrestrial plants: progress and problems. Crit Rev Plant Sci. 22:221–238. 10.1080/713610857
- Kefeli VI, Kalevitch MV, Borsari B. 2003. Phenolic cycle in plants and environment. J Cell Mol Biol. 2:13–18.
- Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB. 2008. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol. 79:217–224. 10.1007/s00253-008-1417-2
- Leonowicz A, Edgehill R, Bollag JM. 1984. The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia praticola and Trametes versicolor. Arch Microbiol. 137:89–96. 10.1007/BF00414446
- Li PD, Wang XX, Li YL, Wang HW, Liang FY, Dai CC. 2010. The contents of phenolic acids in continuous cropping peanut and their allelopathy. Acta Ecologica Sinica. 30:2128–2134. 10.1016/j.chnaes.2009.12.001
- Li R, Chen S, Li X. 2010. Biogas production from anaerobic co-digestion of food waste with dairy manure in a two-phase digestion system. Appl Biochem Biotechnol. 160:643–654. 10.1007/s12010-009-8533-z
- Li Y, Su B, Liu J, Du X, Huang G. 2011. Nitrogen conservation in simulated food waste aerobic composting process with different Mg and P salt mixtures. J Air Waste Manag Assoc. 61:771–777. 10.3155/1047-3289.61.7.771
- Murray AH, Iason GR, Stewart C. 1996. Effect of simple phenolic compounds of heather (Calluna vulgaris) on rumen microbial activity in vitro. J Chem Ecol. 22:1493–1504. 10.1007/BF02027727
- Qu X, Wang J. 2008. Effect of amendments with different phenolic acids on soil microbial biomass, activity, and community diversity. Appl Soil Ecol. 39:172–179. 10.1016/j.apsoil.2007.12.007
- Rosales E, Couto SR, Sanromán A. 2002. New uses of food waste: application to laccase production by Trametes hirsuta. Biotechnol Lett. 24:701–704. 10.1023/A:1015234100459
- Seal AN, Pratley JE, Haig T, An M. 2004. Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J Chem Ecol. 30:1647–1662. 10.1023/B:JOEC.0000042074.96036.14
- Sharma RK, Arora DS. 2010. Production of lignocellulolytic enzymes and enhancement of in vitro digestibility during solid state fermentation of wheat straw by Phlebia floridensis. Bioresource Technol. 101:9248–9253. 10.1016/j.biortech.2010.07.042
- Souto XC, Chiapusio G, Pellissier F. 2000. Relationships between phenolics and soil microorganisms in spruce forests: significance for natural regeneration. J Chem Ecol. 26:2025–2034. 10.1023/A:1005504029243
- Talebnia F, Karakashev D, Angelidaki I. 2010. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol. 101:4744–4753. 10.1016/j.biortech.2009.11.080
- Wang G, Chen C, Li J, Zhou B, Xie M, Hu S, Kawamura K, Chen Y. 2011. Molecular composition and size distribution of sugars, sugar-alcohols and carboxylic acids in airborne particles during a severe urban haze event caused by wheat straw burning. Atmos Environ. 45:2473–2479. 10.1016/j.atmosenv.2011.02.045
- Wang GD, Li QJ, Luo B, Chen XY. 2004. Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat Biotechnol. 22:893–897. 10.1038/nbt982
- Wang HW, Wang XX, Lu LX, Xiao Y, Dai CC. 2012. Effects of applying endophytic fungi on the soil biological characteristics and enzyme activities under continuously cropped peanut. Chin J App Ecol. 23:2693–2700.
- Wang HW, Zhu H, Liang XF, Du W, Dai CC. 2014. Molecular cloning and expression of a novel laccase showing thermo-and acid-stability from the endophytic fungus Phomopsis liquidambari and its potential for growth promotion of plants. Biotechnol Lett. 36:167–173. 10.1007/s10529-013-1347-7
- Wu F, Wang X, Xue C. 2009. Effect of cinnamic acid on soil microbial characteristics in the cucumber rhizosphere. Eur J Soil Biol. 45:356–362. 10.1016/j.ejsobi.2009.04.001
- Zhou X, Wu F. 2012. p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f. sp. cucumerinum Owen. PloS One. 7:e48288. 10.1371/journal.pone.0048288
- Zhou X, Yu G, Wu F. 2012a. Responses of soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) to exogenously applied p-hydroxybenzoic acid. J Chem Ecol. 38:975–983. 10.1007/s10886-012-0156-0
- Zhou X, Yu G, Wu F. 2012b. Soil phenolics in a continuously mono‐cropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities. Eur J Soil Sci. 63:332–340. 10.1111/j.1365-2389.2012.01442.x