Abstract
Salt stress is more and more becoming a serious problem in the world especially if we consider its damaging effect on the plant growth and yield. The cultivation of medicinal plants, such as Aloe vera, might be an alternative for the saline water use and salt-affected soils occupation. Aloe vera, commonly known as aloe, is one of the primary medicinal plants with multipurpose applications going from pharmaceutical to cosmetic aspects with a promising economic return. Aloe plants were cultivated and irrigated, for 14 months, with drinking water (C0) and with two levels of salt (C1 and C2). Changes in growth, hydrogen peroxide (H2O2), lipid peroxidation and phenolic compounds were examined in leaves at harvest. Depressive effects of salt irrigation on the plant growth parameters and a perturbation in inorganic ion contents were found especially with a high level of salt in the irrigation water. The intracellular oxidative stress was evaluated with the H2O2 production. Our results showed that the H2O2 content increased with the accumulation of the toxic ion (Na) in the leaf tissues. In addition, lipid peroxidation, measured by the malondialdehyde (MDA) level, increased as well with salt augmentation in the irrigation water. In response to salt stress, Aloe leaves showed a significant increase in the levels of phenolic compounds too. These results suggest that Aloe can be planted in soils affected by salinity and irrigated with salt water at least at a moderate concentration used in the present study.
Introduction
Salinization plays a major role in soil degradation. It affects 19.5% of the irrigated land and 2.1% of the dry land agriculture existing on the Globe (Nemoto & Sasakuma Citation2002). In Tunisia, soils affected by salts cover about 1.5 million hectares, which is around 10% of the total country area. About 30% of the irrigated areas are affected by salts in different degrees (Hachicha Citation2007). The salinity problem is more acute in arid and semiarid regions due to the need for extensive irrigation, the low annual rainfall that is insufficient to meet evaporative need crops and the relative scarcity of good quality water. Thus, even with water with a relatively good quality, the permanent irrigation practice causes the irrigated soil to be affected by an excess of soluble salts (Bresler Citation1982). Moreover, World prospects indicate that the quality of the irrigation water tends to deteriorate. Hence, using poor quality water is becoming a necessity. That would result in an accumulation of salts in the soil that gets saline.
Crops generally suffer from a high salinity because of the high osmotic pressure that inhibits water suction, and crops symptoms are generally the same as those of moisture stress from the dry conditions. The most typical symptom of saline injury to plants is the reduction of growth (Wu et al. Citation2013; Liu & Chen Citation2014; Zhuo et al. Citation2015), which is a consequence of several physiological responses including the modification of the ion balance, the water status, the mineral nutrition, the photosynthetic efficiency, and the carbon allocation and utilization (Taylor et al. Citation2004; Yildirim et al. Citation2006).
Many reports demonstrated an overproduction of the reactive oxygen species (ROS) in response to salt stress. The most commonly produced ROS are superoxide (O2 –), hydrogen peroxide (H2O2), hydroxyl radicals (OH), and singlet oxygen (1O2). ROS can be associated with the development of the oxidative injury and the disruption of the metabolic functions in plants (Mittler Citation2002). One of the most deleterious effects induced by ROS is lipid peroxidation which can directly cause bio-membrane deterioration. The malondialdehyde (MDA), one of the decomposition products of polyunsaturated fatty acids of membrane, is considered as a reliable indicator of oxidative stress (Demiral & Türkan Citation2005) and in presence of salt stress, enhanced levels of MDA have been observed in many reports (Gondim et al. Citation2012; Nawaz et al. Citation2012; Radi et al. Citation2013). However, low concentrations of ROS, which are byproducts of normal metabolism, do not lead to oxidative damage, thanks to the presence of antioxidant systems that can scavenge ROS (Foyer & Noctor Citation2000). In effect, it is only when the production of ROS exceeds the capacity of these scavenging systems that oxidative damage occurs.
In this case, higher plants have developed different adaptive mechanisms to reduce oxidative damage resulting from salt stress, and this through the biosynthesis of a cascade of antioxidants among them phenolic compounds, which are the most abundant secondary metabolites in plants. These compounds can be classified into soluble compounds such as phenolic acids, phenylpropanoids, flavonoids and quinones (Rispail et al. Citation2005) and non-soluble compounds such as condensed tannins, lignins and cell-wall bound hydroxycinnamic acids. In addition, Phenolic compounds have been implicated to stress resistance against biotic and abiotic factors (Bergmann et al. Citation1994; Waterman & MoleCitation1994) and to play an important role in scavenging the free radicals (Mohamed & Aly Citation2008). The antioxidative property of phenolic compounds streams from their high reactivity as hydrogen or electron donors, and from the ability of the polyphenol-derived radical to stabilize and to delocalize the unpaired electron (Huang et al. Citation2005). Several studies have reported that the total phenol production is stimulated by NaCl (Falleh et al. Citation2008; Mehr et al. Citation2012).
Thus, a concerted effort to understand the effects of salinity on plants and to develop salt-tolerant cultivars is essential to combat soil salinization problems (Rengasamy Citation2006). And obviously, one interesting alternative to use saline water and to occupy salt-affected soils is the cultivation of plants having physiologies adaptive to stress, economic and medicinal values, such as the Aloe vera. Aloe vera (Aloe barbadensis M.), a desert plant with crassulacean acid metabolism (CAM), is a xerophyte with a strong drought resistance and with also a certain degree of tolerance to salt stress although it is not usually taken as a halophyte (Zheng et al. Citation2009). Aloe vera is highly appreciated thanks to its short growth period and its high economic value (Gantait et al. Citation2014). It is, in addition, an important industrially cultivated species, from which a gel of a proven pharmacological and medicinal value is extracted (Hamman Citation2008). This gel has a complex chemical composition, composed primarily of soluble sugars, anthraquinones, polysaccharides, amino acids, vitamins and proteins, many of which are enzymes (Chow et al. Citation2005; Chun-hui et al. Citation2007).
The objective of this research is to investigate the salt stress effects on Aloe plants. To achieve this goal, the growth criteria, the H2O2 production, the membrane integrity and the phenolic compounds were analyzed in the leaves of our interesting plant cultivated under saline conditions.
Materials and methods
Experimental conditions and Aloe plantation
The experiment has been performed under the shelter of the National Research Institute of Rural Engineering, Water and Forestry (INRGREF), located in the North of Tunis (Ariana-Tunisia). The examples were provided by The Laboratory of Ecology and Improvement Sylvo-Pastoral in INRGREF. Plants of 4 to 5 leaves were put in plastic pots with free drainage. Each pot contained 7 kg of soil characterized by a fine and an ultrafine texture. The proportion of fine element (clay + silt) exceeds 90% and the quantity of sand is very small. The content of the organic matter is very low (about 0.7%), and inversely the total carbonate, determined by NF ISO 10693 method, is high (43%). The experimental soil is slightly alkaline (pH 7.6), and the salinity, as measured by the electrical conductivity of the saturated paste extract (ECe), is low (ECe 1.10 dS m–1).
Plant irrigation and soil salinity
Aloe plants have been irrigated with drinking water (ECw 1.25 dS m–1; pH 7.1). The application of salt stress began, six months after their transplantation, with an intake of salt (NaCl) in the irrigation water. For 14 months, plants have been irrigated with drinking water (C0) and with two concentrations of salt: C1 (ECw 3.5 dS m–1, pH 7.56) and C2 (ECw 12 dS m–1, pH 7.52). For each treatment, 12 plants, divided into 3 randomly distributed replicates, were used. The frequency of irrigations varied according to the season: nine irrigations per month during the hot period and two ones per month during the cold period. Each pot was irrigated with 44 mm of water, and at the end of the experiment, the cumulative water intake was about 1782 mm for each pot.
The soil salinity was controlled by the monitoring of the electrical conductivity of the saturated paste extract (ECe) with r = 0.946 at P < 0.05. The soil/water ratio was 1/5 (w/v). A significant linear relationship: CEe = 5.94 * CE 1/5 (n = 36, R 2 = 0.865) was determined.
Growth parameters and mineral analysis
During our experimentation, leaf growth (the number and the length of the leaves) was monitored. And each leaf was counted when the length was about 4 cm. After 14 months, leaves were harvested. Water and gel contents of the samples were determined by drying a known fresh weight (FW) of homogenized samples at 50°C and then at 105°C until there were no further changes in weight under these two temperatures. After cooling to room temperature, the samples were reweighed and then the dry matter and the water-gel contents were calculated. For a mineral analysis, leaf samples were thoroughly washed in tap water and rinsed with distilled water. They were then dried at 50°C to constant weight and ground up to pass through a 2-mm sieve. Mineral extraction was effectuated by digestion, using hot HNO3, according to Zarcinas et al. (Citation1987). The concentrations of Na, K, Ca and Mg were obtained by inductively coupled plasma optical emission spectrometry (ICP-OES) using a Horiba Jobin-Yvon apparatus.
H2O2 determination
The intracellular H2O2 content was determined according to Sergiev et al. (Citation1997). Leaf tissues (500 mg) were homogenized at 4°C with 5 mL 0.1% (w/v) trichloroacetic acid and centrifuged at 12,000 rpm for 15 min. An aliquot (0.5 mL) of the supernatant was added to a solution containing 10 mM potassium phosphate buffer (pH 7.0) (0.5 mL) and 1 M potassium iodide (1 mL). The absorbance was measured at 390 nm using a UV-visible spectrophotometer (Jenway 6305 UV-visible Spectrophotometer, Germany) and the content of H2O2 was calculated based on a standard curve using gradual H2O2 concentrations.
Lipid peroxidation
The level of lipid peroxidation products in the leaf samples was expressed as the MDA content and was determined according to the method of Heath and Packer (Citation1968) with a slight modification. Leaf tissues (1 g) were homogenized in 10% (w/v) trichloroacetic acid (TCA; 10 mL) and centrifuged at 10,000 rpm for 10 min. An equal volume of 10% TCA solution containing 0.5% 2-thiobarbituric acid was added to the supernatant. The sample was incubated at 95°C for 30 min, cooled quickly in an ice-bath and centrifuged at 10,000 rpm for 15 min. The absorbance was measured at 532 nm and corrected for nonspecific absorbance at 600 nm. The concentration of MDA was calculated using 155 mM–1 cm–1 as an extinction coefficient.
Total phenolic contents
The content of total phenolic compounds in methanolic extracts was determined by the Folin-Ciocalteu method (Swain & Hillis Citation1959). An aliquot (1 mL) of the methanolic extract was placed in a volumetric flask (10 mL). A folin-Ciocalteu reagent (0.5 mL) was added. After 3 min, saturated sodium carbonate (1 mL) was added. The solution was mixed well and incubated at room temperature, in the dark, for 1 hour. The absorbance of the resulting blue colour was measured at 725 nm using a UV-visible spectrophotometer. The results were expressed in gallic acid equivalents (mg g–1 of FW) using a gallic acid standard curve.
Statistical analysis
The results presented are the means ± standard deviation obtained from three replicates. The mean effect of salt treatment was tested by one-way analysis of variance. Means were compared by Tukey's test at the 0.05 confidence level using the SPSS program (IBM SPSS statistics, v20). The correlations between Na, MDA, H2O2 and phenolic compounds were evaluated using Person's correlation coefficients.
Results
Soil salinity
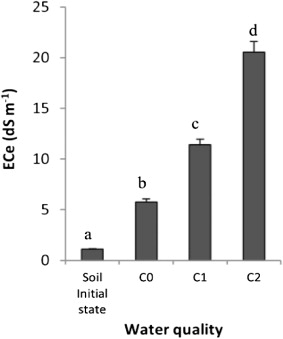
Before the Aloe plantation, the soil had a lower salinity (ECe 1.10 dS m–1). However, 14 months of irrigation with drinking water increased its salinity, which reached 5.77 dS m–1 (). Augmenting salt concentrations in water increased significantly the salinity in the root zone (). In fact, irrigation with 3.50 dS m–1 of salt (C1) induced an increase in soil salinity which ECe was 10.50 dS m–1. Obviously, under the highest salt-water irrigation, the salinity of the soil increased dramatically whose ECe reached 20.55 dS m–1.
Growth parameters and mineral content
High salt concentration in water affected the leaf growth (). Plants irrigated with drinking water (C0) produced in total 173 leaves which are significantly more than the numbers produced by plants irrigated with moderate water (C1) and with high salinity water (C2). In fact, as a consequence to the moderate water irrigation, 145 leaves were produced and only 109 leaves were produced after the high salinity water irrigation during the experiment period. The number of leaves per plants was 14.50, 12.91 and 9.09 for C0, C1 and C2, respectively (). Moreover, the highest rate of a new leaf production occurred in summer, especially during June, July and August, which are the months with the highest temperature (data not shown).
Table 1. The salt effects on the Aloe leaf growth. Irrigations were done with different quality of water: C0 (ECw 1.25 dS m–1), C1 (ECw 3.50 dS m–1) and C2 (ECw 12.00 dS m–1). Values represent means ± standard deviation (SD) of triplicates.
The leaves displayed a highly significant reduction in length of about 30% at the most salinization levels as compared with the control. The significant depressive effects of salt on fresh and dry matters were observed under all treatments (). The leaves fresh weight decreased significantly about 30% under C1 and 56% under C2 compared to the control. However, in plants treated with saline water, no significant reductions were noticed in water-gel contents compared to the control. Indeed, about 95% of the leaves biomass of Aloe vera was made of water and gel in the plants irrigated with drinking water and with water of moderate salinity and about 94% in plants subjected to the highest salt stress. The remaining 5 to 6% of the leaves biomass was made of a photosynthetic tissue, a cuticle and spines surrounding the leaf.
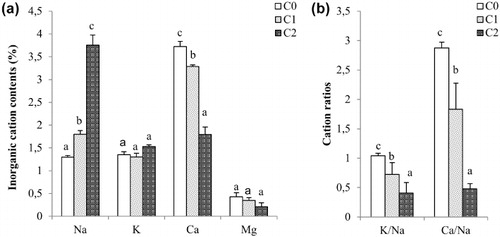
The sodium content augmented significantly (P < 0.05) in the leaf of Aloe vera in the two treatments (). Increasing salt treatments reduced the calcium content but had no significant effect on potassium and magnesium levels. As a result of the change in Na+ content, the treated plants contained significantly lower K+/Na+ and Ca2+/Na+ ratios than plants irrigated with drinking water.
H2O2 production, lipid peroxidation and total phenolic contents
The changes of H2O2 production of Aloe vera are shown in ). Under irrigation with drinking water, plants contained a lower level of H2O2. Salinity significantly increased (P < 0.05) H2O2production. This increase is about 149% for the plants irrigated with C1 and about 269% for the plants irrigated with C2, when compared with plants irrigated with drinking water (C0). A positive correlation (r = 0.945, P < 0.05) was also found between the toxic ion (Na) accumulation and the H2O2 production.
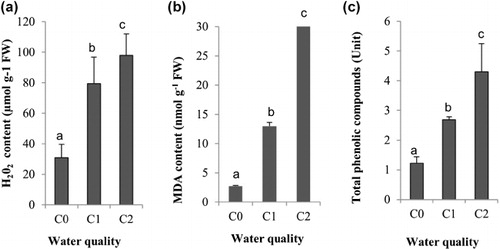
The accumulation of MDA was measured, and our results showed that the salt-stressed plants accumulated significantly (P < 0.05) an MDA higher than that of the controlled plants (). In fact, the highest MDA level was observed in plants having the most concentration of salt. Furthermore, positive correlations were observed between Na accumulation and MDA production (r = 0.983, P < 0.05), and between H2O2 and MDA productions (r = 0.981, P < 0.05).
Like the MDA production, the total phenolic contents were affected significantly by the salt stress (). Indeed, when irrigation was done with a moderate and with a high concentrations of salt (C1 and C2), total phenolic contents increased compared to the control. And these increases were about 219% and 351% respectively. The total phenolic production was then correlated with the H2O2 production.
Discussion
The extension of salinization marks a serious need to find new crop cultivars and medicinal plants with high growth and production under this unfavourable condition. High concentrations of salt resulting from natural processes or from misarrangement in irrigated agriculture provoke the amplification of soil salinity and the inhibition of the plant growth and yield. In our study, the ability of Aloe vera to withstand salinity was tested and plants were cultivated under saline conditions. Before Aloe plantation, our experimental soil had a lower salinity (ECe 1.10 dS m–1) (). However, permanent irrigation with drinking water (C0) increased soil salinity and the ECe became 5.77 dS m–1. The poor quality of irrigation water (C1 and C2) affected severely the soil and increased significantly its conductivity although a leaching was maintained. Thus, excessive salt not only destroys the soil structure (Hachicha & Hallaire Citation2002), but also attracts water and blocks its absorption by the plant roots.
Among the different indices of salt tolerance of plants, the reduction in growth is one of the potential criteria, as indicated in some earlier reports (Keyster et al. Citation2013; Dai et al. Citation2014). Aloe is a xerophyte with a strong drought resistance and a certain degree of tolerance to salt stress as well (Zheng et al. Citation2009). In the present investigation, the effect of salinity on the leaf growth was recorded in Aloe vera plants under different salt stresses (). Our results showed a diminution which is the highest under C2 (12.00 dS m–1) in Aloe leaf number after 14 months of irrigation with saline water. This reduction affected the leaves length too. Changes in Aloe growth induced by salt stress have been demonstrated in many reports (Jin et al. Citation2007; Zheng et al. Citation2009). Yet, Aloe plants subjected to salt stress, showed no significant modification in their content of water and gel. That could be explained by the fact that Aloe vera, a CAM plant, combines the capacity of a great water storage capacity with the efficiency of CO2 fixation. The efficient CO2 assimilation of CAM plants is related to their capacity for osmolyte synthesis, mainly those derived directly from sugar synthesis. Accumulation of osmolytes in the cytoplasm is considered as a mechanism contributing to the water deficit tolerance of the plant. Indeed, even in well watered soils, salinity causes water deficit by reducing the osmotic potential of soil solutes, making it, therefore, difficult for roots to extract water from their surrounding media. Osmolytes, such as proline and glycine betaine, appear to protect not only the quaternary structure of proteins maintaining enzyme activity but also the highly ordered structure of membranes from the damaging effects of environmental stresses (Yoshiba et al. Citation1997; Chen & Murata Citation2008).
The deleterious effects of salinity on Aloe growth were accompanied by an accumulation of the toxic ion (Na) and by changes in the nutrient ion levels (). Indeed, the irrigation with saline water induced an accumulation in the tissue sodium content and inversely a drop in Ca levels in leaves under the two treatments. However, no significant changes in K and Mg levels were noticed. Murillo-Amador et al. (Citation2014) studying the effect of 30–120 mM NaCl on the ion distribution in the different organs of Aloe vera, found at the same times increases in Na and Cl contents and decreases in Ca, K and Mg levels and cations were accumulated more in the roots and the stems than in the leaves. Those results suggest that such a distribution of inorganic cations reduced the osmotic potential of the rooting environment and ensured the normal physiological function and metabolism of the plant.
Similar to the previous reports with Aloe (Jin et al. Citation2007; Murillo-Amador et al. Citation2014), in the present study, the stressed plants maintained a K/Na ratio significantly lower than those of control plants. Owing to the similarity of Na+ to K+, Na+ can replace K+. K+, one of the essential macronutrients taken up by the roots, is generally transported to the shoots through the Xylem. And in order to maintain normal metabolic reactions, plant cells need to keep high K+ levels of about 100 to 200 mM. K+ plays also an important role in maintaining turgor. Na+ levels, on the contrary, should be less than 1 mM in cytoplasm and any excess has to be either excluded out of the cell or sequestered in the vacuolar compartment (Sairam & Tyagi Citation2004). It has been reported that Na is an essential element for the plants using C4 or CAM photosynthetic pathways (Ohnishi et al. Citation1990). These C4/CAM plants use phosphoenolpyruvate (PEP) to fix atmospheric carbon for photosynthesis, and Na is also needed for the regeneration of PEP from pyruvate. Concerning Ca/Na ratio, our results showed lower levels in the treated leaves than those of the controls. Ca is a signalling molecule that plays a significant role in mediating mechanisms involved in the recognition and the response to abiotic stresses in plants. Under stress, plants either accumulate or release intracellular cytosolic Ca, which acts as both a signal and a regulator of a range of physiological processes to adjust to these stresses (Kader & Lindberg Citation2008, Citation2010). In addition, Ca has been reported to restrict the entry of Na+ into the plant cells (Kader & Lindberg Citation2008; Hussain et al. Citation2010).
Reactive oxygen species (ROS) production is a normal biochemical event that occurs in plants. In fact, higher plants, as other aerobic organisms, require oxygen for the efficient production of energy. And during the reduction of O2 to H2O, ROS can be formed (Fath et al. Citation2002; Cvetkovska et al. Citation2005). Environmental stresses limiting CO2 fixation, such as salt stress and drought, reduce the NADP+ regeneration by the Calvin cycle. Consequently, the photosynthetic electron transport chain is over-reduced, producing superoxide radicals and singlet oxygen in the chloroplasts (Bechtold et al. Citation2005; Li & Jin Citation2007). To prevent the over-reduction of the electron transport chain under conditions limiting CO2 fixation, higher plants evolved the photorespiratory pathway to regenerate NADP+ (Ledford & Niyogi Citation2005). As part of the photorespiratory pathway, H2O2 is produced in the peroxisomes, where it can also be formed during the catabolism of lipids as a by-product of ß-oxidation of fatty acids (Foyer & Noctor Citation2005). Under our experimental conditions, we deduced that leaves contained lower levels of H2O2when the plants are irrigated with drinking water (). However, the application of salt stress brought about a significant accumulation of H2O2 that was proportional to the salt accumulation in the leaves.
Free radicals are known to attack the highly unsaturated fatty acids of membrane systems to induce lipid peroxidation, which is an autocatalytic process that may cause peroxidative tissue damage. The level of lipid peroxidation, measured by the MDA content, has been considered as both an indicator of salt-induced oxidation in cell membranes and a tool for determining salt tolerance in plants (Zhang et al. Citation2012; Xing et al. Citation2013). Our results showed that the salt-stressed plants accumulated MDA to levels higher than those of the control plants () and this accumulation was proportional to the toxic ion accumulation and/or H2O2 production. Moreover, the maximum of MDA production was found in plants that accumulated more Na+ and H2O2. However, Jin et al. (Citation2007) demonstrated that when irrigation was done with 60% of seawater, MDA contents had no significant changes in Aloe leaves after 18 months compared to the plants irrigated with drinking water.
It is known that plant adaptability to different types of stresses is associated with an increase in antioxidant capacity such as the phenolic compounds (Falleh et al. Citation2008; Bettaieb et al. Citation2011). The antioxidant activity of the phenolic compounds is mainly due to their redox properties, which can play an important role, either in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or in decomposing peroxides. It has been shown in some recent studies that polyphenols synthesis depends on abiotic factors (Ksouri et al. Citation2008; Naffeti et al. Citation2011). Particularly, when plants were submitted to saline treatment, variations in antioxidant pools were found, notably in polyphenols. The present data demonstrated significant increases in polyphenols production in the leaves of the salt-treated plants compared to the control (). It has also been reported by Ayaz et al. (Citation2000) that salinity induced disturbances of the metabolic process leading to an increase in phenolic compounds. However, other reports showed that these compounds decreased or remained stable under salt stress (Falleh et al. Citation2008; Radi et al. Citation2013).
This study accommodated the collection of more detailed information about the various effects of salt in plants, particularly on xerophile known to grow in such stressed environments owing to their characteristic of ‘adaptive physiologies’. High salts in the water-irrigated Aloe vera affected the growth of plants in terms of leaves number, length, fresh and dry matter. However, water-gel contents were not significantly affected. The Aloe gel has a complex chemical composition, composed primarily of soluble sugars, anthraquinones, polysaccharides, amino acids, vitamins and proteins, many of which are enzymes. Additional studies concerning gel composition should be conducted to know if the quality of Aloe gel will be modified under salt stress and consequently the medicinal and economic values of our plant will be affected.
The reduction in the leaves growth may be due to several reasons such as the disorder in the inorganic cation contents, and/or the oxidative stress install – evaluated by the H2O2 production, and/or the loss of the membrane integrity – evaluated by the MDA accumulation. The phenolic compounds increases provide some cellular protection to the plant necessary for maintaining a normal physiological growth. Aloe, planted in soil affected by at least moderate salinity or irrigated with moderate salt water, can be attractive for industrial production in arid and semiarid areas.
Acknowledgement
This study was carried out under the PISEAUII project for saline water use in the center of Tunisia, the INRGREF/ACSAD project for saline water use in agriculture, and the Tunisia/Egypt project for salinity effects on phenolic compounds in Aloe vera and its antiviral activity.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ayaz FA, Kadioglu A, Turgut A. 2000. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can J Plant Sci. 80:373–378.[doi:10.4141/P99–005]
- Bechtold U, Karpinski S, Mullineaux PM. 2005. The influence of the light environment and photosynthesis on oxidative signaling responses in plant-biotrophic pathogen interactions. Plant Cell Environ. 28:1046–1055.
- Bergmann H, Leinhos V, Machelet B. 1994. Increase of stress resistance in crop plants by using phenolic compounds. Acta Hortic. 381:390–397.
- Bettaieb I, Hamrouni-Sellami I, Bourgou S, Limam F, Marzouk B. 2011. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol Plant. 33:1103–1111. [doi:10.1007/s11738-010-0638-z]
- Bresler E. 1982. Irrigation and soil salinity. In: Yaron D, editor. Salinity in irrigation and water resources. New York: Marcel Dekker; p. 65–102.
- Chen TH, Murata N. 2008. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 13:499–505.[doi:10.1016/j.tplants.2008.06.007]
- Chow JTN, Williamson TA, Yates KM, Goux WJ. 2005. Chemical characterization of the inmunomodulatoring polysaccharide of Aloe vera. Carbohydr Res. 340:1131–1147.[doi:10.1016/j.carres.2005.02.016]
- Chun-hui L, Chang-hai W, Zhi-liang X, Yi W. 2007. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 42:961–970.[doi:10.1016/j.procbio.2007.03.004]
- Cvetkovska M, Rampitsch C, Bykova N, Xing T. 2005. Genomic analysis of MAP kinase cascades in Arabidopsis defense responses. Plant Mol Biol Rep. 23:331–343.[doi:10.1007/BF02788882]
- Dai LY, Zhang LJ, Jiang SH, Yin KD. 2014. Saline and alkaline stress genotypic tolerance in sweet sorghum is linked to sodium distribution. Acta Agr Scand B-S P. 64:471–481.
- Demiral T, Türkan I. 2005. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot. 53:247–257.[doi:10.1016/j.envexpbot.2004.03.017]
- Falleh H, Ksouri R, Chaieb K, Karray-Bouraoui N, Trabelsi N, Boulaaba M, Abdelly C. 2008. Phenolic composition of Cynara cardunculus L., organs and their biological activities. C R Biol. 331:372–379.[doi:10.1016/j.crvi.2008.02.008]
- Fath A, Bethke P, Beligni V, Jones R. 2002. Active oxygen and cell death in cereal aleurone cells. J Exp Bot. 53:1273–1282.[doi:10.1093/jexbot/53.372.1273]
- Foyer CH, Noctor G. 2000. Oxygen processing in photosynthesis: regulation and signaling. New Phytologist. 146:359–388.[doi:10.1046/j.1469-8137.2000.00667.x]
- Foyer CH, Noctor G. 2005. Redox homeostis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 17:1866–1875.[doi:10.1105/tpc.105.033589]
- Gantait S, Sinniah UR, Das PK. 2014. Aloe vera: a review update on advancement of in vitro culture. Acta Agr Scand B-S P. 64:1–12.
- Gondim FA, Gomes-Filho E, Costa JH, Lídia N, Alencar M, Prisco JT. 2012. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol Biochem. 56:62–71.[doi:10.1016/j.plaphy.2012.04.012]
- Hachicha M. 2007. Saline soils and their reclamation in Tunisia. Sécheresse. Jan-Mar;18:45–50. French.
- Hachicha M, Hallaire V. 2002. Evolution structurale de la surface d’un sol tunisien sous différents modes d’irrigation [Structural evolution of a tunisian topsoil under various methods of irrigation: consequences on the water and salts transfer]. Etu Gest Sols. 9:239–249. French.
- Hamman JH. 2008. Composition and applications of Aloe vera leaf gel. Molecules. 13:1599–1616.[doi:10.3390/molecules13081599]
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198.[doi:10.1016/0003-9861(68)90654-1]
- Huang D, Ou B, Prior RL. 2005. The chemistry behind antioxidant capacity assays. J Agr Food Chem. 53:1841–1856.[doi:10.1021/jf030723c]
- Hussain K, Nisar MF, Majeed A, Nawaz K, Bhatti KH, Afghan S, Shahazad A, Zia-ul-Hassnian S. 2010. What molecular mechanism is adapted by plants during salt stress tolerance? Afr J Biotechnol. 9:416–422.
- Jin Z, Wang C, Liu Z, Gong W. 2007. Physiological and ecological characters studies on Aloe vera under soil salinity and sea water irrigation. Process Biochem. 42:710–714.[doi:10.1016/j.procbio.2006.11.002]
- Kader MA, Lindberg S. 2008. Cellular traits for sodium tolerance in rice (Oryza sativa L.). Plant Biotechnol. 25:247–255.[doi:10.5511/plantbiotechnology.25.247]
- Kader MA, Lindberg S. 2010. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav. 5:233–238.
- Keyster M, Klein A, Du Plessis M, Jacobs A, Kappo A, Kocsy G, Galiba G, Ludidi N. 2013. Capacity to control oxidative stress-induced caspase-like activity determines the level of tolerance to salt stress in two contrasting maize genotypes. Acta Physiol Plant. 35:31–40.[doi:10.1007/s11738-012-1045-4]
- Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C. 2008. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol. 331:865–873.[doi:10.1016/j.crvi.2008.07.024]
- Ledford HK, Niyogi KK. 2005. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 28:1037–1045.[doi:10.1111/j.1365-3040.2005.01374.x]
- Li J, Jin H. 2007. Regulation of brassinosteroid signaling. Trends Plant Sci. 12:37–41.
- Liu C, Chen J. 2014. Effects of salt stress on growth, ion concentration, and quality of pineapple fruits. Commun Soil Sci Plant Anal. 45:1949–1960.[doi:10.1080/00103624.2014.909837]
- Mehr ZS, Khajeh H, Bahabadi SE, Sabbagh SK. 2012. Changes on proline, phenolic compounds and activity of antioxidant enzymes in Anethum graveolens L. under salt stress. Intl J Agron Plant Prod. 3:710–715.
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410.[doi:10.1016/S1360-1385(02)02312-9]
- Mohamed AA, Aly AA. 2008. Alterations of some secondary metabolites and enzymes activity by using exogenous antioxidant compound in onion plants grown under seawater salt stress. Am-Euras J Sci Res. 3:139–146.
- Murillo-Amador B, Córdoba-Matson MV, Villegas-Espinoza JA, Hernández-Montiel LG, Troyo-Diéguez E, Garcia-Hernández JS. 2014. Mineral content and biochemical variables of Aloe vera L. under salt stress. PLoS ONE 9:e94870.
- Naffeti M, Sriti J, Hamdaoui G, Kchouk EM, Marzouk B. 2011. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 1:221–225.
- Nawaz A, Amjad M, Jahangir MM, Khan SM, Cui H, Hu J. 2012. Induction of salt tolerance in tomato (Lycopersicon esculentum Mill.) seeds through sand priming. Aust J Crop Sci. 6: 1199–1203.
- Nemoto Y, Sasakuma T. 2002. Differential stress responses of early salt-stress responding genes in common wheat. Phytochemistry. 61:129–133.
- Ohnishi JI, Flugge UI, Heldt HW, Kanai R. 1990. Involvement of Na+ in active uptake of pyruvate in mesophyll chloroplasts of some C4 plants. Plant Physiol. 94:950–959.
- Radi AA, Farghaly FA, Hamad AM. 2013. Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean cultivars to salinity. J Biol Earth Sci. 3:72–88.
- Rengasamy P. 2006. World salinization with emphasis on Australia. J Exp Bot. 57:1017–1023.
- Rispail N, Morris P, Webb KJ. 2005. Phenolic compounds: extraction and analysis. In: Marquez JA, editor. Lotus Japonicus handbook. Netherlands: Springer; p. 349–355.
- Sairam RK, Tyagi R. 2004. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 86:407–421.
- Sergiev I, Alexieva V, Karnov E. 1997. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci. 51:121–124.
- Swain T, Hillis WE. 1959. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituent. J Sci Food Agr. 10:418–425.
- Taylor NL, Day DA, Millar AH. 2004. Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J Exp Bot. 55:1–10.
- Waterman PG, Mole S. 1998. Analysis of phenolic plant metabolites. Oxford: Blackwell Scientific Publications; p. 85–87.
- Wu GK, Liang N, Feng RJ, Zhang JJ. 2013. Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol Plant. 35:2665–-2674.[doi:10.1007/s11738-013-1298-6]
- Xing J, Cai M, Chen S, Chen L, Lan H. 2013. Seed germination, plant growth and physiological responses of Salsola ikonnikovii to short-term NaCl stress. Plant Biosyst. 147:285–297.[doi:10.1080/11263504.2012.731017]
- Yildirim E, Taylor AG, Spittler TD. 2006. Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sci Hortic. 111:1–6.
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi S, Shinozaki K. 1997. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 38:1095–1102.[doi:10.1093/oxfordjournals.pcp.a029093]
- Zarcinas BA, Cartwright B, Spouncer LR. 1987. Nitric acid digestion and multielement analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal. 18:131–146.[doi:10.1080/00103628709367806]
- Zhang Z, Li H, Qiao S, Zhang X, Liu P, Liu X. 2012. Effect of salinity on seed germination seedling growth, and physiological characteristics of Perilla frutescens. Plant Biosyst. 146:245–251.[doi:10.1080/11263504.2011.627386]
- Zheng Q, Liu L, Liu Z, Chen J, Zhao H. 2009. Comparaison of the response of ion distribution in the tissues and cells of the succulent plants Aloe vera and Saliconia europea to saline stress. J Plant Nutr Soil Sci. 172:875–883.[doi:10.1002/jpln.200900122]
- Zhuo Y, Zhang Y, Xie G, Xiong S. 2015. Effects of salt stress on biomass and ash composition of switchgrass (Panicum virgatum). Acta Agr Scand B-S P. 65:300–309.
