Abstract
Mulching is considered a desirable management technology for improving and stabilizing agricultural crop production. The aim of the present study was to evaluate the effect of different mulching practices on hot pepper (Capsicum annuum L.) performance in terms of leaf photosynthetic capacity, photosynthetic light response (PLR) curves, and growth parameters, under greenhouse conditions. Consequently, field experiments were conducted during the 2011 and 2012 growing seasons in Northwest China using four types of mulching practices (without mulch; wheat straw mulch; plastic film mulch; and combined mulch with plastic film and wheat straw). The results showed that mulching increased the leaf net photosynthesis rate, stomatal conductance to water vapor, intercellular CO2 concentration, water-use efficiency, and transpiration rate. Mulching significantly affected the PLR curves, and important parameters (such as the apparent quantum yield, maximum net photosynthetic rate, light compensation point, and light saturation point) were all improved under combined mulching conditions when compared with other mulching practices. Therefore, it is inferred that hot pepper leaf photosynthetic capacity and light-use efficiency were significantly improved under combined mulching, and thereby the combined mulching is recommended for hot pepper cultivation in greenhouses due to working well to facilitate soil condition (moisture and temperature), plant growth, and marketable photosynthetic characteristics.
Introduction
The hot pepper (Capsicum annuum L.) is a vegetable crop commonly grown in greenhouses and consumed in China, the USA, East Indies, Korea, and many other countries for the nutritional value of its fruits (especially, antioxidant compounds and vitamin C) and for their natural colors (carotenoids) (Howard et al. Citation2000; Shao et al. Citation2008). With the adjustment of agricultural structure in China, the pepper production is anticipated to increase during recent years because of both its commercial value and the role this crop production plays in the rural economy (Liang et al. Citation2002). In greenhouses, irrigation is necessary to ensure stable yields of high-quality crops. However, the growing water scarcity in many countries puts pressure on irrigation systems, with the speeding up of urbanization process. Thus, the utilization of the water available in a greenhouse is a prerequisite to obtaining a higher pepper yield (Ertek et al. Citation2007). For a sustainable water use in greenhouses, agronomic measures (such as mulching) that do not negatively affect pepper productivity must be developed worldwide (Shtienberg et al. Citation2010; Qiu et al. Citation2013).
Mulching is an important soil management practice in many parts of the world, and this technique is suitable for sustainable cropping systems, as it provides several benefits to crop production through additional agro-ecosystem services, such as weed and disease control, improved soil quality, and increased nutrient cycling (Debashis et al. Citation2008; Campiglia et al. Citation2011a). Studies in China have indicated that plastic mulch is beneficial for wheat, as this practice increases yield, reduces water use, and improves water-use efficiency (WUE) (Zhang & Yang Citation2001). In addition, the application of mulching also increases the soil organic carbon (SOC) content, and there is a positive linear effect of the mulching application rate on the SOC concentration (Saroa & Lal Citation2003). Dong et al. (Citation2009) reported the crop-yield-enhancing effects of mulching in both saline and non-saline fields.
Photosynthesis is the main driving force influencing dry matter partitioning and organ formation, and it is the basis of plant production. The photosynthetic rate is affected through many factors, such as leaf position, growth stage, and light intensity, and a number of environmental factors and agricultural methods of growth (Borisev et al. Citation2012). Lawlor & Cornic (Citation2002) showed that the leaf photosynthetic capacity is significantly correlated with the relative water content. When plants encounter water deficit, photosynthesis is reduced. Choudhary et al. (Citation2013) observed that profuse vegetative growth in mulching treatments increased solar radiation capture, resulting in a higher rate of photosynthesis. Singh et al. (Citation2009) also reported a significant increase in photosynthetic parameters with cultivar variation in mulched trees. Photosynthetic light response (PLR) curves, which describe the relationship between the leaf net photosynthetic rate and the available photosynthetic photon flux density (PPFD), constitute an analytical tool that helps us to identify important features of photosynthetic capability (Xu et al. Citation2014). Lambers et al. (Citation1998) showed that PLR curves greatly vary between both species and individuals of the same species growing in different environments.
However, the effects of mulching on the physiological and biochemical characteristics of pepper have been primarily studied under open-field conditions, and information associated with the photosynthesis and PLR curves of hot peppers under different mulching conditions in greenhouse culture in China has been less thoroughly studied. Therefore, the objectives of the present study were (1) to study the vegetative growth and photosynthesis of pepper plants under greenhouse conditions, using different mulching practices, (2) to clarify the PLR curves of hot peppers cultivated under different mulching regimes, and (3) to determine the best mulching practices for increasing the leaf photosynthetic capacity and light-use efficiency of pepper plants.
Materials and methods
Plant material and growth conditions
The experiments were conducted in an experimental greenhouse at the Institute of Soil and Water Conservation, the Chinese Academy of Science and Ministry of Water Resources in Yangling (34°12′–34°20′N; 108°–108°7′E, elevation 560 m), Shannxi, China, from March to September 2011 and 2012. The study area has a mean annual temperature of 12.9 °C, with a maximum monthly temperature of 26.7 °C in July, a minimum temperature of −1 to −2 °C in January, and a mean annual rainfall of 637.6 mm. The soil texture was dark loessial soil (46.4% sand, 37.0% silt, and 16.6% clay, on average), with a pH of 7.9. The water-holding capacity of soil was 24% (mass basis) and the soil bulk density was 1.26 g cm−3; organic matter content was 14.66 g kg−1; total N content was 0.82 g kg−1; total P2O5 content was 0.99 g kg−1; available N (1 mol L−1NaOH hydrolysis) was 28.75 mg kg−1; available P (0.5 mol L−1 NaHCO3) was 30.46 mg kg−1; and available K (1 mol L−1 neutral NH4OAc) was 153.68 mg kg−1.
C. annuum (cultivar featured horn pepper, a common variety) seeds were collected in the autumn of 2010 from an experimental greenhouse at the Institute of Soil and Water Conservation, the Chinese Academy of Science and Ministry of Water Resources in Yangling (34°12′–34°20′N; 108°–108°7′E, elevation 560 m), Shannxi, China. After drying outside in direct sunlight for one week, the seeds were placed in a sealed container and stored in our laboratory.
The experiment was initiated on 5 March 2011 and 12 March 2012. Approximately 1.0 kg of air-dried soil was packed into each pot (diameter: 10 cm; depth: 15 cm). A vertical plastic pipe, placed adjacent to the inner wall of each pot, was used to supply water to the base of the pot. The seeds were sown in each pot on 10 March 2011 and 15 March 2012. C. annuum seedlings at the three-leaf stage were transplanted into the soil in the greenhouse on 20 May 2011 and 27 May 2012 at a density of 40,000 plants ha−1. Four rows of pepper plants (with six per row) at an inter-row spacing of 50 cm and inter-plant spacing of 50 cm were transplanted in each plot in two years.
Experimental design and treatments
The four treatments were replicated in three fully randomized blocks (6 m2 per plot): control (CK, conventional practice un-mulch), plastic film mulch (FM, 0.01 mm transparent polythene film mulch), combined mulch with plastic film and wheat straw (CM, plastic film covered in planting row and then wheat straw covered in operation row, and the 5 cm length of wheat straw with 2500 kg ha−1), and straw mulch (SM, 3 cm length of wheat straw with 5000 kg ha−1). The plastic membrane was set vertically between plots at approximately 100 cm underground to prevent water interpenetration. Fertilizers containing the same amount of N (75 kg ha−1), P (75 kg ha−1), and K (75 kg ha−1) were applied to each plot on 19 May and 20 July 2011, and 26 May and 27 July 2012. The treatments were established on 18 June 2011 and 26 June 2012. The peppers were harvested on 21 September 2011 and 28 September 2012. After harvesting, the plastic film was removed, and the wheat straw was incorporated into the soil.
Photosynthetic gas-exchange parameters
The gas-exchange parameters (net photosynthetic rate, Pn; stomatal conductance, Gs; transpiration rate, Tr; and intercellular CO2 concentration, Ci) and environmental factors (photosynthetically active radiation, PAR; air relative humidity, RHa; air temperature, Ta) were measured during three consecutive, completely sunny days (6–8 August 2011 and 21–23 August 2012), when the plants were in the fruiting phase. Five newly expanded, healthy leaves lying at similar middle positions in each plot were selected and repetitively measured using a portable infrared gas analyzer in the open system mode (LI-Cor 6400; Li-Cor Inc., Nebraska, USA). Diurnal variations were measured every 2 h from 8:00 am to 18:00 pm under natural sunlight. The leaf temperature was set at 27 °C, with a 500-μmol s−1 flow rate, 60% relative humidity, and 360-μmol mol−1 CO2 (Ref CO2). The leaves attached to the stem were inserted into the chamber (2 cm × 3 cm). The detecting head of the LI-6400 was held horizontally to receive enough sunlight within the chamber, which is transparent and rectangular. The WUE was calculated as WUE = Pn/Tr (Fischer & Turner Citation1978). The diurnal variation in the mean values of the parameters was calculated from the measured values obtained between 08:00 and 18:00 for three consecutive days.
The PLR curve measurement
The PLR curves were obtained on the fully developed leaf of the middle part of each plant on sunny days between 9:00 and11:00, using an LI-6400 portable photosynthesis system with a red-blue LED light source (6400-02B). The leaf was exposed to the highest PAR (2000 μmol m−2s−1) for 1200 s before Pn was determined; therefore the leaf was adapted for 600 s to a series of decreasing light intensities for the determination of Pn at each light level. PAR of 0, 40, 80, 120, 160, 200, 300, 400, 600, 800, 1000, 1200, 1600, and 2000 μmol m−2 s−1 was applied under the same conditions [360 μmol (CO2) mol−1 Ca, 27 °C Tleaf, and 55% RH] inside the chamber. The PLR curves were fitted using a modified rectangular hyperbolic model (Ye Citation2007; Ye & Yu Citation2008). The parameters were established using the nonlinear regression module of the SPSS statistical package (Version 17.0 for windows, SPSS, Chicago, IL, USA). The regression equation is expressed as(1) where LCP is the light compensation point, and α, β and γ are coefficients independent of PAR. The dark respiration rate (RD) was obtained using Equation (1), where PAR = 0. As the PAR increased, photosynthetic rate also increased. When reached a certain light intensity, leaf photosynthetic rate was equal to the respiratory rate; then, the light intensity was called LCP. When the light reached a certain intensity, photosynthetic rate no longer increased and presented the light saturation phenomenon. So, when the photosynthetic rate achieved the first maximum point of the curve, the light intensity was called light saturation point (LSP). The LSP and P′max were calculated using the following formulas:
(2)
(3)
Q is a measure of photosynthetic efficiency expressed in moles of photons absorbed per mole of CO2 fixed or O2 evolved. And it was calculated from each regression line fit to different numbers of points starting at low PPFD. Therefore, in our research, Q was estimated from the linear part of the PLR curves, occurring at a rate of 0–200 μmol (photon) m−2 s−1 (0, 40, 80, 120, 160, 200) PAR range (Singsaas et al. Citation2001).
Soil conditions
The soil temperature in each plot was automatically recorded every hour for the entire growing seasons at 20 cm depths in 2011 and 2012, using a portable LCD soil temperature meter (Mod. TPJ-21, Zhejiang Top Instrument Co., Ltd., China).
The soil water content (SWC; 0–40 cm) was measured through Time Domain Reflectometry (TDR); the TDR probes were embedded to a depth of 40 cm in the soil. And three TDR probes were used in each plot with three replicated measurements obtained for each probe. Soil moisture was maintained around 85% of field moisture capacity, and each crop was irrigated with the drip irrigation system. Each plot of the water-deficient ullage was determined on the basis of the 85% field moisture capacity and the measured soil water content value. So the amount of the water we irrigated is determined by the following formula: M = S × H × R × (W1−W2), where M is the amount of the water we irrigated; S, the area of the plot; H, the soil depth (here we use 40 cm as the TDR probes were embedded to a depth of 40 cm in the soil); R, the soil bulk density; W1, the 85% of the field moisture capacity; W2, the soil water content we measured using the TDR. So each plot received different amounts of water and when we irrigated them, we used the water-meter to control the amount of the water. SWC was measured for each experimental plot on the observation days (the days we measured photosynthetic gas-exchange parameters and the PLR curve) and also on the day before we irrigated. The TDR was calibrated by gravimetry before each measurement.
Yield
Fresh pepper fruits were recorded as per the quantity of produce harvested at seven-day intervals and total yield was determined after cumulating all the pickings and converting the numbers into yield per hectare in both 2011 and 2012.
Statistical analysis
The data were analyzed using SPSS 17.0 for Windows (USA). A one-way analysis of variance was performed to evaluate the influence of mulching on photosynthetic parameters. Subsequently, the least significant difference (LSD) was used to identify statistically significant differences (p < .05) between the mean values of the four different mulching treatments. Pearson linear correlations between fresh fruit yield and net photosynthetic rate (Pn) of hot peppers were also conducted using SPSS 17.0.
Results
Photosynthetic performance under mulching treatments
Diurnal variation of the environmental factor parameters
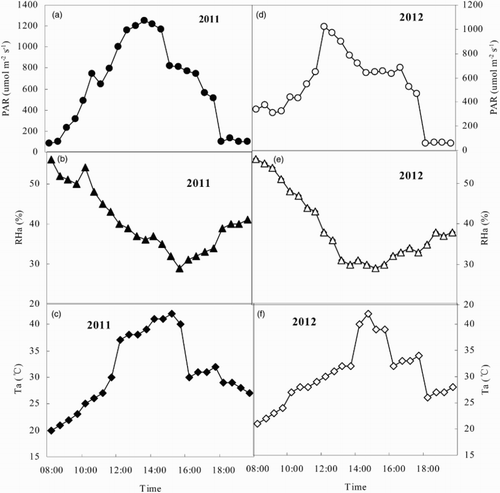
The diurnal changes in PAR, RHa, and Ta all presented one-peak pattern curves in both 2011 and 2012 (). The PAR variations ranged from 84 to 1250 μmol m−2 s−1 in 2011 and 54 to 1017 μmol m−2 s−1 in 2012. The highest PAR values were obtained at 12:00, but declined to the lowest values at 18:00 in 2011 and 2012, and the trends in Ta changes were similar to the diurnal changes in PAR. The Ta reached a minimum before sunrise and subsequently increased to the highest value at 14:00, consistent with enhanced PAR. The RHa reached the highest value (56% in 2011 and 57% in 2012) at 8:00, and subsequently decreased with continually increasing PAR and Ta, and the lowest value was obtained at 14:00 (29% in 2011 and 30% in 2012).
Effect of mulching on gas-exchange parameters
The diurnal patterns of leaf gas-exchange under different mulching practices were observed in 2011 and 2012 (, ). The daily highest Pn values were obtained at 14:00 in all the mulching treatments in 2011 and at 12:00 in FM, SM, and CM treatments and 10:00 in CK in 2012 ((a) and 2(b)). The daily average Pn was highest after CM treatment (9.70 μmol m−2 s−1 in 2011 and 11.02 μmol m−2 s−1 in 2012) during both years observed. On average, compared to the CK treatment, CM and SM increased Pn 13.19% and 3.03%, but decreased 3.97% in FM in 2011 and increased 42.75%, 9.07%, and 26.17% under CM, FM, and SM, respectively, in 2012. Gs was the highest at 10:00 in CK and at 12:00 in SM, FM, and CM treatments in 2011. However, Gs was highest at 12:00 in all the treatments in 2012. Similar to Pn, Gs increased in SM, FM, and CM treatments, and the daily average Gs was also highest in CM in both 2011 and 2012 ((c) and 2(d), ). Ci presented a double-peak curve on a sunny day ((e) and 2(f)). The highest Ci was obtained at 10:00 and 16:00, and the lowest Ci was recorded at 14:00 in all the treatments in 2011. In contrast, the highest Ci was recorded at 10:00 and 12:00 in CK and at 10:00 and 16:00 in other mulching treatments, and the lowest Ci was obtained at 08:00 in FM and SM, but at 14:00 in CM treatment in 2012. The Ci value increased in FM and CM, but declined under SM conditions. Tr increased, peaking at 14:00 in all of treatments in 2011 and at 12:00 in SM and FM in 2012 ((g) and 2(h)). Mulch treatments (SM, FM, and CM) significantly increased Tr and WUE ((i) and 2(j)) values compared with CK (). Mulch greatly increased Pn, GS, Ci, Tr, and WUE, particularly under CM and SM treatments ().
Figure 2. Diurnal changes of leaf gas-exchange parameters of hot pepper under different mulching practices in a greenhouse in 2011 and 2012 (Pn: photosynthetic rate; Gs: stomatal conductance; Ci: intercellular CO2 concentration; Tr: transpiration rate; WUE: water use efficiency; CK: un-mulched control; FM:plastic film mulch; CM: combined mulch with plastic film and wheat straw; SM: wheat straw mulch).
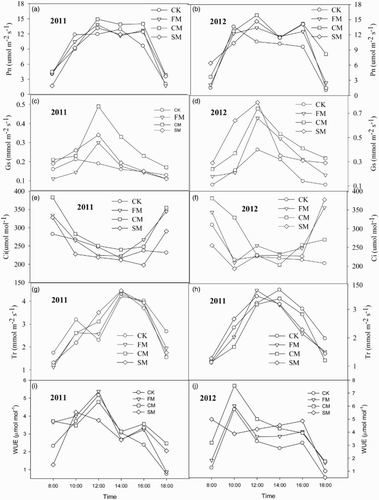
Table 1. Leaf photosynthesis capacity of hot pepper in response to mulching in a greenhouse in 2011 and 2012.
The relationship of fresh fruit yield with Pn in response to mulching
In two years, mulching significantly increased the fresh fruit yield (). Compared with CK control, the yield was increased 55.77%, 160.58%, and 135.58% in 2011 and 37.6%, 106%, and 44.8% in 2012 under FM, CM, and SM conditions, respectively. The highest hot pepper fresh fruit yield was obtained under CM treatment, which also produced the highest daily average Pn. There was no significant difference between CM and SM treatments in 2011, while no difference between SM and FM was observed in 2012. The correlation analysis () showed that the fresh fruit yield was significantly and positively correlated with Pn during 2011 and 2012 in response to all mulching treatments for all fruit growth stages.
Table 2. Influences of mulching on the fresh fruit yield of hot peppers in a greenhouse in 2011 and 2012.
Table 3. Correlation coefficients of the fresh fruit yield and net photosynthetic rate (Pn) of hot peppers in a greenhouse in 2011 and 2012 under different mulching practices.
PLR performance under mulching practices
PLR in response to mulching
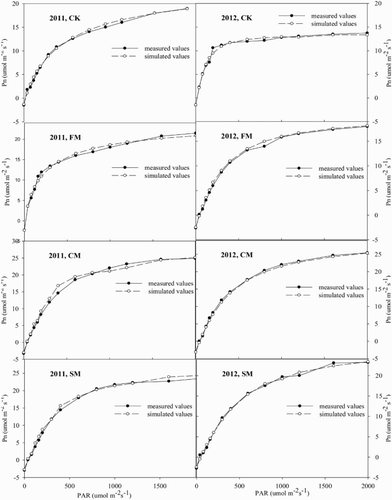
The photosynthetic responses to light under the four mulching treatments in 2011 and 2012 are shown in . Under a PAR lower than 500 μmol m−2 s−1, the PLR curve in FM was higher than that in the other treatments in 2011, but there were no variations between CM and SM. However, the PLR curve was highest in CM application when the PAR was higher than 500 μmol m−2 s−1. In contrast, the PLR curve in CK was higher than that in mulching practices when the PAR was lower than 500 μmol m−2 s−1 in 2012. Compared with CK control, the PLR curve was higher under mulching practices with increasing PAR, which was highest for CM, intermediate for SM, and lowest for FM ().
Effect of mulching on PLR curve parameters
Mulching practices significantly influenced PLR curve parameters during the two years studied; however, the exact influence varied among years (, ). The coefficients of determination (R2) between simulated and measured values were 0.996, 0.986, 0.944, and 0.992 in CK, FM, CM, and SM, respectively, in 2011. Similarly, the R2 was 0.989, 0.999, 0.932, and 0.997 in CK, FM, CM, and SM, respectively, in 2012 (). As shown in , the PN–PAR response curves were well fitted by the modified rectangular hyperbolic model as indicated by R2 values, which were greater than 0.932 in the present study; thus it was used to simulate the other parameters of the PLR curve in both 2011 and 2012.
Figure 4. Soil temperature (0–20 cm) and soil water content (0–40 cm) of hot peppers grown in the greenhouse under different mulching practices during the entire fruit growth stage in 2011 and 2012.
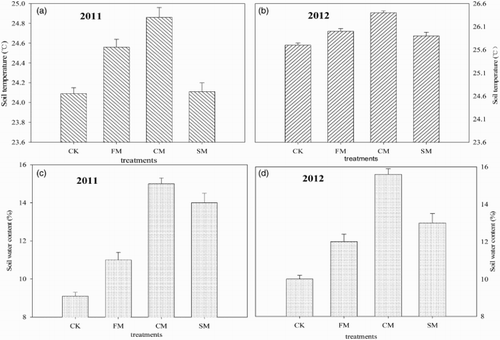
Table 4. Effects of mulching on PLR curve parameters of hot pepper in a greenhouse in 2011 and 2012.
As shown in , compared with CK, the P′max values for the three mulching-based groups were significantly increased and also showed differences (p < .05), which were highest for CM, intermediate for SM, and lowest for FM in both 2011 and 2012. Mulching increased the Q value, but there were no significant difference between the practices in the two years observed. In contrast, mulching treatments sharply decreased Rd, and the reduction was higher in CM and FM in 2011 and in CM and SM in 2012. Mulching practices significantly increased the LCP in 2011, and a rate of 11.22%, 56.24%, and 44.69% was obtained in FM, CM, and SM, respectively. Compared with CK applications, the LCP was increased 45.17%, 62.73%, and 30.42% under FM, CM, and SM treatments, respectively, in 2012. The LCP showed a similar trend under all mulching treatments in 2012. SM treatment was higher than CK treatment in LSP, but CM and FM had little effect during 2011 and 2012.
Soil conditions under mulching practices
Soil moisture in response to mulching
Soil moisture under the different mulching practice was influenced strongly by the composition of the mulch material employed in trials (). The highest value was obtained under CM in both 2011 and 2012. In all cases, the soil moisture determined in CK condition was always lower than that obtained across all mulch materials, and followed a pattern similar to them in both 2011 and 2012. In comparison to CK, the average mean soil moisture was increased by 22.22%, 66.67%, and 55.56% under FM, CM, and SM conditions, respectively, in 2011; 20.00%, 56.00%, and 30.00% in 2012 for the entire growth season.
Soil temperature in response to mulching
Soil temperature was affected significantly by mulching in two years (). In all cases, it was higher under FM, CM, and SM in comparison to CK. And the highest value was obtained under CM, while the lowest value was attained under CK condition. On average, soil temperature was high 0.47°C, 0.77°C, and 0.02°C in FM, CM, and SM in 2011, respectively. In 2012, FM, CM, and SM increased 0.30°C, 0.70°C, and 0.20°C compared to CK practice, respectively.
Discussion
As photosynthesis is the basic process that determines the primary productivity in terrestrial ecosystems, which is also the primary physiological process and the foundation of crop-yield formation (Zou et al. Citation2006). Covshoff & Hibberd (Citation2012) reported that increasing photosynthetic capacity raises yield potential, while Long et al. (Citation2006) reported negative relationships between leaf photosynthetic rate and crop yield. And the most important reason for these contradicting results may be that measuring photosynthesis on one small part of one leaf is not at all indicative of the photosynthesis of the entire plants. The current study obtained a positive correlation () between leaf photosynthetic rate and fruit yield, which indicated that increasing the rates of net photosynthesis capacity by agricultural practices such as mulching may bring about increases in crop yields of hot pepper crops during the fruit-bearing stages ().
In the current study, the photosynthetic capacity of plants was estimated using leaf gas-exchange measurements based on CO2 assimilation and PLR measurement based on the PAR. The results obtained from this two-year (2011 and 2012) experiment demonstrated that the daily average Pn of hot peppers cultivated in a solar greenhouse was increased with mulching compared with non-mulching, with effectiveness in the order of CM>SM>FM>CK (), and the differences among the treatments were significant. Cai et al. (Citation2007) reported that mulching enhanced plant gas exchange through increasing soil moisture content, while Choudhary et al. (Citation2013) argued that mulching increased photosynthetic capacity through increased solar radiation capture. In the present study, the soil moisture and temperature were improved under mulch growth conditions () compared with control. And the correlation coefficients in showed that soil water content was significantly positive correlated with these physiological parameters except that with Ci and WUE in 2011. Therefore, we can conclude that soil moisture was better in mulch growth conditions () for pepper, resulting in increased stomata conductance, and transpiration increased through the stomatas opening more widely, while maintaining the internal CO2 concentration in the leaf, which contributed to the increment in net photosynthesis rate (Pn) (). Ibarra-Jimenez et al. (Citation2008) also reported that plastic mulch is useful for farming crops because this technique increases soil temperature and plant photosynthesis, consistent with the results obtained in the present study. However, Zhu et al. (Citation2012) stated that photosynthesis of hot peppers is influenced through many factors, such as light intensity, soil moisture, temperature, and nutrients. Zou et al. (Citation2006) indicated that the net photosynthesis rate is most correlated with leave characteristics (leave length, leave width, petiole length, and so on) of hot pepper. Ali et al. (Citation2008) showed that the decline in the growth of plants subjected to stressful environments is often associated with a reduction in the photosynthetic capacity. In the present study, the environmental factors influenced the Pn under the four treatments, as the Pn was measured in the same atmospheric environment (). On average, CM treatment was more favorable than FM and SM material in leaf gas-exchange (), as CM mulch practice was more favorable either in preserving soil water or in improving soil temperature, consistent with Li et al. (Citation2004) and Liang et al. (Citation2012). Consistently, Shao et al. (Citation2011) demonstrated that in greenhouses, water availability is an important factor affecting plant growth and yield, and the hot pepper is considered a horticultural crop most susceptible to water stress. Thus, the most effective soil water conservation in CM may result in the higher crop physiology on growth.
Table 5. Correlation coefficients of soil water content to physiological parameters of hot pepper in 2011 and 2012.
The PLR curve is important in predicting carbon fixation in nature because the variation in the light environment of a leaf is one of the most important factors affecting photosynthetic rate. In the present study, the results of the modified rectangular hyperbolic model were encouragingly consistent with observations for the PLR (), indicating that the obtained PLR parameters were an adequate fit. Mulching increased the P′max values and the Q in the two years observed. Q is an estimate of the maximum efficiency of light harvesting during the assimilation of CO2 (Bernacchi et al. Citation2003). This result () suggests that mulching improved the water status of the leaves, allowing chloroplasts in the leaves to more efficiently use the absorbed light energy and thereby enhancing the capacity of CO2 assimilation in the Calvin cycle (Yu et al. Citation2004). Therefore, under the higher water status in the mulching treatments, the efficiency of light utilization of pepper was significantly higher than that of no-mulched plants. We also showed that there was a significant increase in LCP and LSP in mulching-treated plants compared with non-mulching conditions, suggesting that mulching-induced light utilization is associated with the efficiency which this intercepted radiation is utilized for net dry matter production (Lachapelle & Shipley Citation2012). Differences in the effects of mulching on these parameters were evident during the two years observed; the combination of plastic film and straw showed higher improvements in the soil environment than plastic film and straw mulches alone (). In addition, the leaf traits (leave length, leave width, petiole length, and so on) varied under different mulching conditions, and the soil environment in which the plant grows and the shape of the PLR curve also varied under these growth conditions. It is therefore important to extend the results from PLR curves based on mulching-practices-changed water status of the leaves, which increased the efficiency of light utilization under different light environments.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Ali B, Athar H, Ashraf M. 2008. Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul. 56:107–116. doi: 10.1007/s10725-008-9290-7
- Bernacchi CJ, Calfapietra C, Davey PA. 2003. Photosynthesis and stomatal conductance responses of poplars to free air CO2 enrichment (PopFACE) during the first growth cycle and immediately following coppice. New Phytol. 159:609–621. doi: 10.1046/j.1469-8137.2003.00850.x
- Borisev M, Krstic B, Gvozdenovic D, Gvozdanovic-VARGA J. 2012. Photosynthesis and water use efficiency relations to yield of the pepper varieties (Capsicum annnuum L.). Bulg J Agric Sci. 18:589–594.
- Cai CT, Cai ZQ, Yao TQ. 2007. Vegetative growth and photosynthesis in coffee plants under different watering and fertilization managements in Yunnan, SW China. Photosynthetic. 45:455–461. doi: 10.1007/s11099-007-0075-4
- Campiglia E, Mancinelli R, Radicetti E. 2011a. Influence of no-tillage and organic mulching on tomato (Solanum lycopersicum L.) production and nitrogen use in the Mediterranean environment of central Italy. Sci Hortic-Amsterdam. 130:588–598. doi: 10.1016/j.scienta.2011.08.012
- Choudhary VK, Bhambri MC, Pandey N, Sharma HG. 2013. Effect of drip irrigation and mulches on physiological parameters, soil temperature, picking patterns and yield in Capsicum (Capsicum annuum L.). Arch Agron Soil Sci. 58:277–292. doi: 10.1080/03650340.2010.517197
- Covshoff S, Hibberd JM. 2012. Integrating C4 photosynthesis into C3 crops to increase yield potential. Curr Opin Biotech. 23:209–214. doi: 10.1016/j.copbio.2011.12.011
- Debashis C, Shantha N, Pramila A, Gupta VK, Tomar RK. 2008. Effect of mulching on soil and plant water status, and the growth and yield of wheat (Triticum aestivum L.) in a semi-arid environment. Agr Water Manage. 95:1323–1334. doi: 10.1016/j.agwat.2008.06.001
- Dong HZ, Li WJ, Tang W, Zhang DM. 2009. Early plastic mulching increases stand establishment and lint yield of cotton in saline fields. Field Crop Res. 111:269–275. doi: 10.1016/j.fcr.2009.01.001
- Ertek A, Sensoy S, Gedik I, Kuciikyumuk C. 2007. Irrigation scheduling for green capsicum (Capsicum annuum L.) grown by field condition by using class A pan evaporation. Am Eurasian J Agr Environ Sci. 2:349–358.
- Fischer RA, Turner NC. 1978. Plant productivity in the arid and semiarid zones. Annu Rev Plant Physiol. 29:277–317. doi: 10.1146/annurev.pp.29.060178.001425
- Howard LR, Talcott ST, Brenes CH. 2000. Changes in photochemical and antioxidant activity of selected pepper cultivars (Capsicum species. L) as influenced by maturity. J Agr Food Chem. 48:1713–1720. doi: 10.1021/jf990916t
- Ibarra-Jimenez L, Zermeno-Gonzalez A, Munguia-Lopez J, Quezada-Martin MAR, De L, Rosa-Ibarra M. 2008. Photosynthesis, soil temperature and yield of cucumber as affected by colored plastic mulch. Acta Agr Scand B-S P. 58:372–378.
- Lachapelle PP, Shipley B. 2012. Interspecific prediction of photosynthetic light response curves using specific leaf mass and leaf nitrogen content: effects of differences in soil fertility and growth irradiance. Ann Bot. 109:1149–1157. doi: 10.1093/aob/mcs032
- Lambers H, Chapin FS, Pons TL. 1998. Plant physiological ecology. New York: Springer.
- Lawlor DW, Cornic G. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x
- Li FM, Wang P, Wang J, Xu JZ. 2004. Effects of irrigation before sowing and plastic film mulching on yield and water uptake of spring wheat in semiarid Loess Plateau of China. Agr Water Manage. 67:77–88. doi: 10.1016/j.agwat.2004.02.001
- Liang YL, Chen ZJ, Wang ZM. 2002. The status and function of facility agriculture on ecological environment constructing. J Soil Water Conserv. 16:32–35. ( In Chinese with English abstract).
- Liang YL, Wu X, Zhu JJ, Zhou MJ, Peng Q. 2012. Response of hot pepper (Capsicum annuum L.) to mulching practices under planted greenhouse condition. Agr Water Manage. 99:111–120. doi: 10.1016/j.agwat.2011.07.010
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x
- Qiu RJ, Song JJ, Du TS, Kang SZ, Tong L, Chen RQ, Wu LS. 2013. Response of evapotranspiration and yield to planting density of solar greenhouse grown tomato in northwest China. Agr Water Manag. 130:44–51. doi: 10.1016/j.agwat.2013.08.013
- Saroa GS, Lal R. 2003. Soil restorative effects of mulching on aggregation and carbon sequestration in a Miamian soil in Central Ohio. Land Degrad Dev. 14:481–493. doi: 10.1002/ldr.569
- Shao GC, Guo RQ, Liu N, Yu SE, Xing WG. 2011. Photosynthetic, chlorophyll fluorescence and growth changes in hot pepper under deficit irrigation and partial root zone drying. Afr J Agr Res. 6:4671–4679.
- Shao GC, Zhang ZY, Liu N. 2008. Comparative effects of deficit irrigation (DI) and partial rootzone drying (PRD) on soil water distribution, water use, growth and yield in greenhouse grown hot pepper. Sci Hortic. 119:11–16. doi: 10.1016/j.scienta.2008.07.001
- Shtienberg D, Elad Y, Bornstein M, Ziv G, Grava A, Cohen S. 2010. Polyethylene mulch modifies greenhouse microclimate and reduces infection of Phytophthora infestans in tomato and Pseudoperonospora cubensis in cucumber. Phytopathol. 100:97–104. doi: 10.1094/PHYTO-100-1-0097
- Singh VK, Singh G, Bhriguvanshi SR. 2009. Effect of polyethylene mulch on soil nutrient level root, leaf and fruiting characteristics of mango (mangifera indica). Indian J Agr Sci. 79:411–417.
- Singsaas EL, Qrt DR, DeLucia EH. 2001. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia. 128:15–23. doi: 10.1007/s004420000624
- Xu JZ, Yu YM, Peng SZ, Yang SH, Liao LX. 2014. A modified nonrectangular hyperbola equation for photosynthetic light-response curves of leaves with different nitrogen status. Photosynthetica. 52:117–123. doi: 10.1007/s11099-014-0011-3
- Ye ZP. 2007. A new model for relationship between light intensity and the rate of photosynthesis in Oryza sativa. Photosynthetica. 45:637–640. doi: 10.1007/s11099-007-0110-5
- Ye ZP, Yu Q. 2008. A coupled model of stomatal conductance and photosynthesis for winter wheat. Photosynthetica. 46:637–640. doi: 10.1007/s11099-008-0110-0
- Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S. 2004. A role of brassinosteroids in the regulation of photosynthesis in Cucummis sativus. J Exp Bot. 55:1135–1143. doi: 10.1093/jxb/erh124
- Zhang BJ, Yang WP. 2001. Studies on the dynamic change of soil water of dibbling wheat in film-mulched field. J North West Sci Technol. 29:70–73.
- Zhu JJ, Peng Q, Liang YL, Wu X, Hao WL. 2012. Leaf gas exchange, Chlorophyll fluorescence and fruit yield in hot pepper (Capsicum anmuum L.) Grown under different shade and soil moisture during the fruit growth stage. J integr agr. 11:927–937. doi: 10.1016/S2095-3119(12)60083-5
- Zou XX, Ma YQ, Liu RY, Zhang ZQ, Chen WC. 2006. Study of relation between net photosynthesis rate and agronomic character in pepper (Capsicum annuum L.). Southwest China J Agr Sci. 19:270–275.