ABSTRACT
In grain legumes, the N requirements of growing seeds are generally greater than biological nitrogen fixation (BNF) and soil N uptake during seed filling, so that the N previously accumulated in the vegetative tissues needs to be redistributed in order to provide N to the seeds. Chickpea, field bean, pea, and white lupin were harvested at flowering and maturity to compare the relative contribution of BNF, soil N uptake, and N remobilisation to seed N. From flowering to maturity, shoot dry weight increased in all crops by approximately 50%, root did not appreciably change, and nodule decreased by 18%. The amount of plant N increased in all crops, however in field bean (17 g m−2) it was about twice that in chickpea, pea, and lupin. The increase was entirely due to seeds, whose N content at maturity was 26 g m−2 in field bean and 16 g m−2 in chickpea, pea, and lupin. The seed N content at maturity was higher than total N accumulation during grain filling in all crops, and endogenous N previously accumulated in vegetative parts was remobilised to fulfil the N demand of filling seeds. Nitrogen remobilisation ranged from 7 g m−2 in chickpea to 9 g m−2 in field bean, and was crucial in providing N to the seeds of chickpea, pea, and lupin (half of seed N content) but it was less important in field bean (one-third). All the vegetative organs of the plants underwent N remobilisation: shoots contributed to the N supply of seeds from 58% to 85%, roots from 11% to 37%, and nodules less than 8%. Improving grain legume yield requires either reduced N remobilisation or enhanced N supply, thus, a useful strategy is to select cultivars with high post-anthesis N2 fixation or add mineral N at flowering.
Introduction
Grain legumes are important crops which are grown worldwide primarily for their high seed protein content and are used as human food, feed for animals, and industrial demands (Gulmezoglu & Kayan Citation2011). In addition to being important food crops, grain legumes also release nitrogen into the soil because of their N2-fixing capacity and enhance yields of subsequent crops, playing an important role in cereal cropping systems. The accumulation of nitrogen in legume plants depends on the N fixed by biological nitrogen fixation (BNF) and on the N assimilated from the soil. However in grain legumes, the N requirements of growing seeds are generally greater than BNF and soil N uptake during seed filling, so that the N previously accumulated in the vegetative tissues needs to be redistributed in order to provide N to the seeds (Salon et al. Citation2001; Schiltz et al. Citation2005). Remobilisation of nutrients such as N and P from vegetative tissues to reproductive organs plays an important role for legume grain yield. The extent of the contribution of N remobilisation to seed N yield varies markedly among legumes, ranging from 0% to 90% because of genotype and environmental factors (Kurdali Citation1996; Davies et al. Citation2000; Schiltz et al. Citation2005; Soltani et al. Citation2006). Kumarasinghe et al. (Citation1992) hypothesised that in bean plants, N translocation and BNF during grain filling are complementary. They suggested that when N2 fixation was high enough to satisfy virtually all the N demand of the rapidly filling seeds, the remobilisation of N from the vegetative tissues was not necessary. Thus the differences in N remobilisation among grain legume crops can also be attributed to differences in their N2 fixation efficiency after flowering.
According to Salon et al. (Citation2001), all vegetative organs undergo N remobilisation, however the efficiency with which it can be transferred to growing seeds and the rate of N remobilisation are higher in leaves and stems than in roots, although little research has been carried out on the N remobilisation from roots (Van Kessel Citation1994).
The aim of this study was to assess the complementarity between N remobilisation and BNF during seed filling in four grain legumes by comparing the relative contribution to seed N by BNF, soil N uptake, and N remobilisation from vegetative aerial part and roots. The crops used were chickpea (Cicer arietinum L.), field bean (Vicia faba L. var. minor), pea (Pisum sativum L.), and white lupin (Lupinus albus L.), which are some of the most commonly cultivated grain legumes.
Materials and methods
The research was carried out in 2012 and in 2013, at the Research Centre of the Department of Agriculture, Food and Environment of the University of Pisa, Italy, which is located approximately 4 km from the sea (43° 40′ N, 10° 19′ E) and 1 m above sea level. The climate of the area is hot-summer Mediterranean (Csa) with a mean air temperature of 14.9°C and a mean rainfall of 971 mm.
In both years, experimental treatments consisted of four legume crops and two harvesting stages. The legumes were chickpea (cv. ‘Pascia'), field bean (cv. ‘Chiaro di Torrelama'), pea (cv. ‘Iceberg'), and white lupin (cv ‘Multitalia'). The harvesting stages were full flowering and physiological maturity. Flowering was defined when plants had more than one node with flowers open and one node with one pod set (Knott Citation1987, Citation1990). Durum wheat (Triticum durum L. ‘Claudio') was used as a non-fixing reference crop (RC) to determine plant-available soil N and to estimate BNF. Harvest times of durum wheat were the same as legume crops.
In both years the research was carried out in an open-air facility consisting of 48 growth boxes (24 for legume crops and 24 for durum wheat) of 300-L volume (0.50 m2 and 0.6 m depth), spaced 20 cm apart, and embedded in expanded clay to prevent daily fluctuations in soil temperature. In both years, approximately six months before seeding, growth boxes were filled with soil collected from a field previously cultivated with rapeseed.
Differences in soil properties for the two years were negligible, and the averaged soil properties were: 74.4% sand (2 > ∅ > 0.05 mm), 20.2% silt (0.05 > ∅ > 0.002 mm), 5.4% clay (∅ < 0.002 mm), 8.0 pH, 1.3% organic matter (Walkley and Black method), 0.5 g kg−1 total nitrogen (Kjeldahl method), 8.8 mg kg−1 available P (Olsen method), 72.4 mg kg−1 available K (BaCl2–TEA method), and 0.4 mg kg−1 NO3–N.
All the legume crops and durum wheat were sown on 14 February 2012 and 4 February 2013. Just prior to sowing, field bean and pea seeds were inoculated with Rhizobium leguminosarum biovar. viciae, chickpea seeds with Bradyrhizobium sp. (cicer) and white lupin seeds with Bradyrhizobium lupinus. The seeding rate was 32 seeds m−2 for chickpea, 56 seeds m−2 for field bean and pea, and 40 seeds m−2 for white lupin. For all legume crops a 30-cm row spacing was used. The seeding rate of durum wheat was 400 seeds m−2 with a 16-cm row spacing. All legume crops and durum wheat were fertilised pre-planting with urea, triple mineral phosphate and potassium sulphate, at rates of 30 kg ha−1 of N, 65 kg ha−1 of P, and 125 kg ha−1 of K. In both years all the crops were irrigated from flowering to maturity to prevent water stress. A total of 100 mm of water was applied in May and June. The crops were kept free of weeds by hand hoeing when necessary.
Flowering occurred between 7 and 14 May 2012 and 9 and 17 May 2013, and physiological maturity occurred between 22 June and 9 July 2012 and between 1 and 22 July 2013. At both stages, plants from each box were cut at ground level and were partitioned into leaf + stem, pod wall, and seed. All dead leaves were collected. Roots from each box were separated from the soil by gently washing with a low flow from sprinklers. They were then immersed in a 10% sodium hexametaphosphate and sodium bicarbonate dispersant solution for 18 h. Nodules were separated from roots; total root fresh weight was recorded and taproots and rootlets were separated and weighed. The dry weight of all plant parts was determined by oven-drying at 60°C to constant weight, and samples were analysed for N concentration by the micro Kjeldahl method. Nitrogen content was obtained by multiplying N concentration by dry weight.
The two most commonly used methods for estimating N2 fixation during the growth period are 15N-isotope dilution and N difference. With the N difference method, the total N content of the non-fixing crop is subtracted from the total N content of the N2-fixing legume. The two methods deliver the same results when comparing BNF in soil with a low N concentration (Herridge et al. Citation2008). Thus, the N2 fixation was estimated using the N difference method as improved by Evans and Taylor (Citation1987): [N yield (legume)−N yield (RC)] + [N soil (legume at harvest)−N soil (RC at harvest)].
We assumed that all N lost from vegetative parts between flowering and maturity was translocated to the seeds, thus N apparent remobilisation was calculated as the difference between total plant N content at flowering and total N content of vegetative plant parts (leaf + stem + pod wall + root + nodule) at maturity (Koutroubas et al. Citation2009).
In both years, the experiment was arranged in a split plot design with four main plot treatments (four legume crops) and two subplot treatments (two stages) with three replicates. Significantly different means were separated at the 0.05 probability level by the least significant difference test (Steel et al. Citation1997).
Results and discussion
There were no significant differences between years in all growth variables or interaction ‘Year × Crop × Stage', ‘Year × Crop', and ‘Year × Stage'. This is because differences in temperature between the two years were negligible and crops were irrigated when necessary. Accordingly, only interaction ‘Crop × Stage' is reported.
The four crops studied had the same growth cycle but differed in terms of grain and biomass yields, root weight, nodule mass, nitrogen uptake, and fixed nitrogen. Production and amounts of N2 fixation were consistent with those found in field conditions by other authors in similar Mediterranean environments (Thomson et al. Citation1997; Lopez-Bellido et al. Citation2011).
The four crops have an indeterminate growth habit, which results in the continuation of vegetative growth after flowering and podding have commenced. From flowering to maturity, shoot dry matter (stems + leaves + pod walls) statistically increased in all crops by approximately 50%, root dry matter did not change appreciably, and nodule dry matter decreased by approximately 18% (). Field bean was the most productive in seeds (577 g m−2) followed by pea (487 g m−2), chickpea (397 g m−2), and white lupin (379 g m−2).
Figure 1. Dry matter of shoots (a), roots (b), and nodules (c) of chickpea, field bean, pea, and white lupin at flowering and maturity stages. Values are means of 2012 and 2013. Vertical bars denote LSD at P ≤ 0.05.
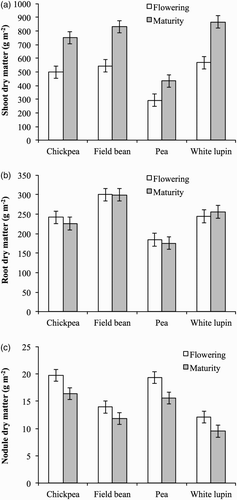
The percentage of photosynthetic carbon allocated to nodulated roots is considered to be an indicator of the sink strength of nodulated roots compared with that of shoots (Jeuffroy & Warembourg Citation1991). Our results suggest that after flowering, nodulated roots lose their sink strength; and both roots and nodules are limited by the carbon supply, because of the large demand for assimilates for seed filling (Voisin et al. Citation2003). However, the growth of roots may have been completed at flowering, and during seed filling only maintenance of C was necessary. In legume plants, nitrogen nutrition during the seed filling relies on both symbiotic fixation and soil mineral N absorption. Even though symbiotic fixation decreases during the seed filling, it still seems to be effective and to stop only at the physiological maturity stage (Sparrow et al. Citation1995; Voisin et al. Citation2002). In addition, when mineral N is available in soil during seed filling, soil mineral N absorption can continue and contribute to plant nitrogen accumulation (Voisin et al. Citation2002). As a consequence, a considerable percentage (25–40%) of the total amount of N in plants can be accumulated during the seed filling period (Crozat et al. Citation1994). In our research, during seed filling, the amount of plant N increased in all four crops, thus confirming that they are able to maintain N acquisition during reproductive development. However, the extent of the increase differed markedly among crops and in field bean (17 g m−2) was about twice the amount in chickpea, pea, and white lupin (approximately 8 g m−2). In all crops the increase was entirely due to seeds, whose N content at maturity was 26 g m−2 in field bean and approximately 16 g m−2 in chickpea, pea, and white lupin. At maturity, seeds contained between 55% and 70% of total plant N, although they made up only from 25% to 44% of the total dry matter.
Nitrogen concentration and content of vegetative plant parts drastically decreased during seed filling in all legume crops. The decrease in N concentration was higher in nodules (from 5.6 to 3.1 g kg−1) and shoots (from 3.0 to 1.2 g kg−1) than in roots (from 1.8 to 1.0 g kg−1) without statistical differences among crops. In all species the decrease in N content was higher in shoots than in roots and in nodules (). In white lupin, the decreases in both shoot and root N content were statistically higher than those in the other three crops. The decrease in nodule N content did not differ among species.
Figure 2. Nitrogen content of shoots (a), roots (b), and nodules (c) of chickpea, field bean, pea, and white lupin at flowering and maturity stages. Values are means of 2012 and 2013. Vertical bars denote LSD at P ≤ 0.05.
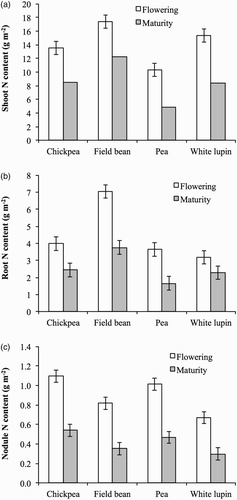
From flowering to maturity, BNF represented between 83% and 90% of the exogenous N in all legume crops. However, the amount of N fixed during seed filling differed markedly among legumes and in field bean was more than twice the amount in pea and white lupin, and three times the amount in chickpea (). Competition for carbon by developing pods and seeds is likely to result in decreased C supply to the Rhizobia bacteria, resulting in the decrease in N fixation which occurs during early flowering (Hooda et al. Citation1986; Kurdali Citation1996). Herridge and Pate (Citation1977) reported that at the time of seed filling, the ability of the nodules of grain legumes to fix N decreases, because the plant feeds the developing seed rather than the nodule, and BNF decreases. We found that during seed filling, BNF was very important for N acquisition by the plants, representing 26% of total BNF in chickpea, 35% in pea and white lupin, and 42% in field bean.
Figure 3. BNF, nitrogen remobilisation, and nitrogen soil uptake during seed filling in chickpea, field bean, pea, and white lupin. Values are means of 2012 and 2013. Vertical bars denote LSD at P ≤ 0.05.
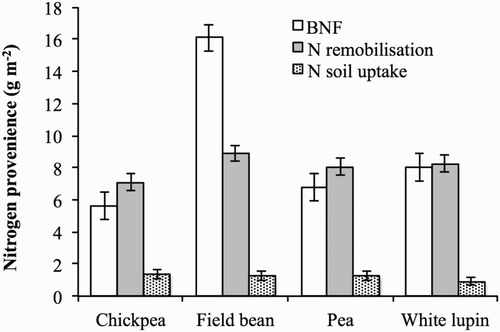
The demand for N by developing seeds is known to be high, and in our study, the N content of the seeds at maturity was higher than total N accumulation during seed filling in all four crops. Accordingly, soil N uptake and BNF were not able to sustain the demand for N of filling seeds, and endogenous N previously accumulated in vegetative parts was remobilised to fulfil this demand. Despite the great differences among legumes in grain yield, grain N content and total N content at flowering and maturity, nitrogen remobilisation during seed filling differed little among species ranging from 7.1 g m−2 in chickpea to 8.9 g m−2 in field bean. However, the importance of N remobilisation for N economy in the plants differed markedly among legumes. Indeed, N remobilised was equal to BNF in white lupin, was about 23% higher in chickpea and pea, and was 50% lower in field bean.
Koutroubas et al. (Citation2009) found that most of the variation in N remobilisation in chickpea could be accounted for by the differences in total N content at the beginning of seed growth. This finding is likely for varieties within the same species, but not among different crops. In our research the total N content of field bean at flowering was higher than that of pea, while N remobilisation was similar. This is because legume crops differed in their N remobilisation efficiency. Field bean had the highest N content at flowering, but was also the species with the lowest N remobilisation efficiency (35%) during seed filling, while pea had the lowest N content at flowering but the highest N remobilisation efficiency (54%).
Davies et al. (Citation2000) hypothesised that a higher pod number and seed yield could be indicative of a higher requirement for seed N, and that the ability to remobilise high pre-podding N may be related to this N sink demand. In our research this hypothesis was confirmed in field bean, which was the most productive plant both in terms of seed and N yield, and had the highest N remobilisation, but not for pea which had a higher seed yield than white lupin but the same N remobilisation.
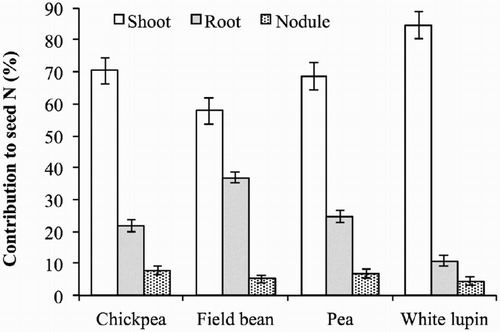
Our results confirm that the vegetative parts of plants are important sources of N for seed development during periods when N requirements exceed those provided by soil and N2 fixation (Zapata et al. Citation1987; Kurdali et al. Citation1997). Remobilised N was crucial to provide N to the seeds of chickpea, pea, and white lupin for which N remobilisation supplied half of the seed N content at maturity but was less important in field bean where it contributed only a third (). All the vegetative organs of the legume plants underwent N remobilisation (), however the N remobilisation efficiency varied among the organs. In chickpea and pea, shoots and roots remobilised the same percentage of the N allocated at flowering, while in field bean, the N allocated in shoots remobilised less than that in roots (30% and 47%), and in white lupin, the remobilised N was higher in shoots than in roots (46% and 28%). Finally, nodule N content at flowering was remobilised by 51–57% in all crops. However, owing to the different N content at flowering, shoots were the major contributors to the N supply of seeds for all four legume crops () although their relative importance ranged from 85% in white lupin to 70% in chickpea and pea, to only 58% in field bean. The N contribution of roots to N seeds was only 11% in white lupin and 23% in chickpea and pea but up to 37% in field bean. Finally, nodules overall contributed less than 8%.
Figure 4. Relative contribution of BNF, nitrogen remobilisation, and nitrogen soil uptake to seed nitrogen content in chickpea, field bean, pea, and white lupin. Values are means of 2012 and 2013. Vertical bars denote LSD at P ≤ 0.05.
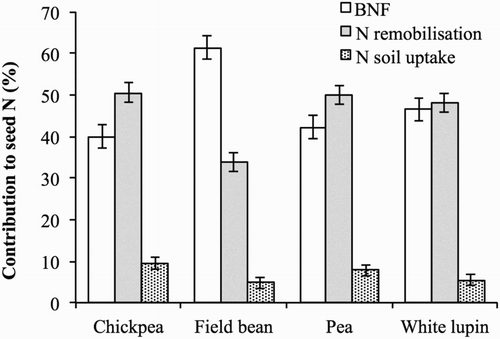
Figure 5. Relative contribution of shoots, roots, and nodules to nitrogen remobilised to seeds in chickpea, field bean, pea, and white lupin. Values are means of 2012 and 2013. Vertical bars denote LSD at P ≤ 0.05.
In conclusion, our results highlight that the vegetative parts of grain legume crops are important sources of N for seed development during periods when N requirements exceed those provided by soil and N2 fixation. Remobilised N and exogenous N had the same impact in terms of N supply to seeds in chickpea, pea, and white lupin, while in field bean, that was the most productive crop, the exogenous N was more important than endogenous. All vegetative organs underwent N remobilisation, however, owing to the different N contents at flowering, shoots were the largest contributors to the N supply of seeds in all crops. However, the contribution of nodulated roots markedly differed among species and in field bean it was almost like that of the shoots. Since nitrogen remobilisation induces senescence, which reduces the seed filling period (Salon et al. Citation2001), the improving of seed yield in grain legume requires either reduced N remobilisation or enhanced exogenous N supply to seeds. The latter might be obtained either by prolonging symbiotic fixation or by increasing the root assimilation of soil mineral N. Thus, a useful strategy for increasing legume seed yield is to select cultivars for high post-anthesis N2 fixation and N uptake, or add mineral N at flowering.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Crozat Y, Aveline A, Coste F, Gillet JP, Domenach AM. 1994. Yield performance and seed production pattern of field-grown pea and soybean in relation to N nutrition. Eur J Agron. 3:135–144. doi: 10.1016/S1161-0301(14)80119-6
- Davies SL, Turner LC, Palta JA, Siddique KHM, Plummer JA. 2000. Remobilisation of carbon and nitrogen supports seed filling in chickpea subjected to water deficit. Austr J Agric Res. 51:855–866. doi: 10.1071/AR00018
- Evans J, Taylor AC. 1987. Estimating dinitrogen (N2) fixation and soil accretion of nitrogen by grain legumes. J Austr Inst Agric Sci. 53:78–82.
- Gulmezoglu N, Kayan N. 2011. Dry matter and nitrogen accumulation during vegetative and grain filling of lentil (Lens culinaris Medic.) as affected by nitrogen rates. Not Bot Hort Agrobot Cluj Napoca. 39:196–202.
- Herridge DF, Pate JS. 1977. Utilization of net photosynthate for nitrogen fixation and protein production in an annual legume. Plant Physiol. 60:759–764. doi: 10.1104/pp.60.5.759
- Herridge DF, Peoples MB, Robert M. 2008. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 311:1–18. doi: 10.1007/s11104-008-9668-3
- Hooda RS, Rao AS, Luthra YP, Sheoran IS, Singh R. 1986. Partitioning and utilization of carbon and nitrogen for dry matter and protein production in chickpea (Cicer arietinum L.). J Exp Bot. 37:1492–1502. doi: 10.1093/jxb/37.10.1492
- Jeuffroy M, Warembourg FR. 1991. Carbon transfer and partitioning between vegetative and reproductive organs in Pisum sativum L. Plant Physiol. 97:440–448. doi: 10.1104/pp.97.1.440
- Knott CM. 1987. A key for stages of development of the pea (Pisum sativum). Ann Appl Biol. 111:233–245. doi: 10.1111/j.1744-7348.1987.tb01450.x
- Knott CM. 1990. A key for stages of development of the faba bean (Vicia faba). Ann Appl Biol. 116:391–404. doi: 10.1111/j.1744-7348.1990.tb06621.x
- Koutroubas SK, Papageorgiou M, Fotiadis S. 2009. Growth and nitrogen dynamics of spring chickpea genotypes in a Mediterranean-type climate. J Agric Sci. 147:445–458. doi: 10.1017/S0021859609008739
- Kumarasinghe KS, Danso SKA, Zapata F. 1992. Field evaluation of fixation and N partitioning in climbing bean (Phaseolus vulgaris L.) using 15N. Biol Fert Soils. 13:142–146.
- Kurdali F. 1996. Nitrogen and phosphorus assimilation, mobilization and partitioning in rainfed chickpea (Cicer arietinum L.). Field Crops Res. 47:81–92. doi: 10.1016/0378-4290(96)00034-2
- Kurdali F, Kalifa K, Al-Shamma M. 1997. Cultivar differences in nitrogen assimilation, partitioning and mobilization in rain-fed grown lentil. Field Crops Res. 54:235–243. doi: 10.1016/S0378-4290(97)00056-7
- Lopez-Bellido RJ, López-Bellido L, Benítez-Vega J, Munoz-Romero V, Lopez-Bellido FJ, Redondoc R. 2011. Chickpea and faba bean nitrogen fixation in a Mediterranean rainfed Vertisol: effect of the tillage system. Eur J Agron. 34:222–230. doi: 10.1016/j.eja.2011.01.005
- Salon C, Munier-Jolain NG, Duc G, Voisin AS, Grandgirard D, Larmure A, Emery RJN, Ney B. 2001. Grain legume seed filling in relation to nitrogen acquisition: a review and prospects with particular reference to pea. Agronomie. 21:539–552. doi: 10.1051/agro:2001143
- Schiltz S, Munier-Jolain N, Jeudy C, Burstin J, Salon C. 2005. Dynamics of exogenous nitrogen remobilization from vegetative organs in pea revealed by 15N in vivo labeling throughout seed filling. Plant Physiol. 137:1463–1473. doi: 10.1104/pp.104.056713
- Soltani A, Robertson MJ, Manschadi AM. 2006. Modeling chickpea growth and development: nitrogen accumulation and use. Field Crops Res. 99:24–34. doi: 10.1016/j.fcr.2006.02.006
- Sparrow SD, Cochran VL, Sparrow EB. 1995. Dinitrogen fixation by seven legume crops in Alaska. Agron J. 87:34–41. doi: 10.2134/agronj1995.00021962008700010007x
- Steel RGD, Torrie JH, Dickey DA. 1997. Principles and procedures of statistics: a biometrical approach. 3rd ed. New York, NY: McGraw-Hill.
- Thomson BD, Siddique KHM, Barr MD, Wilson JM. 1997. Grain legume species in low-rainfall Mediterranean-type environments I. Phenology and seed yield. Field Crop Res. 54:173–187. doi: 10.1016/S0378-4290(97)00047-6
- Van Kessel C. 1994. Seasonal accumulation and partitioning of nitrogen by lentil. Plant Soil. 164:69–76. doi: 10.1007/BF00010112
- Voisin AS, Salon C, Jeudy C, Warembourg FR. 2003. Root and nodule growth in Pisum sativum L. in relation to photosynthesis. Analysis using 13C labelling. Ann Bot. 92:557–563. doi: 10.1093/aob/mcg174
- Voisin AS, Salon C, Munier-Jolain NG, Ney B. 2002. Quantitative effect of soil nitrate, growth potential and phenology on symbiotic nitrogen fixation of pea (Pisum sativum L.). Plant Soil. 243:31–42. doi: 10.1023/A:1019966207970
- Zapata F, Danso SKA, Hardarson G, Fried M. 1987. Nitrogen fixation and translocation in field-grown fababean. Agron J. 79:505–509. doi: 10.2134/agronj1987.00021962007900030020x
