ABSTRACT
The potential effect of rhizobial inoculation on root knot nematodes in chickpea, mungbean and pigeonpea were studied under field condition. The seed treatment with respective rhizobium strains increased the nodulation, leghemoglobin content, bacteriod population, plant growth, yield and nitrogen uptake of three three food legumes compared to the plants without the rhizobium treatment. The nematode (1500 juveniles/kg soil) incited oval galls on the roots of the three legumes, and suppressed plant growth and yield. The galling, egg mass production and soil population of the nematode was greater on the plants without the rhizobium treatment. The pure culture and culture filtrate of the rhizobium strains suppressed the egg hatching and induced mortality to the juveniles of M. incognita over control. The nematode infection reduced the nodulation, bacteroid population and leghemoglobin contents of the nodules and NPK uptake by the plants. Hence, the rhizobia treatment shall be integrated to common agronomic practice of food legume cultivation so as to enhance crop productivity and to protect roots from nematode attack.
Introduction
India is the world’s leading producer and consumer of food legumes, especially the pulses with annual production of 68.82 million tons (FAOSTAT, Citation2014). Mungbean, Vigna radiata (L) Wilczek, chickpea, Cicer arietinum (L), and pigeon pea, Cajanus cajan (L) Millsp., are important food legumes (pulses), and an excellent source of low cost and high quality protein (Khan and Jairajpuri Citation2010). Due to poverty and the vegetarian dietary preference of the majority of the population, demand for pulses in India has increased tremendously during the past three decades (Ali and Naimuddin Citation2010). The pulse productivity rate in India is considerably low in comparison to the average global mean productivity of 496.4 kg/ha (FAOSTAT, Citation2014). Attack of pests and pathogens is one of the important factors for a lower productivity rate in the country. The root-knot nematode, Meloidogyne spp. is one of the important parasites that attacks almost all food legumes, and causes significant yield loss to these crops (Bridge et al. Citation2005; Ali and Naimuddin Citation2010). Chickpea, mungbean and pigeon pea are quite susceptible to the infection of Meloidogyne spp. and exhibit 9%–24% (Khan et al. Citation2002), 11%–29% (Ahmed et al. Citation2009) and 7%–19% yield loss (Khan et al. Citation2012), respectively. Among the Meloidogyne spp. that attack these three food legumes, M. incognita and M. javanica are most important with regard to occurrence and damage (Sharma et al. Citation1992). These two Meloidogyne species are distributed throughout the world, and besides food legumes, attack enumerable number of crops (Sasser Citation1977).
The legume plants develop a symbiotic relationship with the nodule forming bacteria, Rhizobium, Bradyrhizobium, Mesorhizobium etc. (Maróti and Kondorosi Citation2014). This relationship results in the formation of nodules on the roots which function to fix free nitrogen biologically (Herridge et al. Citation2008). The biologically fixed nitrogen is readily utilized by the plant (Soussi et al. Citation1998). The cultivation of food legumes in a field also increases the availability of soil nitrogen to a subsequent crop by 15–40 kg/ha (Kanwar and Rego Citation1983). The root-knot nematodes while attacking the roots of legume crops also affect the development of rhizobial nodules and vice-versa (Taha Citation1993). Researches have shown that the development of nodules in terms of number and size of nodules is suppressed in the roots infected with Meloidogyne spp. (Nath et al. Citation1979; Khan et al. Citation2002). The root invasion by the nematode results in the direct or indirect damage to the nodules that leads to the conversion of functional nodules into nonfunctional ones (Khan et al. Citation2002). The nematode infection also affects the bacteroid population (Huang Citation1987), leghemoglobin contents and nitrogenase activity in the nodule (Chahal and Chahal Citation1988). As a result, in addition to the direct damage caused by root-knot nematode to a leguminous plant, the plant growth is additionally affected due to suppression of root nodulation and the resulting decline in the N uptake. Another aspect of rhizobium-nematode interaction is that root nodulation may curtail the pathogenesis of root-knot nematode leading to a significant decline in the galling and reproduction of Meloidogyne spp. (Taha Citation1993). The present study was carried out to examine the effect of seed treatment with root nodule bacteria, Mesorhizobium ciceri (Jarvis et al. Citation1997), Bradyrhizobium japonicum (Kirchner Citation1896) and Rhizobium spp. (Frank Citation1889) on the infection by M. incognita (Kofoid & White) Chitwood on three important food legumes, chickpea, mungbean and pigeon pea, respectively. The effects were quantified by estimating the root nodulation, leghemoglobin content and rhizobia population in the nodule, NPK acquisition, biomass production and grain yield. The effect of the root nodulation was also examined on the nematode disease severity, reproduction and soil population of M. Incognita in two different fields during consecutive two years.
Materials and methods
Experimental plots
Thirty six microplots (2.0 × 1.0 m dimension) were prepared in a field plot. The soil of the plot was alluvial sandy loam and contained 63.9% sand, 21.3% silt, 14.8% clay, 7.9 g/ kg organic matter, 0.83 g/ kg N, 16 mg/ kg P, 2.1 mg/ kg K, pH 7.4, 461 ml/ kg water holding capacity, 3.1 m mhol/ cm cation exchange capacity and 1.4 m mhol/ cm anion exchange capacity. The experiment was repeated next year in an adjoining field with almost similar characteristics (±2%–5% variation). The background population of nematodes in the field soil was determined by collecting, 30 cores of soil (250 g soil/core) at a depth of 5–10 cm in a stratified manner before the laying of plots. Five samples, from the composite sample, each of the 1 kg soil were processed using the Cobb decanting and sieving method followed by the Baermann funnel technique (Southey Citation1986; Khan Citation2008).
Inoculum of root-knot nematode and root nodule bacteria
A culture of M. incognita was prepared from egg masses collected from the eggplants grown in a pure culture bed. The egg masses were incubated in the Baermann funnels at 25°C for 6–8 days. The nematode suspension was standardized to 1.5 × 103 juveniles/L water, and was spread in a small area (150 × 150 × 150 mm) at 32 spots (1L/spot) within a plot (8 spots/row, 4 rows/plot) one day before the sowing of seeds (2–3 seeds/spot). In the control plots (without nematode), 1L distilled water (without nematode juveniles) was added at the 32 spots in a microplot. A space of 25 cm plant to plant and row to row was maintained. The seed sowing of chickpea cv. BG-256, mungbean cv. T-44 (first week of November) and pigeon pea cv UPAS-120 (first week of June) was done in different seasons. A population of 1500 juveniles of M. incognita/ plant has been found to cause severe galling and significant loss in pulses (Khan et al. Citation2002, Citation2016).
Pure cultures of rhizobia strains of chickpea (MECP-12), mungbean (MEMB-03) and pigeon pea (RZPP-09) strains were procured from the Division of Microbiology, IARI, New Delhi. The bacterial strains were subcultured on the yeast manitol broth (YMB). The bacterial strains were characterized by determining the maximum tolerance concentration (MTC) to amoxicillin, chloramphenicol, Cloxacillin, co-trimoxazole, doxycycline hydrochloride, erythromycin, flucanazole, methacillin, nalidixic acid, nitrofurantoin, novobiocin, penicillin, streptomycin and tetracycline (Bauer et al. Citation1966). The antibiotic profiling revealed the MTCs of 31 µg amoxicillin, 25 µg oxycyclin and 23 µg ovbabiocin/ ml for M. ciceri; 26 µg chloramphenicol, 24 µg methacillin and 28 µg tetracycline/ ml for B. japonicum; and 19 µg streptomycin, 30 µ gtetracyclin and 33 µg erythromycin/ ml for Rhizobium sp. A combination of three antibiotics at the respective MTCs was added to the YMA to make the medium specific to rhizobia strains. The strain specific YMA mediums were used throughout the study to monitor soil populations of the applied rhizobia.
Treatments and plant culture
Two-three days old cultures of rhizobia strains (× 109–10 CFU/ml) were applied to the seeds (each at 5 ml/kg seed). The seeds treated with the respective rhizobia vstrain were sown in the 36 microplots (described in the above section of Experimental Plots). In each microplot, 2–3 seeds were sown at 32 spots in a plot (8 spots/ row, 4 rows/ plot), where 1L distilled water with or without nematode juveniles had been added. Four treatments, each of rhizobium (treated and untreated seeds) and nematode infested (inoculated and uninoculated with M. incognita) were maintained for each of the three legumes. The three legumes crops were grown during October to January (chickpea and mungbean) and June to September (pigeon pea). Each treatment was represented by three plots, which were randomly distributed in the field. The experiment was repeated in an adjoining field under identical agroclimatic conditions in the following year. Two weeks after sowing, the plots were thinned to one seedling at each spot, and irrigated by the drain irrigation. Four months after sowing, 10 plants from each plot were randomly harvested, and galls, egg masses/root system, fecundity (eggs/egg mass) and dry matter production and yield were determined.
The nodule parameters viz., nodulation (number of functional, non functional and total nodules/root system) and fresh weight of nodules, leghemoglobin content, bacteriod population and nematode invasion on the nodule were determined on 2 months old plants. To determine nodule infection by M. incognita, the nodules were immersed in the phloxine B solution (0.15 g/l water) for 20 min, the gelatinous material secreted by the females stained pink color. The nodules were examined under a stereomicroscope to observe galls and/or egg masses formed on the nodules.
Soil population of root-knot nematode and root-nodule forming bacteria
Five soil samples (260g each) were collected from the root zone of 5 plants (at 5–15 cm depth and within 10–20 cm diameter of main root) at 0 (planting), 1, 2, 3 and 4 months of sowing in each plot inoculated or not inoculated with the nematode. At each sampling occasion, the soil was collected from five different plants. The soil samples (250 g) were processed separately to extract nematode juveniles using Cobb’s sieving and decanting method described above.
To estimate the rhizospheric population of the applied rhizobia strains in the plots, 1 g soil was taken from each of the 5 samples/ plot and processed accordingly the dilution plate method (Waksman Citation1922). The petri plates having solidified YMA supplemented with respective rhizobia strain specific combination of MTCs of three antibiotics were inoculated with 0.25 ml suspension of 10−6 dilution (2 plates/ sample, 10 plates/ plot, 30 plates/ treatment). Native rhizobial strains or the background populations of morphologically similar rhizobia were also determined in YMA supplemented and not supplemented with the MTCs of three respective antibiotics. The medium without antibiotics revealed 0.8–1.9 × 103 CFUs of Mesorhizobium strains/g soil. The medium with MTCs of respective three antibiotics did not detect the rhizobia at 10−6 dilution.
Nematode hatching and mortality in vitro
The overnight cultures of rhizobia strains on YMB were centrifuged twice at 2800 g for 20 min. The pellets were transferred to a volume of sterile double distilled water (SDDW) equal to the broth culture. The population of the bacteria in the pure culture was estimated using the dilution plate method and it ranged 108–1010 CFUs of B. japonicum, M. ciceri or Rhizobium sp. per ml. The supernatant was collected in a beaker and filtered through a sterile 0.2 µm syringe filter (Berggren et al. Citation2001). The 5 ml each of the culture and culture filtrate were transferred to glass cavity blocks separately, to which surface sterilized egg masses (0.5% NaOCl for 2–3 min) of relatively same size were added. Two sets of blocks in which egg masses were immersed in distilled water, and YMB (uninoculated) served as control. The cavity blocks, placed inside petri plates containing 10 ml distilled water. The plates were covered with the lids to avoid evaporation of water from the cavity blocks during the incubation at 25–27 °C for 7 days in an incubator. Five blocks were maintained for each treatment. After incubation, the nematode larvae present in the suspension were counted.
To examine the effect on mortality of juveniles, 1 ml bacterial culture and the culture filtrate were placed in a glass cavity block to which 1 ml suspension containing 40–50 freshly hatched and surface sterilized juveniles of M. incognita (0.5% NaOCl for 2–3 min.) were added. The juveniles kept in the broth alone (without the bacterium) or in distilled water served as control. Five blocks/ treatment were placed on glass supports in petri plates containing distilled water and covered with a lid. The assembly was incubated at 25–27°C for 2 days. After incubation, the dead juveniles (immobile) were counted. The juvenile mortality was verified by transferring the larvae to distilled water.
Root nodulation, leghemoglobin contents rhizobia and population in the nodules
Two months old chickpea, mungbean and pigeon pea plants were randomly and carefully uprooted from each plot (5 plants/ plot), and the root system was thoroughly washed in water to remove adhering soil particles. The nodules were counted and weighted. Five sterilized nodules from each root system (0.5% sodium hypochloride solution) were crushed with 10 ml distilled water in a sterilized test tube and shaken on a rotatory shaker for 10 min (100 rpm). Thereafter, the suspension was serially diluted to 10−6 and the population of rhizobia was estimated using the dilution plate method described above on the YMA supplemented with the MTCs of three antibiotics. Nodules were separated from roots, the level of leghaemoglobin was immediately analysed. Nodules (500 mg) were homogenized in aliquots of Drabkin’s reagent (10 ml) and leghaemoglobin was quantified spectrophotometrically at 540 mµ, as described by Wilson and Reisenauer (Citation1963). Bovin haemoglobin was used as a standard, and values are expressed as milligrams per gram of nodule fresh weight (Appleby and Bergerson Citation1980).
Plant growth, yield and NPK
The pods were collected from 4 month old randomly selected plants at harvest and dried in the sun for a week to estímate the grain yield per plant. The plants were also dried to determine the dry weights of shoot and root. Thereafter, the shoots and roots were ground, and sieved through 0.2 mm mesh sieve. The 0.5 g powder (shoot + root) was digested in the mixture of nitric acid and perchloric acid (4:10), and the total N content was determined by the colorimetric method (Jackson Citation1973). The P content was estimated by the ammonium molybdate method (Olsen and Sommers Citation1982) and K content by flame photometry (Jackson Citation1973).
Statistical analysis
Fifteen observations taken for a variable from the three plots of a treatment (5/ plot) were averaged to calculate means. The data of the two years were pooled because the year differences were not significant (P ≤ 0.05). The data (30 replicates, 15/ year) on biomass, yield and nodulation were subjected to a two-factor analysis of variance (seed treatment × nematode inoculation). The data on galls, egg masses, fecundity and soil population were analyzed by a single factor analysis of variance. Least significant difference (L.S.D.) for hatching and mortality was calculated at P ≤ 0. 05, 0.01 and 0.001, whereas for the rest of the variables, the probability level P ≤ 0.05 was used. Tukey test was applied to some data and standard errors were calculated.
Results and discussion
Nematode hatching and mortality
The hatching of juveniles from the egg masses of M. incognita incubated in the broth alone (without bacteria) was equal to the distilled water, hence it was averaged for ease in the interpretation (). The egg hatching decreased by 9%–19% in the culture and culture filtrate of rhizobia strains compared with the control (P ≤ 0.05). Inhibition in the hatching was relatively greater in the culture filtrate. No difference in the antihatching impact among the B. japonicum, M. ciceri and Rhizobium sp. was observed. A 12%–18% mortality in the nematode juveniles due to the culture and culture filtrate of the rhizobia strains was recorded (, P ≤ 0.001). Since rhizobia do not parasitize nematodes (Barker et al. Citation1971; Chahal and Chahal Citation1988), the inhibition in the hatching or larval survival was apparently due to the toxic metabolites synthesized by the bacteria (Siddiqui et al. Citation2007). A toxin named as ‘rhizobitoxine’ is reported to be synthesized by R. japonicum which may adversely affect the nematode pathogenesis (Chakraborty and Purkayastha Citation1984). Another toxin namely, bacteriocin was synthesized by rhizobia strains which may be involved in the nematode suppression (Roslycky Citation1967). Ehteshamul-Haque and Gaffar (Citation1993) have also reported the production of toxins by inoculants rhizobia that suppressed root-rot disease.
Table 1. In vitro effects of bacterial cells and cell free culture filtrate of some Pseudomonas species, Mesorhizobium ciceri, Bradyrhizobium japonicum and Rhizobium spp. on the hatching and mortality of Meloidogyne incognita.
Galling and reproduction of root-knot nematode
Numerous oval shape galls developed on the roots of chickpea (68–75 galls/ root system), mungbean (76–84 galls) and pigeon pea (59–50 galls) grown in the plots inoculated with M. incognita (). The galling was 9%–15% less on the plants that had received seed treatment with the rhizobia. The egg mass production was also lower (7%–11%) on the plants treated with the rhizobium. However, no impact of rhizobium treatment on the gall/egg-mass ratio was recorded. Fecundity of M. incognita (eggs/egg mass) was not affected by the rhizobium treatments (). The degree of susceptibility of three pulses to the nematode infection varied, and greater galling was recorded on the mungbean and chickpea in comparison to pigeon pea, confirming results by (Ali and Naimuddin Citation2010; Khan et al. Citation2011).
Table 2. Effects of inoculation with Meloidogyne incognita on the galling, egg mass production and fecundity of the root-knot nematode, and on the dry plant weight, yield and NPK contents of mungbean cv.T-44.
Soil population of the nematode and applied bacteria
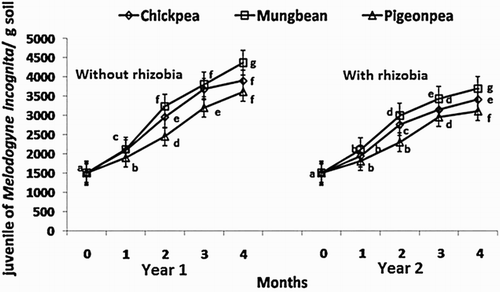
The background soil population of nematodes at the site was 173 ± 61 larvae/kg soil, which included Aphelenchoides, Cephalenchus, Ditylenchus, Polenchus, Xiphinema, Rotylenchus, Helicotylenchus, Meloidogyne, Hirschmanniella, Hoplolaimus etc. In the plots where inoculation with M. incognita was done, soil population of the nematode significantly (P ≤ 0. 05) and gradually increased with a maximum at 4 months over an initial population of 1500 juveniles/kg soil (). The increase of nematode population in the root zone of untreated plants was in the order: mungbean > chickpea > pigeon pea. In the rhizobium treated plants, the increase in the nematode population was 9%–19% less than the untreated plants.
Figure 1. Effect of inoculation withMesorhizobiumciceri, Bradyrhizobiumjaponicum and Rhizobium spp. on the monthly soil population of juveniles of Meloidogyne incognita.
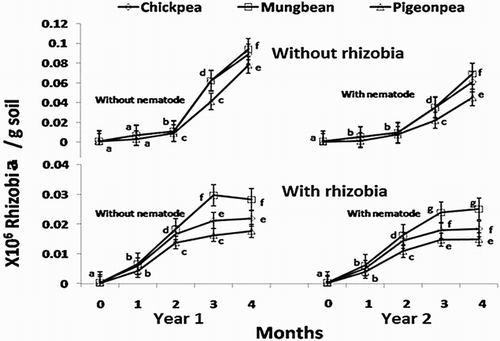
The soil population of B. japonicum, M. ciceri and Rhizobium sp. in the root zone of mungbean, chickpea and pigeon pea increased over time with the highest population recorded at 4 months stage (). Crop wise, the population increase was in the order of: mungbean > chickpea > pigeon pea. The rhizobia populations in the nematode infested plots were 9%–26% less than the plots without the infestation of M. incognita. The qualitative and/or quantitative alteration in the root exudates of profusely nodulated plants may have contributed in the suppression of nematode pathogenesis. Some antagonism to M. incognita may also have been imparted by the altered microbial composition in the root zone soil resulted due to the bacterial treatments (Khan et al. Citation2012).
Plant dry weight, yield and nutrient uptake
The seed treatment with the rhizobium strains improved the plant growth and yield of chickpea, mungbean and pigeon pea by 7%–11% and 8%–13%, respectively, compared to untreated plants with relatively lesser enhancement in the pigeon pea (). In the untreated plots, infection with M. incognita decreased yield of chickpea (18% and 24%), mungbean (16% and 21%) and pigeon pea (9% and 15%) compared to untreated and non-infested plants. However, in the plots where plants had received seed treatment with rhizobium, the nematode damage in the mungbean (16%–18%), chickpea (10%–13%) and pigeon pea (6%–9%) was less than the untreated plants (P ≤ 0.05).
The nitrogen contents of plants were greater with rhizobium treatments (). Whereas, its NPK contents of nematode infected plants without the rhizobium treatment, decreased by 15%, 24% and 26% (mungbean), 12%, 10% and 15% (chickpea) and 11%, 15% and 10% (pigeon pea), lesser nitrogen, phosphorus and potassium respectively, over control (). In the rhizobium treated plants, the decrease in the NPK contents induced by M. incognita was less than the untreated plants.
Table 3. Effects of inoculation with Meloidogyne incognita on the plant growth, yield and root nodulation of mung bean, chick pea and Pigeon pea.
The plants that received rhizobium treatments showed fewer galls on the roots. Due to the rhizobium treatment, profuse nodulation took place that resulted in greater nitrogen contents of plants. Adequate availability of N helps the plants to grow healthier and stronger. The healthy plants might have developed a stronger natural defense, which would have worked against the nematode as shown by Khan et al. Citation1997. Exogenic application of ammonium nitrate (@50 kg N/ ha) promoted the plant growth and substantially suppressed the nematode density in forest soils (Sun et al. Citation2013). Sharma et al. (Citation2005) reported that increasing levels of soil nitrogen resulted in 50% decrease in the nematode population.
Effect of root-knot nematode on the root nodulation, Lb contents and bacteriod population
In the plots where seed treatment with the rhizobium was not given, 4–8 nodules/root system were formed (). However, the seed treatment with the respective rhizobium strains greatly increased the total nodules/root system of chickpea (23% and 18%), mungbean (31% and 14%) and pigeon pea (24% and 10%) in the plots without the nematode infestation (P ≤ 0.05, ). Nematode infection caused decreased nodulation in the three pulses. In the nematode infested plots, where the seeds were not treated with the rhizobium, the roots were almost devoid of nodules. Whereas, in the rhizobium treated plants the nodulation was fairly good, it was 14%–25% less than uninoculated rhizobium treated plants. The nematode infection induced conversion of functional nodules in the non-functional nodules as the later were increased by 66%–200%, over control (). Quite a few nodules/root system were also found invaded by the nematode evidenced by the presence of a small gall on the nodule ().
Table 4. Effects of inoculation with Meloidogyne incognita on the leghemoglobin of nodules and nutrient uptake of mung bean, chick pea and Pigeon pea plants.
The greater nodulation on the treated plants reduced the root mass available for the invasion by the nematode juveniles, especially of the second generation. As a result lesser galls caused by the second generation J2 developed on the roots. In a previous study similar effect of rhizobium treatments on the galling incited by M. incognita on green gram was recorded. (Khan et al. Citation2002). Further, reproduction of the nematode females, which caused galls on the nodules would have been curtailed due to shedding of the infected nodules prematurely or at maturity. The invasion of nodules by Meloidogyne spp. causes the nodules to dysfunction and disintegrate prematurely (Taha and Kassab Citation1979). Under normal conditions, the rhizobial nodules mature in 25–45 days and may disintegrate thereafter (Ott et al. Citation2005). Further, the rhizobial oozing may have caused the direct suppressive effect on the nematode pathogenesis. In vitro study, we observed a significant inhibition in the egg hatching or juvenile survival of M. incognita was recorded in the pure culture or culture filtrate of B. japonicum, M. ciceri and Rhizobium sp.
The suppressive effect of M. incognita on the nodulation would have come by inhibiting the formation and development of the nodules or premature conversion of functional nodules into nonfunctional ones (Ali and Naimuddin Citation2010). Nodule formation may have also been suppressed due to damage to root hairs by the nematode larvae (Ali et al. Citation1981; Khan et al. Citation2002). Meloidogyne is an endoparasitic nematode and its larvae penetrate up to stellar tissue (Eisenback Citation1985). Hence, in a case of nodule invasion, the vascular bundles, bacteriod zone and cortex of the nodule would have been severely damaged during the internal movement of the nematode larvae (Taha and Kassab Citation1979).
The leghemoglobin content of nodules increased due to seed treatment with rhizobium in the three pulses compared to untreated plants (P ≤ 0.05, ). Nematode infection caused 21%–62% decrease in the Lb contents in the untreated plants over control. However, seed treatment with the rhizobia strains yielded 17% (mungbean), 12% (chickpea) and 7% (pigeon pea) improvement in the Lb contents of nematode infected plants over control (). The rhizobia population in the nodules of treated plants was also significantly greater than the untreated plants (), and the increase was in the order of mungbean (18%) > pigeon pea (16%) > chickpea (13%) (). Infection with M. incognita caused 19%–27% decrease in the population of rhizobia in the nodule over uninoculted untreated control with greater effect was recorded on mungbean followed by chickpea and pigeon pea (P ≤ 0.05).
Significant decrease in the rhizobia population and Lb contents of the nodule in nematode infected roots have revealed that invasion and feeding of the nematode larvae would have affected the rhizobia multiplication and prevented normal development of the nodule. The nematode larvae attack young lateral roots and root hairs, thereby reducing the specific root mass for invasion by the rhizobia. Due to these adverse effects, the nodulation on the nematode infected roots was significantly decreased in the infested plots. The absorption capacity of the roots is generally impaired due to formation of galls caused by Meloidogyne spp. (Wilcox-Lee and Lorea Citation1987), as a result plants fail to acquire nutrients present in the soil evidenced by 18%–28% lesser NPK contents of M. incognita infected plants.
The study has demonstrated antagonistic interaction between root-nodule bacteria and root-knot nematode. The seed treatment with B. japonicum, M. ciceri and Rhizobium sp. significantly reduced the galling and reproduction of M. incognita on chickpea, mungbean and pigeon pea, respectively. The nodulation was also adversely affected by the nematode invasion. Hence, seed treatment with a suitable strain of rhizobium should be taken as an integral practice in the pulses cultivation. Generally, small/ marginal farmers ignore the use of rhizobium in pulse cultivation in many Asian and African countries. This treatment may significantly improve the plant growth and yield, and will also provide a partial protection to the root system from the root-knot nematode invasion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. Mujeebur Rahman Khan is a Professor, and Chairperson of the Department of Plant Protection, Aligarh Muslim University. His research area includes management of plant diseases (fungi & nematodes) and impact of climate change on disease epidemiology. He has over 200 publications to his credit.
Dr. Fayaz A. Mohiddin is Assit. Professor in SKUAST, Srinagar. He is working on biocontrol of plant diseases of agricultural importance.
Mr. Faheem Ahamad is a research scholar in the Department of Plant Protection, Aligarh Muslim University, and is working on the nematode problems in irrigated Rice.
References
- Ahmed N, Abbasi MW, Shaukat SS, Zaki MJ. 2009. Physiological changes in leaves of Mungbean plants infected with Meloidogyne javanica. Phytopathol Mediterr. 48:262–268.
- Ali MA, Trabulsi IY, Abd-Elsamea ME. 1981. Antagonistic interaction between Meloidogyne incognita and Rhizobium leguminosarum on cowpea. Plant Dis. 65:432–435. doi: 10.1094/PD-65-432
- Ali SS, Naimuddin AM. 2010. Nematode infestation in pulses. In: Khan MR, Jairajpuri MS, editors. Nematode infestation part-I food crops. National Academy of Sciences, India; p. 288–325.
- Anonymous. 2014. Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). http://www.fao.org/faostat/en/#data/QC
- Appleby CA, Bergerson FJ. 1980. Methods for evaluating biological nitrogen fixation. New York: John Wiley and Sons; p. 315.
- Barker KR, Lehman PS, Huisingh D. 1971. Influence of nitrogen and Rhizobium Japonicum on the activity of Heteroidera Glycines 1. Nematologica. 17(3):377–385. doi: 10.1163/187529271X00602
- Bauer AW, Kirby WMM, Sherris JC, Turch M. 1966. Antibiotic susceptibility testing by a standardized single disc method. Amer J Clinical Path. 45:494–496.
- Berggren I, Van Vuurde JWL, Martensson AM. 2001. Factor influencing effect of Pseudomonas putida Rhizobacteria on initial infection of pea roots by Rhizobium leguminosorum bv. viceae. App Soil Ecol. 17:97–106. doi: 10.1016/S0929-1393(01)00130-5
- Bridge J, Coene DL, Kwoseh CK. 2005. Nematode parasite of tropical root and tuber crops. In: Luc M, Sikora RA, Bridge J, editors. Plant parasitic nematodes in tropical and subtropical agriculture. Oxfordshire: CABI Publishing; p. 221–258.
- Chahal PPK, Chahal VPS. 1988. Effects of different population levels of Meloidogyne incognita on nitrogenase activity, leghaemoglobin and bacteroid contents of chickpea (Cicer arietinum) nodules formed by Rhizobium spp. Zentralbla Mikrobiol. 143:63–65.
- Chakraborty U, Purkayastha RP. 1984. Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Can J Microbiol. 30:285–289. doi: 10.1139/m84-043
- Ehteshamul-Haque S, Ghaffar A. 1993. Use of rhizobia in the control of root rot diseases of sunflower, okra, soybean and mungbean. J Phytopathol. 138:157–163. doi: 10.1111/j.1439-0434.1993.tb01372.x
- Eisenback JD. 1985. Diagnostic characters useful in the identification of four most common species of root-knot nematodes (Meloidogyne spp.). In: An advanced treatise on Meloidogyne. Vol. 1. North Carolina State University Graphics, Raleigh, North Caroline; p. 95–112.
- Frank B. 1889. Über die Pilzsymbiose der Leguminosen. Ber Dtsch Bot Ges. 7:332–346.
- Herridge DF, Peoples MB, Boddey RM. 2008. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 311:1–18. doi: 10.1007/s11104-008-9668-3
- Huang JS. 1987. Interactions of nematodes with rhizobia. In: Veech JA, Dickson DW, editors. Vistas in nematology. Hyattsville: Society of Nematologists; p. 301–306.
- Jackson ML. 1973. Soil chemical analysis. New Delhi: Prentice Hall of India Pvt Ltd; p. 25–214.
- Jarvis BDW, Berkum PV, Chen WX, Nour SM, Fernandez M, Pcleyet-Marel JC, Gillis M. 1997. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium mediterraneum, and Rhizobium to Mesorhizobium gen. nov. Rhizobium ciceri, tianshanense. Int J Syst Bacteriol. 47:895–898. doi: 10.1099/00207713-47-3-895
- Kanwar JS, Rego TJ. 1983. Fertilizer use and watershed management in rainfed areas for increasing crop production. Ferti News. 28:33–43.
- Khan MR. 2008. Plant Nematodes- methodology, morphology, systematics, biology and ecology. New Hampshire (USA): Science Publishers; p. 360.
- Khan MR, Jairajpuri S. 2010. Nematode infestation in industrial crops-national scenario. In: Nematode infestations part II: industrial crops. New Delhi: National Academy of Sciences, India; p. 1–19.
- Khan MR, Khan MW, Anver MA, Haque Z. 2012. Laboratory and field performance of some soil bacteria used as seed treatments on Meloidogyne incognita in chickpea. Nematol Medit. 40:143–151.
- Khan MR, Khan MW, Singh K. 1997. Management of root-knot disease of tomato by the application of fly ash in soil. Plant Pathol. 46:33–43. doi: 10.1046/j.1365-3059.1997.d01-199.x
- Khan MR, Kounsar K, Hamid A. 2002. Effect of certain rhizobacteria and antagonistic fungi on root-nodulation and root-knot nematode disease of green gram. Nematol Medit. 30:85–89.
- Khan MR, Majid S, Mohidin FA, Khan N. 2011. A new bioprocess to produce low cost powder formulations of biocontrol bacteria and fungi to control fusarial wilt and root-knot nematode of pulses. Biol Control. 59:130–140. doi: 10.1016/j.biocontrol.2011.04.007
- Khan MR, Mohidin FA, Khan U, Ahamad F. 2016. Native Pseudomonas spp. suppressed the root-knot nematode in in-vitro and in-vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol Control. 101:159–168. doi: 10.1016/j.biocontrol.2016.06.012
- Kirchner O. 1896. Die Wurzelknöllchen der Sojabohne. Beitr Biol Pflanz. 7:213–224.
- Maróti G, Kondorosi É. 2014. Nitrogen-fixing Rhizobium-legume symbiosis: are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front Microbiol. 5:1–6.
- Nath RP, Banerjee AK, Haider MG, Sinha BK. 1979. Study of the nematodes of pulse crop in India. I. Pathogenecity of Meloidogyne incognita on gram. Ind Phytopath. 32:28–32.
- Olsen SR, Sommers LE. 1982. Phosphorus. In: Page AL, Miller LH, Keeney DR, editors. Methods of soil analysis, part 2. Madison (WI): American Society of Agronomy; p. 403–430.
- Ott T, van Dongen JT, Gunther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK. 2005. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Cur Biol. 15:531–535. doi: 10.1016/j.cub.2005.01.042
- Roslycky EB. 1967. Bacteriocin production in the rhizobia bacteria. Can J Microbiol. 13:431–432. doi: 10.1139/m67-057
- Sasser JN. 1977. Worldwide dissemination and importance of the root-knot nematodes, Meloidogyne spp. J Nematol. 9:26–29.
- Sharma SB, Smith DH, Mc Donald D. 1992. Nematode constraints of chickpea and pigeon pea production in the semi-arid tropics. Plant Dis. 76:868–874. doi: 10.1094/PD-76-0868
- Sharma GC, Thakur BS, Kashyap AS. 2005. Impact of NPK on the nematode populations and yield of plum (Prunus salicina). Acta Hort (ISHS). 696:433–436. doi: 10.17660/ActaHortic.2005.696.77
- Siddiqui ZA, Baghel G, Akhtar MS. 2007. Biocontrol of Meloidogyne javanica by Rhizobium and plant growth-promoting rhizobacteria on lentil. World J Microbiol Biotechnol. 23:435–441. doi: 10.1007/s11274-006-9244-z
- Soussi M, Ocana A, Lluch C. 1998. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). J Exp Bot. 49:1329–1337. doi: 10.1093/jxb/49.325.1329
- Southey JF. 1986. Plant nematology. London: Her Majesty’s Stationary.
- Sun X, Zhang X, Zhang S, Dai G, Han S, Liang W, Bond-Lamberty B. 2013. Soil nematode responses to increases in nitrogen deposition and precipitation in a temperate forest. PloS one. 8:e82468. doi: 10.1371/journal.pone.0082468
- Taha AHY, Kassab AS. 1979. The histopathological reactions of Vigna sinensis to separate and concomitant parasitism by Meloidogyne javanica and Rotylenchulus reniformis. J Nematol. 11:117–123.
- Taha YA. 1993. Nematode interactions with root-nodule bacteria. In: Khan MW, editors. Nematode interactions. London: Chapman and Hall; p. 170–202.
- Waksman SA. 1922. A method of counting the number of fungi in the soil. J Bacteriol. 7:339–341.
- Wilcox-Lee, Lorea DR. 1987. Effect of nematode parasitism on plant water relation. In: Veech JA, Dickson WD, editors. Vistas on nematotogy. Maryland: SON Publication; p. 260–266.
- Wilson DO, Reisenauer HM. 1963. Determination of leghemoglobin in legume nodules. Anal Biochem. 6:27–30. doi: 10.1016/0003-2697(63)90004-6