ABSTRACT
In this study, the effects of potassium doses (control, 150, 300 and 450 mg K2SO4 kg−1) and salt stress (control, 100 mM NaCl) on the yield and some element content of four medicinal and aromatic (Coriandrum sativum, Anethum graveolens, Ocimum basilicum and Foeniculum vulgare) plants were investigated in climate chamber. Both salinity and K fertiliser levels affected the fresh and dry weight of all evaluated plants. Anethum graveolens, Ocimum basilicum species are more sensitive to salinity, particularly at the vegetative productive stages. The highest fresh and dry weights of leaves, stems, roots and herb in Coriandrum sativum and Foeniculum vulgare species was observed in 300 mg kg−1 fertiliser applications. In general, there was a relatively consistent and positive correlation between root element content and aerial parts element content. The result of the present study showed that NaCl treatment caused an increase in Na+ concentration, and a decrease in K+ and Ca+2 concentration in Coriandrum sativum, Anethum graveolens, Ocimum basilicum and Foeniculum vulgare. There was an interaction between K2SO4 application and salinity effects on Na+ concentration in the all evaluated plants. Given the experimental results, especially Foeniculum vulgare and Coriandrum sativum species were the most resistant to salt stress.
Introduction
Medicinal and aromatic plants (MAPs) are of interest for human uses from the prehistoric times to the present day. Considering the importance and the role of medicinal plants in various industries and increasing saline soil and water resources in the country, produced valuable plants should be properly managed (Gholizadeh et al. Citation2016). Identify medicinal plants resistant to drought and salinity, and selected in the seedling and germination stage is considered a reliable and low-cost in this research is considered. Several studies on the germination of plants reflects the fact that increasing salinity, germination, root length, shoot and seedling dry weight significantly reduced (Kaya et al. Citation2006). Fertilisation is one of the significant agricultural practices used to improve yield and quality of the traditional crops. Potassium has an important place in fertilisation due to its physiological and biochemical functions in plants, and it is one of the most up taken and accumulated elements for plant growth and development (Cakmak Citation2002; Hu and Schmidhalter Citation2005). There is abundant evidence that Na and the Na/Ca ratio affects K uptake and accumulation within plant cells and organs and that salt tolerance is correlated with selectivity for K uptake over Na. This provides the basis for hypothesis which exists in the literature and was examined in this study, that K application can reduce salinity damage to plants (Bar-Tal et al. Citation1991).
Coriander (Coriandrum sativum L.) Has been proven to possess role and activities concerned with the treatment of cough, dysentery, sore throat, convulsion, insomnia and anxiety (Katar et al. Citation2016). Fennel (Foeniculum vulgare) is a Mediterranean medicinal and aromatic plant, of which fruits are used in the treatment of digestive disorders in addition to the bitter fennel usages as food flavour, in liqueurs and in the perfumery industry (Rani and Das Citation2016). Dill (Anethum graveolens L.) Seeds have medicinal and aromatic values with respect to the antimicrobial, antispasmodic, antidiabetic, antihypercholesteromic, and anti-inflammatory activities and flavour to cakes and pastries, soups, salads, potatoes, meats, and pickles (Orhan et al. Citation2013).
Sweet basil (Ocimum basilicum L.) Is an important aromatic plant cultivated in many parts of the world for its essential oil. Essential oils of sweet basil are used for treatment of dry mouth and dental complaints, diarrhea and chronic dysentery, respiratory disorders, etc. (Suppakul et al. Citation2003; Sartoratotto et al. Citation2004; Lee et al. Citation2005; Wannissorn et al. Citation2005; Telci et al. Citation2006; Politeo et al. Citation2007).
The main objectives of this study were to: (i) study the effects of salinity and K fertilisation interactions on Coriandrum sativum, Anethum graveolens, Foeniculum vulgare and Ocimum basilicum and nutrient uptake; (ii) test the possibility that salinity damage can be reduced by elevating K fertilisation rate; and (iii) the response of all evaluated plants to K fertilisation under saline and non-saline conditions was studied by growing in a pot experiment.
Materials and methods
Growth conditions
Plant material and experimental design
The experiment was carried out in a climate chamber (27°C) at the Experimental Mudurnu Süreyya Astarcı Vocational School of Abant Izzet Baysal University, between 30 of April and 17 August 2015. Coriander (Coriandrum sativum), dill (Anethum graveolens), fennel (Foeniculum vulgare) and basil (Ocimum basilicum) species were selected as the experiment materials. The seeds of these plants obtained from Ankara Research Institute, Turkey. Seeds were sown in plastic pots (400 mm diameter) filled with 4 kg of soil mixture containing red soil, sand and torf at 1:1:1 ratio. A total of 96 pots was used in this study. Ten seeds were sown in each pot, after 15 days of germination; plants were tinned to 4 plants per pot. Generally, it took 8–11 days for the first emergence of seedlings and within 13–15 days the germination completed in all the species. However, Anethum graveolens took 18 days to germinate and more than 22 days for the maximum possible germination. The average day and night temperatures of the entire growth period of the crop were 27°C.
The pots were watered to the field capacity with deionized water up to 109 days after sowing. Experiment was conducted in a split plot design using completely Randomized Complete Block Design (RCBD) with two factors; 0 (control) and 100 mM (salinity) concentration of NaCl in main plots and 0 (control), 150, 300, 450 mg kg−1 as K2SO4 (%50 K2O) in sub-main plots with three replicates. The soil used had 30.71% CaCO3, 3.25% organic matter, 0.85 mg kg−1 phosphorus, 127 mg kg−1 potassium, 0.03% total soluble salts, clay-loam and neutral (pH=7.2) (Anonymous Citation2017).
As base fertiliser, 50 ml triple süper fosfat and 50 ml ammonium nitrate (per pot) were applied at sowing time in accordance with the soil analysis. As experimental factors, potassium sulfate fertilisers were applied with sowing. After germination, plants were irrigated with different salinity levels, NaCl as treatment (). Plants were irrigated when 50% of available soil water was extracted. This volume of water compensated for water loss due to evapo-transpiration. Four plants were harvested from each pot 60 days after the start of NaCl treatments. While coriander and fennel were harvested at the beginning of the flowering stage, basil and dill were harvested at the start of fading.
Table 1. Detailed work schedule of salinity application.
Plants were uprooted carefully and washed with distilled water. After recording the fresh weights of both aerial parts and roots, they were oven-dried at 40°C for one week and dry weights recorded. To evaluate the physical quality aspects of these medicinal plants and height, of the aerial parts and roots were measured while to evaluate the chemical quality aspects, and pH values of the soil were determined.
Determination of ions in aerial plants and roots
To prepare the samples for the element content determination, 5 g of dry samples were extracted with 50 mL deionized water, in ultrasonic water bath during 30 min. Then, extracts were filtered with 0.22 μm cellulose acetate filter and prepared for the analysis. Before sample analysis, the standard Dionex anion mix and Dionex cation mix were used for calibration. The ions in aerosol samples were determined by using Dionex ICS 1100 Series ion chromatography. The results were checked by using the ERM-CA408 simulated rainwater (low contents) (Wang et al. Citation2016).
Statistical analysis
Data for all attributes were subjected to the COSTAT computer package (Cohort Software, Berkeley, USA) for calculating analysis of variance. The mean values were compared with the least significant difference test (LSD) following Snedecor and Cochran (Citation1980).
Results
Plant development
Salty growth medium caused a marked inhibitory effect on fresh and dry weights of both aerial parts and roots of these plants (). According to the results of these plants, the effect of salinity, aerial parts were statistically significant. Comparing control plants, fresh weight reductions in 100 mM NaCl were approximately 75%, 60%, 65% in herb, leaf and root respectively, when these plants were exposed to about two months of salinity. Anethum graveolens, Ocimum basilicum species are more sensitive to salinity, particularly at the vegetative productive stages. Ocimum basilicum was effected negatively by increasing NaCl doses, and did not application NaCl after 50 mM dose.
Table 2. Effect of different potassium sulphate (K2SO4) doses on plant height (cm), fresh and dry herb (g plant–1), fresh and dry leaf (g plant–1) of evaluated plants under salinity and control conditions.
Increasing potassium doses of the growth medium did not significantly affected on fresh and dry weights of both aerial parts and roots of these plants (). There was a significant positive effect to fresh and dry weights response to K application (150–300 mg kg−1) in Coriandrum sativum, Anethum graveolens, Ocimum basilicum and Foeniculum vulgare. The lowest doses of potassium (150 mg kg−1) demonstrated highest fresh and dry herb in Anethum graveolens, Ocimum basilicum, Coriandrum sativum compared to other potassium. The highest fresh and dry weights of leaves and shoots in Coriandrum sativum, Foeniculum vulgare species was observed in 300 mg kg−1 fertiliser applications. After this concentration (300 mg kg−1), the fresh and dry herb weights of herb, leaf, shoot and root were found to be lower than control applications in Coriandrum sativum, Foeniculum vulgare, but all potassium dose applied in Anethum graveolens, Ocimum basilicum contained the highest fresh and dry weights of leaves, stems, roots and herb compared to control application (). Also, Increasing potassium doses did not significantly affected both plant and root height in these plants.
The highest fresh and dry root weights were observed in 150 mg kg−1 fertiliser applications Anethum graveolens, Coriandrum sativum and Ocimum basilicum species. However, there is no correlation between potassium application and plant and root height of in these plants (, ). The results in this study showed that the application of potassium from 0 to 450 mg kg−1 had significantly effect on these plants under salinity stress at vegetative productive stages (, ).
Table 3. Effect of different potassium sulphate (K2SO4) doses on fresh and dry shoot (g plant–1), fresh dry root (g plant–1) and root length (cm) of evaluated plants under salinity and control conditions.
Data in this study showed that potassium had positive effect on aerial parts in these plants under salinity stress of the dionize water. According to salinity conditions with no potassium, the results indicated that application of 450 mg kg−1 potassium at the salty conditions increased the fresh and dry yield of these medicinal plants (, ). When salinity stress and potassium applications were evaluated together, the highest interaction values were obtained from 150 to 300 mg kg−1 potassium application for Coriandrum sativum, Anethum graveolens, Ocimum basilicum and Foeniculum vulgare.
Soil pH
There was no significant effect of salt stress on soil pH of these plants and the pH values of soils did not vary at salty conditions. Increasing potassium levels of the growth medium caused marked decrease pH values. While the highest pH value in Anethum graveolens was observed in 100 mM NaCl applications, the lowest pH value was observed in 50 mM NaCl applications in Ocimum basilicum. However, Ocimum basilicum was more sensitive to salinity, the salt application was left at 50 mM. pH values ranged from 7.62 to 7.69 ().
Table 4. Salinity and pH values of growth media of different potassium levels and salinity and control conditions.
Ion Contents
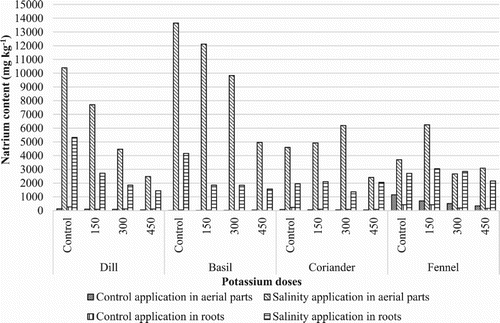
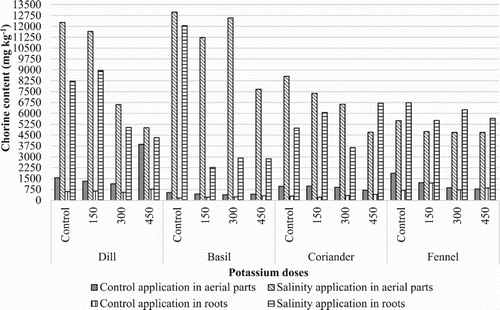
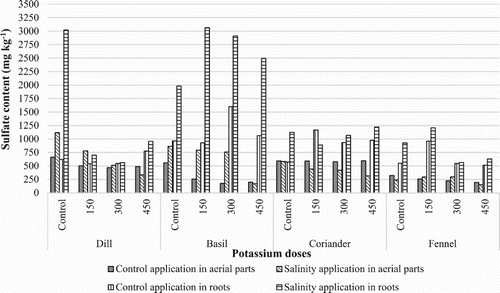
The present study was conducted for the evaluation of ions such as Calcium (Ca+2), Magnesium (Mg+2), Potassium (K+), Sodium (Na+), Chloride (Cl−) and Sulfate () in these plants. The results indicated that these aerial parts and roots contained highest concentration of Cl−, Mg+2 and K+ and lowest concentration of
and Ca+2 respectively ().
Figure 3. Concentration of Ca+2 in evaluated plants (mg kg−1) by increasing K2SO4 doses under salinity and control conditions.
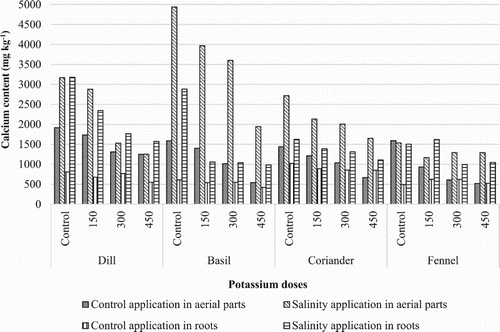
In general, there was a relatively consistent and positive correlation between root element content and aerial parts element content.
In this study, while the concentration of Na+ ranged from 8 to 13645 mg kg−1, Cl− was present in the range of 177–12981 mg kg−1 in aerial parts and roots (, ). The concentration of both ions in aerial parts and roots decreased with increasing levels of K2SO4 and the highest concentration was present in control application followed by 150 mg kg−1, 300 mg kg−1, and 450 mg kg−1 K2SO4 application in aerial parts except Ocimum basilicum. While the highest Na+ content in Ocimum basilicum aerial parts were observed in control application under salt stress, the lowest Na+ content in Foeniculum vulgare aerial parts were observed in control application under salt stress. The concentration of both ions in aerial parts and roots increased on salty conditions (). However, concentration of Cl− was considerably higher in the aerial parts than that in the roots, the aerial parts Cl− concentration was almost double than that in the roots. The highest Cl− content in Ocimum basilicum aerial parts were observed in control applications.
When these plants exposed to salinity for two months, Na+ concentration increases aerial parts and roots of all evaluated medicinal plants. When salinity stress and potassium applications were evaluated together, the highest Na+ and Cl− concentrations were obtained from control application for Ocimum basilicum, Foeniculum vulgare aerial parts, and the lowest Na+ content values were obtained from 450 mg kg−1 K2SO4 application for Coriandrum sativum, Anethum graveolens, Ocimum basilicum, Foeniculum vulgare.
The concentration of Ca+2 in this study ranged from 521 to 4935 mg kg−1 (). Increasing K2SO4 from 0 to 450 mg kg−1 in the media, Ca+2 content of in aerial parts decrased differently in Coriandrum sativum, Anethum graveolens, Ocimum basilicum (). Under salt stress, these plants demonstrated highest Ca+2 concentrations compared to different dose potassium applications in aerial parts and roots. However, concentration of this ion was considerably more in the aerial parts than those in the roots at salinity treatments. The highest Ca+2 content was in Ocimum basilicum aerial parts under salt stress.
When salinity stress and potassium applications were evaluated together, the highest Ca+2 content values were obtained from control application for all evaluated medicinal plant aerial parts and roots.
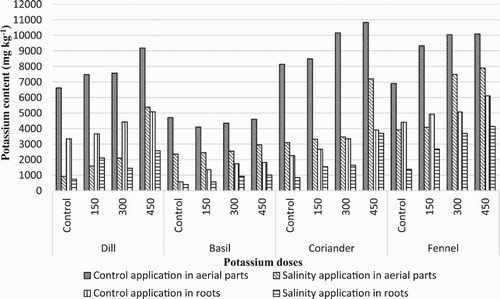
K+ concentrations of these plants varied between 404 and 10831 mg kg−1 for all evaluated medicinal plants (). The highest doses of potassium sulphate (450 mg kg−1) application demonstrated highest K+ concentration compared to other potassium and control aerial parts and root applied. When these plants exposed to salinity for two months, K+ concentration decreased aerial parts and roots of all evaluated medicinal plants. When salinity stress and potassium applications were evaluated together, the highest K+ content values were obtained from 450 mg kg−1 K2SO4 application for all evaluated medicinal aerial parts and roots. The highest K+ content was found in Foeniculum vulgare and Coriandrum sativum.
Figure 4. Concentration of K+ in evaluated plants (mg kg−1) by increasing K2SO4 doses under salinity and control conditions.
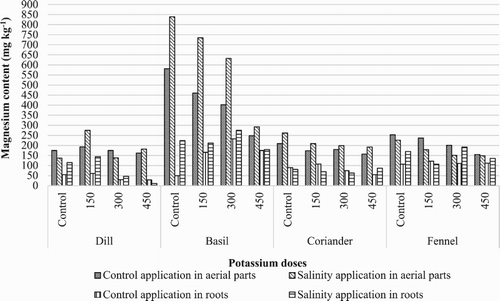
Mg+2 concentrations of these plant herbs varied between 11 and 839 mg kg−1 (). While the highest values were obtained from control application in O. Basilicum aerial parts, the lowest values were obtained from 450 mg kg−1 doses of potassium sulphate application in Anethum graveolens roots under salt stress. Also increasing potassium sulphate from 0 to 450 mg kg−1 in the pot, the highest Mg+2 content values were obtained from control application for Coriandrum sativum, Ocimum basilicum, Foeniculum vulgare. When these plants exposed to salinity for two months, Mg+2 concentration increased aerial parts and roots of all evaluated medicinal plants. In all evaluated medicinal plants, aerial parts contained higher Mg+2 concentrations than plant roots in both salinity and potassium conditions. The highest Mg+2 content was found in Anethum graveolens. When salt stress and potassium applications were evaluated together, the highest Mg+2 content were obtained from control application expect Anethum graveolens aerial parts, the lowest Mg+2 contents were 450 mg kg−1 K2SO4 application except Coriandrum sativum roots.
Figure 5. Concentration of Mg+2 in evaluated plants (mg kg−1) by increasing K2SO4 doses under salinity and control conditions.
concentrations of all evaluated medicinal plants ranged from 151 to 3061 mg kg−1 (). While its maximum content was presented in control application under salt stress, and its minimum content was presented in 450 mg kg−1 potassium sulphate application under salt stress. When these plants exposed to salinity for two months,
concentration increased in aerial parts and roots of all evaluated medicinal plants. In all evaluated medicinal plants, roots contained higher Mg+2 concentrations than aerial parts in both salinity and potassium conditions. The highest
content was found in Ocimum basilicum and Anethum graveolens. When salinity stress and potassium applications were evaluated together, the highest
content values were obtained from 150 mg kg−1 potassium sulphate application for Ocimum basilicum, Foeniculum vulgare.
Discussion
In the earlier studies in the literature, salt stress results in a considerable decrease in the fresh and dry weights of leaves, stems, tillers, fertile tillers, roots and grain yield survival of plants. (Chartzoulakis and Klapaki Citation2000; Hasegawa et al. Citation2000; Hosseini and Rezvani Moghadam Citation2006; Akbari et al. Citation2007; Mahdavi et al. Citation2007; Hamidi and Safarnejad Citation2010).
Abou el-Magd et al. (Citation2008) reported the use of saline water to irrigation decreased the vegetatif growth and green yield of sweet fennel. Similar results were reported on fennel plants (Amin Citation1994; Graifenberg et al. Citation1996; Ahmad Citation1999), on coriander plants (Neffati et al. Citation2011). The present findings are also in line with Hajar et al. (Citation1996) who reported decreased fresh weight of shoots and roots in Nigella sativa at higher salinity levels.
It causes an imbalance of the cellular ions resulting in osmotic stress (Meloni et al. Citation2003) which makes water uptake difficult (Taiz and Zeiger Citation1998). This might be due to low osmotic potential and low ionic concentrations of the control (Munns et al. Citation1995; Kaya et al. Citation2006; Alebrahim et al. Citation2009). In agreement with other studies, we demonstrated that growth was stimulated by NaCl (, ). Our data indicate that the root was less sensitive to salinity than the shoot, as also reported for other vegetables (Munns and Termot Citation1986; Lauchli and Epstein Citation1990; Graifenberg et al. Citation1993, Citation1996).
In this study, there was a significant positive in aerial parts and roots response to potassium sulphate application (0–450 mg kg−1) in Coriandrum sativum, Anethum graveolens, Ocimum basilicum and Foeniculum vulgare (). This result was in agreement with many other reports on the other plants, in which potassium plays an important role in balancing membrane potential and turgor, activating enzymes, regulating osmotic pressure, stoma movement, and tropisms. Potassium is essential for many physiological processes, such as photosynthesis, translocation of photosynthesis into sink organs, maintenance of turger, activation of enzymes, and reducing excess uptake of ions such as natrium and iron in saline soils (Mengel and Kirkby Citation2001; Cherel Citation2004).
Similar to the present findings, Heidari and Jamshidi (Citation2011) reported that millet plants have the potential to increase grain yield with K+ fertiliser applications under saline conditions. Supplemental fertilisation has been shown to overcome to some extent the nutritional deficiency in plants (El-baky et al. Citation2003). Tavakkoli et al. (Citation2011) indicated that addition of potassium to the nutrient solution diminished the negative effect of NaCl on growth and grain yield of barley by increasing N uptake as well as N metabolism. Similar results were also obtained on spinach plants irrigated with saline well water (4200 mg kg−1), when chicken manure was applied, significant increases were obtained in plant height, leaf number and plant fresh weight (El-Missery Citation2003). Akram et al. (Citation2009) observed an improvement in growth of sunflower due to the foliar spray of K2SO4 and KNO3 at 1.25% under saline concentration of 150 mM NaCl. Ebert et al. (Citation2002) found that supplying of Ca(NO3)2 at 10 mM had a beneficial effect on growth and metabolism of NaCl treated guava seedlings.
The result of the present study showed that NaCl treatment caused an increase in Na+ concentration, and a decrease in K+ and Ca+2 concentration in Coriandrum sativum, Anethum graveolens, Ocimum basilicum, Foeniculum vulgare (, , ). Seemingly, this result is similar to findings of the other researchers (Maathuis and Amtmann Citation1999; Munns Citation2002; Colmer et al. Citation2005). Also in this study, increasing potassium doses Cl− and contents decreased all evaluated plants (, ). Salinity often leads to an unfavourable uptake of cations (ion imbalance stress), inducing a reduction of Ca+2 and K+ concentration in some species (Lynch and Lauchli Citation1984; Cramer et al. Citation1989; Francois et al. Citation1991; Graifenberg et al. Citation1995). On the other hand, In fennel, Ca+2 concentration was not affected by increased Na+ concentration, while K+ in the bulb decreased with increasing Na+ (Graifenberg et al. Citation1996). Jabeen and Ahmad (Citation2011) reported that foliar application of KNO3 alleviate the toxicity of Na+ by decreasing the chances of its accumulation in plant parts. Talei et al. (Citation2012) indicated that under salinity stress, the sodium, iron, zinc and copper contents significantly increased, while nitrogen, phosphorus, potassium, calcium, magnesium and manganese content decreased in high salinity levels. They also said that, under salinity stress, tolerant accessions could produce higher K+, P, N, Mg+2 and Ca+2 and lower Na+, Fe+2, Zn+2 and Cu+2 than sensitive accessions.
The results of present investigation are in agreement with the findings of many workers in different plant species (Abdel-Rahman Citation1999; Sultana et al. Citation2001; Tabatabaei and Fakhrzad Citation2008; Cha-um et al. Citation2010) who found that nutrients were absorbed by the leaves when applied onto the shoot.
The results in this study showed that the application of potassium sulphate from 0 to 450 mg kg−1 had significantly effect on these plants under salinity stress at vegetative productive stages. Supplementary potassium sulphate partially decreased the negative effects of high NaCl concentrations. Possibly, by controlling the permeability of root cell membranes, adequate K+ nutrition reduces excess uptake of Na+ by root in saline conditions. Therefore, adequate potassium nutrition is important for the maintenance of good growth and yield under saline conditions. Given the experimental results, especially Foeniculum vulgare and Coriandrum sativum came to fore in terms of salt stress. Salinity stress also induces ion deficiency or imbalance due to the competition of nutrients such as K+, Ca+2 and Mg+2 with toxic ions Na+ and Cl−. Under saline conditions K+, Ca+2, Mg+2 concentrations decreased in the aerial parts and roots, while the Na+2, Cl− and concentration increased. There was an interaction between K+ application and salinity effects on Na+ concentration in the all evaluated plants. Application of potassium sulphate increased uptake of K+ and reduced that of Na+ in the aerial parts and roots. On the basis of the results presented here, our results support the view that K2SO4 can contribute to protect plants against salinity by alleviating the salt induced oxidative stress. For optimum growth and ion uptake, the application of 450 mg kg−1 K2SO4 dose was suitable for all evaluated plants under saline conditions. Further research is needed to investigate this effect under field conditions, where the problem is more complex.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Gülsüm Yaldız has obtained a PhD in the subject of medicinal and aromatic plants. Presently, she is positioned as Assistant Professor at the Department of Field Crops, Faculty of Agriculture and Natural Sciences in Abant Izzet Baysal University. Her main subjects of interest include good agricultural practices, natural medicinal product, biological and chemical properties of plants.
Ferit Özen is presently working as lecturer at Programme of Medicinal and Aromatic Plants, Department of Crop and Animal Production in Abant Izzet Baysal University Mudurnu Sureyya Astarci Vocational School. He has given courses about medicinal and aromatic plants’ breeding and growing.
Mahmut Çamlıca is presently working as research assistant at the Department of Medicinal and Aromatic Plants, Faculty of Agriculture and Natural Sciences in Abant Izzet Baysal University. His studies mainly focused on the natural medicinal and aromatic plant product, salinity and good agricultural practices.
Ferit Sönmez has obtained a PhD in the subject of soil microbiology and plant nutrition interactions. Presently, he is an academic staff and serving as an Assistant Professor in the Department of Seed Science and Technology, Izzet Baysal University, Bolu, Turkey. He has been completed eight research projects in soil science and plant nutrition. He has published 32 research papers in the international journals and proceeding books. He is involved in research on the areas of soil pollution, plant nutrition, organic fertilizer, plant-growth-promoting rhyzobacteria and soil conditioners.
Additional information
Funding
References
- Abdel-Rahman AAM. 1999. Productivity of some rice varieties as influence by different concentrations of foliar spray of urea under saline soil conditions. Egyptian J Agric Res. 77:242–251.
- Abou El-Magd MM, Zaki MF, Abou-Hussein SD. 2008. Effect of organic manure and different levels of saline irrigation water on growth, green yield and chemical content of sweet fennel. Aust J Basic Appl Sci. 2:90–98.
- Ahmad ME. 1999. Studies on the development and production of sweet fennel [Ph.D. thesis, Faculty of Agriculture]. Cairo: Al-Azhar University.
- Akbari G, Modarres Sanavy SAM, Yousefzadeh S. 2007. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars (Triticum aestivum L.). Pak J Biol Sci. 10(15):2557–2561. doi: 10.3923/pjbs.2007.2557.2561
- Akram MS, Ashraf M, Akram NA. 2009. Effectiveness of potassium sulfate in mitigating salt-induced adverse effects on different physio-biochemical attributes in sunflower (Helianthus annuus L.). Flora. 204(6):471–483. doi: 10.1016/j.flora.2008.05.008
- Alebrahim MT, Janmohammadi M, Sharifzade F, Tokasi S. 2009. Evaluation of salinity and drought stress effects on germination and early growth of maize (Zea mays L.) inbred lines (short technical report). Electron J Crop Prod. 1(2):35–43.
- Amin IS. 1994. Effect of different levels of salinity on the growth and volatile oil constituents of Foeniculum vulgare L. (sweet fennel). Egyptian J Appl Sci. 9(4):129–142.
- Anonymous. 2017. Bolu Directorate of Provincial Food Agriculture and Livestock. Bolu.
- Bar-Tal A, Feigenbaum S, Sparks DL. 1991. Potassium-salinity interactions in irrigated corn. Irrig Sci. 12:27–35. doi: 10.1007/BF00190706
- Cakmak I. 2002. Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil. 247:3–24. doi: 10.1023/A:1021194511492
- Chartzoulakis K, Klapaki G. 2000. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hortic. 86:247–260. doi: 10.1016/S0304-4238(00)00151-5
- Cha-um S, Siringam K, Juntawong N. 2010. Water relations, pigment stabilization, photosynthetic abilities and growth improvement in salt stressed rice plant treated with exogenous potassium nitrate application. Int J Plant Prod. 4(3):187–198.
- Cherel I. 2004. Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot. 55:337–351. doi: 10.1093/jxb/erh028
- Colmer TD, Munns R, Flowers TJ. 2005. Improving salt tolerance of wheat and barley: future prospects. Aust J Exp Agric. 45(11):1425–1444. doi: 10.1071/EA04162
- Cramer G, Epstein E, Lauchli A. 1989. Na–Ca interactions in barley seedlings: relationship to ion transport and growth. Plant Cell Environ. 12:551–558. doi: 10.1111/j.1365-3040.1989.tb02128.x
- Ebert G, Eberle J, Ali-Dinar H, Lüdders P. 2002. Ameliorating effects of Ca(NO3)2 on growth, mineral uptake and photosynthesis of NaCl-stressed guava seedlings (Psidium guajava L.). Sci Hortic. 93:125–135. doi: 10.1016/S0304-4238(01)00325-9
- El-Baky A, Hanaa H, Mohamed Amal A, Hussein MM. 2003. Influence of salinity on lipid peroxidation, antioxidant enzymes, and electrophoretic patterns of proteins and isoenzymes in leaves of some onion cultivars. Asian J Plant Sci. 2:1220–1227. doi: 10.3923/ajps.2003.1220.1227
- El-Missery MMA. 2003. Effect of organic fertilization on yield and quality of some vegetable crops under saline conditions [M.Sc. thesis, Faculty of Agriculture]. Cairo: Ain Shams University.
- Francois LE, Donovan TI, Maas EV. 1991. Calcium deficiency of artichoke buds in relation to salinity. HortScience. 26:549–553.
- Gholizadeh F, Abotaleb–Tavakkoli M, Pazoki A. 2016. Evaluation of salt tolerance on germination stage and morphological characteristics of some medicinal plants artichoke, flax, safflower and coneflower. Int J Farm & Allied Sci. 5(3):229–237.
- Graifenberg A, Botrini L, Giustiniani L, Lipucci di Paola M. 1996. Salinity affects growth, yield and elemental concentration of fennel. HortScience. 31(7):1131–1134.
- Graifenberg A, Giustiniani L, Temperini O, Lipucci di Paola M. 1995. Allocation of Na, Cl, K and Ca within plant tissues in globe artichoke (Cynara scolymus L.) under saline-sodic condition. Sci Hortic. 63:1–10. doi: 10.1016/0304-4238(95)00797-W
- Graifenberg A, Lipucci di Paola M, Giustiniani L, Temperini O. 1993. Yield and growth of globe artichoke under saline-sodic conditions. HortScience. 28:791–793.
- Hajar AS, Zidan MA, Al-Zahrani HS. 1996. Effect of salinity stress on the germination, growth and some physiological activities of black cumin (Nigella sativa L.). Arab Gulf J Sci Res. 14(2):445–454.
- Hamidi H, Safarnejad A. 2010. Effect of drought stress on alfalfa cultivars (Medicago sativa L.) in germination stage. Amer-Eurasian J Agric Environ Sci. 8(6):705–709.
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. 2000. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 51:463–499. doi: 10.1146/annurev.arplant.51.1.463
- Heidari M, Jamshidi P. 2011. Effects of salinity and potassium application on antioxidant enzyme activities and physiological parameters in pearl millet. Agric Sci China. 10:228–237. doi: 10.1016/S1671-2927(09)60309-6
- Hosseini H, Rezvani Moghadam P. 2006. Effect of water and salinity stress in seed germination on Psylliom (Plantago ovata). Iran J Field Crop Res. 4(1):15–22.
- Hu Y, Schmidhalter U. 2005. Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci. 168:541–549. doi: 10.1002/jpln.200420516
- Jabeen N, Ahmad R. 2011. Foliar application of potassium nitrate affects the growth and nitrate reductase activity in sunflower and safflower leaves under salinity. Notulae Botanicae Horti Agrobotanici. 39(2):172–178.
- Katar D, Kara N, Katar N. 2016. Yields and quality performances of coriander (Coriandrum Sativum L.) genotypes under different ecological conditions. Turk J Field Crops. 21(1):79–87.
- Kaya MD, Okcu G, Atak M, Cikili Y, Kolsarici O. 2006. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron. 24:291–295. doi: 10.1016/j.eja.2005.08.001
- Lauchli A, Epstein E. 1990. Plant response to saline and sodic conditions. In: KK Tanji, editors. Agricultural salinity assessment and management. New York: American Society of Civil Engineers Manuals and Reports on Engineering Practice; p. 113–137.
- Lee S-J, Umano K, Shibamoto K, Lee K-G. 2005. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 91:131–137. doi: 10.1016/j.foodchem.2004.05.056
- Lynch J, Lauchli A. 1984. Potassium transport in saltstressed barley roots. Planta. 161:295–301. doi: 10.1007/BF00398718
- Maathuis FJ, Amtmann A. 1999. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot. 84(2):123–133. doi: 10.1006/anbo.1999.0912
- Mahdavi B, Modares Sanavi SAM, Balochi HR. 2007. The effect of sodium chloride on the germination and seedling growth figures grass pea (Lathyrus sativus L.). Iran J Biol. 20:363–374.
- Meloni DA, Oliva MA, Martinez CA, Cambraia J. 2003. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot. 49(1):69–76. doi: 10.1016/S0098-8472(02)00058-8
- Mengel K, Kirkby EA. 2001. Principles of plant nutrition. 5nd ed. Dordrecht: Kluwer Academic Publishers.
- Munns R. 2002. Comparative physiology of salt and water stress. Plant Cell Environ. 25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x
- Munns R, Schachtman DP, Condon AG. 1995. The significance of a two-phase growth response to salinity in wheat and barley. Aust J Agric Research. 22:561–569.
- Munns R, Termot A. 1986. Whole-plant responses to salinity. Aust J Plant Physiol. 13:143–160. doi: 10.1071/PP9860143
- Neffati M, Sriti J, Hamdaoui G, Kchouk ME, Marzouk B. 2011. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 124:221–225. doi: 10.1016/j.foodchem.2010.06.022
- Orhan EI, Senol FS, Ozturk N, Celik SA, Pulur A, Kan Y. 2013. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem Toxicol. 59:96–103. doi: 10.1016/j.fct.2013.05.053
- Politeo O, Jukic M, Milos M. 2007. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 101:379–385. doi: 10.1016/j.foodchem.2006.01.045
- Rani S, Das S. 2016. Foeniculum Vulgare: phytochemical and pharmacological review. Int J Adv Res (Indore). 4(7):477–486. doi: 10.21474/IJAR01/1143
- Sartoratotto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. 2004. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol. 35:275–280. doi: 10.1590/S1517-83822004000300001
- Snedecor GW, Cochran WG. 1980. Statistical methods. 7th ed. Ames: Iowa State University Press.
- Sultana N, Ikeda T, Kashem MA. 2001. Effect of foliar spray of nutrient solutions on photosynthesis, dry matter accumulation and yield in sea water-stressed rice. Environ Exp Bot. 46:129–140. doi: 10.1016/S0098-8472(01)00090-9
- Suppakul P, Miltz J, Sonneveld K, Bigger SW. 2003. Antimicrobial properties of basil and its possible application in food packing. J Agric Food Chem. 51:3197–3207. doi: 10.1021/jf021038t
- Tabatabaei SJ, Fakhrzad F. 2008. Foliar and soil application of potassium nitrate affects the tolerance of salinity and canopy growth of perennial ryegrass (Lolium perenne var ‘Boulevard’). Am J Agric Biol Sci. 3(3):544–550. doi: 10.3844/ajabssp.2008.544.550
- Taiz L, Zeiger E. 1998. Plant physiology. 2nd ed. Sunderland (MA): Sinauer Associates Inc.
- Talei D, Mihdzar AK, Khanif MY, Valdiani A, Puad MA. 2012. Response of king of bitters (Andrographis paniculata Nees.) seedlings to salinity stress beyond the salt tolerance threshold. Aust J Crop Sci. 6(6):1059–1067.
- Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, Glenn K. 2011. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J Exp Bot. 62(6):2189–2203. doi: 10.1093/jxb/erq422
- Telci I, Bayram E, Yilmaz G, Avci B. 2006. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biocheml Syst Ecol. 34:489–497. doi: 10.1016/j.bse.2006.01.009
- Wang M, Fu Y, Liu H. 2016. Nutritional quality and ions uptake to PTNDS in soybeans. Food Chem. 192:750–759. doi: 10.1016/j.foodchem.2015.07.002
- Wannissorn B, Jarikasem S, Siriwangchai T, Thubthimthed S. 2005. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia. 76:233–236. doi: 10.1016/j.fitote.2004.12.009