ABSTRACT
The main objective of this study was to investigate the performance of faba bean landraces originating from different regions of Greece under both organic and conventional farming systems focusing mainly on yield, biological nitrogen fixation (BNF), and competitiveness to weeds. Faba bean exhibited a high ability to fix nitrogen from the atmosphere, as indicated by the percentage of N2 derived from the atmosphere which exceeded 75% in all evaluated varieties, and the total amount of biologically fixed N up to full anthesis, which fluctuated from 118.5 to 193.9 kg ha−1 in the various cropping systems and cultivars. The weed density was appreciably higher in the organic plots, without significant differences among the faba bean cultivars, while wild mustard and corn poppy were the most competitive weeds. The application of inorganic starter fertiliser in the conventionally-treated plots had no negative effect on biologically-fixed nitrogen by faba bean plants, while the herbicide pendimethalin had no negative impact on the nodulation process. Protein concentrations in faba bean cultivars fluctuated from 27.3% to 31.4%. The evaluated landraces could be utilised in breeding programmes due to their earliness, and their high performance in terms of protein content, BNF ability, and productivity.
Introduction
Faba bean (Vicia faba L.) is one of the most important legume species (Li et al. Citation2017) and it is cultivated for food, feed and green manure purposes (Hauggaard-Nielsen et al. Citation2009; Bilalis et al. Citation2010; Link and Ghaouti Citation2012; Sulas et al. Citation2013; Di Paolo et al. Citation2015; Lavania et al. Citation2015). Faba bean seeds contain about 30% protein (Lizarazo et al. Citation2015), while plants due to their high nitrogen fixation ability, can play a significant role as a N source in cropping systems (Sulas et al. Citation2013). According to Hauggaard-Nielsen et al. (Citation2009), faba bean is a legume with high capacity for N2 fixation. As a total, this species has been reported to fix nitrogen to amounts up to 200 kg ha−1 (Neugschwandtner et al. Citation2015). Therefore, the inclusion of faba bean in crop rotations could reduce the need for the use of inorganic nitrogen fertilisers in subsequent crops.
In faba bean crops, symbiotic nitrogen fixation (BNF) due to root colonisation by rhizobia is dependent on nitrogen fertilisation (Mohamed and Babiker Citation2012), molybdenum (Mo) uptake (Van Zwieten et al. Citation2015), phosphorus fertilisation (Amanuel et al. Citation2000), Rhizobium inoculation (Habtemichial et al. Citation2007), salinity (Abd-Alla et al. Citation2001), drought (Neugschwandtner et al. Citation2015), sulphur fertilisation (Habtemichial et al. Citation2007; Cazzato et al. Citation2012), broomrape infestation (Bouraoui et al. Citation2016) and faba genotypes (Maalouf et al. Citation2015).
The importance of BNF by legumes in crop nutrition is well documented (Dubova et al. Citation2015), primarily due to its use as a low-cost alternative solution to inorganic N fertilisers (Amanuel et al. Citation2000). As stated by Köpke (Citation1995), to maximise BNF, it is extremely important to select species or varieties suitable for each cultivation site. Consequently, effort should be made to develop faba bean varieties with elevated BNF ability. Furthermore, crop varieties specifically bred for organic farming is needed (Lammerts van Bueren and Myers Citation2011), to improve crop performance and increase yields in crops aimed at organic food production. The landraces from different geographical regions constitute a valuable germplasm pool that can be exploited and used to increase productivity and resilience to different environments. Furthermore, we hypothesise that the local landraces due to their higher genotypic heterogeneity, could constitute a valuable germplasm source for breeding new varieties suitable for organic farming systems. However, little information is available on the nodulation and nitrogen fixation ability of faba bean landraces or varieties, especially under conditions of organic farming. The main objective of this study was to investigate the performance of faba bean landraces originating from different regions of Greece under both organic and conventional farming systems focusing mainly on yield, biological nitrogen fixation (BNF), and competitiveness to weeds.
Materials and Methods
Site description
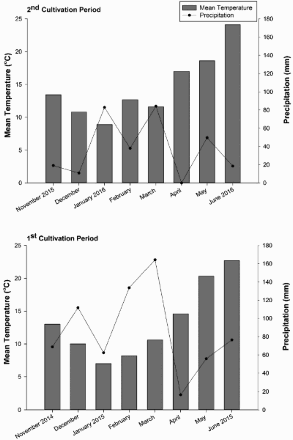
Two field experiments were conducted at the experimental farm of the Agricultural University of Athens in Kopais, located in central Greece (23°05′41″E, 38°23′51″N, altitude 95 m). The mean air temperature and precipitation during the growing seasons (1st exp.: November 2014-June 2015; 2nd exp.: November 2015-June 2016) are presented in . At the beginning of each experiment, soil samples were collected from the plow layer (0–30 cm) to determine their physical and chemical properties. The soil texture, which was determined by the Bouyoucos hydrometer method (Bouyoucos, Citation1962), was clay (43.7% clay, 25.6% silt, and 30.7% sand), with 10.83% organic matter (determined by the Nelson and Sommers method, Citation1982). The pH and the electrical conductivity of the soil were 8.12 and 0.75 mS cm−1, respectively, as determined in soil to water suspensions (1:2 v/v) using conductivity and pH metres. The total-N, P and K concentrations in the soil, which were determined as described in a previous paper (Kontopoulou et al., Citation2015), were 0.27% (w/w), 28, and 137 mg kg−1, respectively. Moreover, naturally occurring Rhizobium leguminosarum bv. viciae strains were isolated in the soil according to a method described by Tampakaki et al. (Citation2017).
Experimental layout
The field experiments were laid out as split-plot designs with four replications, the farming system as main plot (conventional or organic) and the cultivars as sub-plot. In the 1st experimental year (EY), the evaluated varieties were the commercial variety ‘Aquadulche’ and three Greek landraces, i.e. ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, and ‘AUAMANIfb001’ which originated from Andros Island in the Aegean Sea, Lefkada Island in the Ionian Sea, and Mani in southern Peloponnese, respectively. Taking into consideration the results of the 1st EY and the availability of the experimental fields, the commercial variety ‘Aquadulche’ was compared only with the landrace ‘AUALEFKADAfb001’ in the 2nd EY. The plots with the different faba bean cultivars were randomised within each main plot. The size of each sub-plot size was 10.5 m2 (3 × 3.5 m) and comprised 10 rows with plants spaced at 30 cm apart. Faba bean seeds were sown by hand to a depth of 2–3 cm on 20 November 2014 (1st EY) and 12 November 2015 (2nd EY). Before sowing, 570 kg ha−1 of an inorganic NPK fertiliser (N: P2O5: K2O ratio 11: 15: 15), and 7.6 ton ha−1 of sheep manure were applied in the conventional and the organic plots, respectively. The corresponding N application rates were 62.7 and 63.4 kg ha−1, respectively. The rate of sheep manure application is on dry weight (DW) basis. The concentrations of main nutrients in the sheep manure were as follows: 0.84% total-N, 0.3%, P2O5, 0.7% K2O5, 0.38% CaO, and 0.24% MgO, on DW basis. In the conventional plots, pendimethalin (Stomp Aqua 455 CS; BASF, Athens, Greece) was applied directly after sowing at a rate of 1.14 kg active ingredient ha−1. No herbicide was applied in the organic plots. The herbicide applied in the conventional plots is widely used in faba bean crops, because weeds constitute the main constraint for faba bean production in both organic and conventional agriculture.
Soil measurements
At the flowering and harvest stage, soil samples were collected in all plots from the plough layer using a cylindrical auger (diameter: 10 cm, height: 20 cm). Then, each soil sample was air-dried and sieved to <2 mm for chemical analyses. For the determination of and
concentrations, each sample of sieved soil was extracted using a KCl solution as described by Keeney and Nelson (Citation1982). Afterwards, the nitrate and ammonium concentrations in the sample extracts were determined by applying the cadmium reduction and the indophenol blue methods, respectively (Keeney and Nelson, Citation1982) using a microplate spectrophotometer (Anthos Zenyth 200; Biochrom, USA).
Crop and weed measurements
Weed density was recorded by counting seedlings in 0.4 × 0.4 m quadrants at 120 days after sowing (DAS). Two quadrats per plot were placed in a representative site within each plot and the weeds growing in this area were identified and recorded. After the weed measurements, all remaining weeds were removed by hoeing and hand weeding.
Phenology for each faba bean cultivar was monitored weekly and dates were recorded when 50% of the plants in a plot reached flowering as well as when the crop reached full ripening. The plant height was also measured at the flowering stage. Moreover, two root samples from each plot were collected by using a cylindrical auger (diameter: 10 cm, height: 20 cm). Soil samples were placed for 24 h in a ‘Calgon’ solution (dispersing agent) prepared by adding 40 g (NaPO3)6 and 10 g Na2CO3 per 1000 mL of water. Subsequently, the roots were separated from the soil by soaking in water and then gently washing them over a series of sieves with mesh sizes of 2.0 and 0.5 mm, as described by Anderson and Ingram (Citation1993). Then, the number of nodules per L of soil was counted. Root dry biomass was also measured after drying the samples for 48 h at 70°C. Plant height and above-ground biomass from 10 plants randomly selected from each plot were also recorded. The biomass was determined after drying at 70°C for 72 h. Moreover, at each plot, an area of 1 m2 was manually harvested weekly, starting from the beginning of April until the middle of May, and fresh pod yield as well as pod length, and pod number per plant, were measured. The pods were harvested when the seeds reached the full size but were still green. Total nitrogen (N) was determined by applying the Kjeldhal method (Bremer Citation1960). Protein content was determined in dry seeds by near infrared reflectance spectroscopy (NIRS) technique. Briefly, each sample was ground to pass a 1-mm sieve (Kinematica, PX-MFC 90D, Luzern, Switzerland) and then kept in hygroscopic environment until analysis. Each sample was scanned in triplicate on a near infra-red spectrometer (Rapid Analyzer XDS, FOSS, Hillerød, Denmark) with a spectral range from 400 to 2498 nm. The calibration equation with RSQ = 0.972 (coefficient of determination) for crude protein prediction in faba bean samples was used.
Biological nitrogen fixation measurements
Biological nitrogen fixation was estimated using the natural 15N abundance method. The 15N content of the plant samples was determined in the Stable Isotope Facility of UC-Davis, CA, USA, by CF-IRMS (Europa Scientific, Crewe, UK). The obtained values were used to compute the differences (δ15Ν) between the abundance of 15N in each sample and the natural 15N abundance in the atmospheric N, which is a known constant (0.3663%). The δ15Ν values were estimated as parts per thousand (‰) deviations relative to the nominated international standard of atmospheric N2 (0.3663%) using the following equation (Bedard-Haughn et al. Citation2003):(1) Subsequently, the proportion of N derived from the atmosphere (%Ndfa) was estimated by substituting the δ15N (‰) of the N2-fixing legume and a non-N2-fixing reference plant grown on the same soil, as calculated using (1), into the following equation suggested by Unkovich et al. (Citation2008):
(2) where ‘B’ is the δ15N in shoots of faba bean plants grown on an inert medium and starved of N throughout their life, thereby being fully dependent upon N2 fixation. The B value used in the current study (−0.50) is that suggested by Unkovich et al. (Citation2008) based on published works. The reference plant used to determine the corresponding δ15Ν values in this study was wild mustard (Sinapis arvensis). Reference plants were separately collected from organic and conventional plots.
To estimate the total amounts of biologically-fixed N2 in the aboveground biomass of faba bean (BNF, kg ha−1), the %Ndfa values obtained from (2) were substituted in the following equation (Collino et al. Citation2015):(3) where DB is the above-ground dry biomass of faba bean (kg ha−1) and Nt is the total-N concentration (% w/w) in plant samples.
Statistical analyses
The experiments of both years were statistically analyzed as two-factorial split-plot experimental designs with four replications per treatment. Initially, the data were subjected to factorial analysis of variance. When the farming system and/or the faba bean varieties had a significant impact but the interaction between them was insignificant, the means between the two tested farming systems and/or the faba bean varieties were separated using the Duncan's Multiple Range Test (P < 0.05). The same test was used to separate means of all four treatments when also the interaction was significant. All statistical analyses were carried out using the STATISTICA software package, version 9.0 for Windows (StatSoft Inc., Tulsa, USA).
Results
Soil nutrient status
In both experiments and on both sampling dates (flowering stage and harvest stage) the soil and
concentrations were not influenced either by the evaluated varieties or by the farming system (data not shown). In the 1st EY (experimental year), the soil
and
concentrations at the stage of full anthesis were 15.9 and 5.81 mg kg−1, respectively. Similar
and
concentrations in soil were obtained also in the 2nd EY (17.2 and 4.88 mg kg−1, respectively, at the stage of full anthesis).
Weed flora
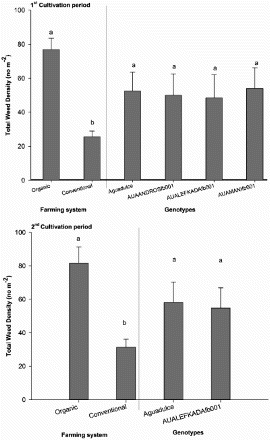
The total weed density was significantly higher in the plots treated according to organic farming practices in comparison with those treated conventionally, while it was not influenced by the evaluated faba bean varieties (). In both experimental years, no interaction was found between the faba bean varieties and the farming system. The total weed density ranged from 25.62 to 76.87 plants m−2 and from 31.33 to 81.56 plants m−2 in 2014 and 2015, respectively. Cleavers (Galium aparine L.), corn poppy (Papaver rhoeas L.), common henbit (Lamium aplexicaule L.), common fumitory (Fumaria officinalis L.), corn chamomile (Anthemis arvensis L.), common knotgrass (Polygonum aviculare L.), speedwell (Veronica spp.) and wild mustard (Sinapis arvensis L.) were the dominant broad-leaved weeds. The highest weed density was recorded for wild mustard and corn poppy and ranged from 8.75 to 43.12 plants m−2 and from 3.12 to 26.87 plants m−2, respectively (data not shown). The grass weeds wild oat (Avena sterilis L.) and black-grass (Alopecurus myosuroides Huds.) were rarely recorded.
Figure 2. Effects of the farming system (organic system vs. conventional) and faba bean variety (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) on total weed density (no m−2) in both experimental years. For each factor, bars followed by different letters indicate significant differences according to the Duncan's multiple range test (P < 0.05).Vertical bars indicate the standard errors of the means.
Phenological and plant development parameters
In the first experimental year, the time from sowing to 50% flowering and to full ripening fluctuated from 117 to 132 days and from 193 to 202 days, respectively (). The number of days to 50% flowering differed significantly among the evaluated faba bean varieties. The landrace ‘AUALEFKADAfb001’ required significantly more days to 50% flowering (132 d) in both farming systems and both experimental years (data shown only for the 1st EY), in comparison with all other varieties. However, the longest time to full ripening was recorded in the commercial variety ‘Aquadulce’ followed by ‘AUALEFKADAfb001’ (202 and 197 d, respectively, in the 1st EY), while the other two landraces needed significantly less time (193 d in the 1st EY). In the first EY, plant height ranged from 65.6 to 74.4 cm without significant differences due to the farming system or the variety. Similar values were recorded in the 2nd EY.
Table 1. Effects of the farming system (organic vs. conventional) and faba bean varieties (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) on days to 50% flowering, days to full ripening and plant height (cm) at the flowering stage in the first experimental year.
Root growth and nodulation
The root biomass was significantly influenced by the faba bean variety (). The highest root dry weight (RDW) was recorded in ‘AUALEFKADAfb001’under both farming systems (7.27 g plant−1 and 7.52 g plant−1 on average in the 1st and the 2nd EY, respectively). On the other hand, the lowest RDW was found in ‘AUAMANIfb001’ (4.44 g plant−1) and ‘Aquadulce’ (4.26 g plant−1 and 4.19 g plant−1 in the first and the second EY, respectively). No interaction was found between the farming system and the variety, with respect to the fresh and dry root biomass. The number of nodules per soil volume unit ranged from 26.2 to 58.5 nodules L−1 in the 1st EY and from 35.5 to 64.3 nodules L−1 in the 2nd EY. The highest nodulation was recorded in roots of ‘AUALEFKADAfb001’ in both farming systems and both experimental years followed by ‘Aquadulche’ in the conventional farming system (). However, ‘Aquadulche’ formed the lowest number of nodules when cultivated following organic farming practices and this interaction was significant in both experimental years.
Table 2. Root fresh weight (FW) and dry weight (DW) in four faba bean varieties (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) as influenced by the farming system (organic or conventional) in the first and the second experimental year.
Table 3. Number of nodules per volume of soil (No L−1) in four faba bean varieties (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) as influenced by the farming system (organic or conventional) in the first and the second experimental year.
Shoot dry biomass, tissue N concentration and biological N2 fixation (BNF)
The percentage of N derived from the atmosphere was slightly, but significantly higher in faba bean plans cultivated according to conventional farming practices in comparison with those cultivated in organic plots. Ndfa values in both experimental years ranged from 76.73% to 91.01% (). With respect to the impact of the faba bean variety on Ndfa, a significant interaction was observed between the farming system and the varieties. In particular, the commercial variety rendered significantly lower Ndfa values in the organic plots than in those treated conventionally, while all landraces in the 1st EY rendered similar Ndfa values with those of ‘Aquadulche’ in the conventional plots. The shoot dry biomass of ‘Aquadulche’ was also higher in the conventional than in the organic plots, while that of the landraces was similar in both farming systems. With respect to the varieties, the conventionally-grown ‘AUALEFKADAfb001’ plants accumulated significantly more shoot dry biomass than all other varieties (1st EY) or organically-grown ‘AUALEFKADAfb001’ and ‘Aquadulce’ (2nd EY). The total nitrogen concentration in the shoot was not influenced either by the variety or by the farming system. Using these data, it was estimated that ‘AUALEFKADAfb001’ fixed significantly higher amounts of atmospheric N2 per unit of cultivated area than the other varieties in both experiments, as indicated by the obtained BNF values (). Overall, the total amounts of biologically fixed N2 ranged from 118.6 to 193.9 kg ha−1 across the different faba bean varieties.
Table 4. Impact of the farming system (organic vs. conventional) and the variety (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) on the proportion of plant N derived from atmospheric N2-fixation (Ndfa) at full flowering, shoot dry biomass, shoot total-N concentration, and amounts of biologically-fixed Ν (BNF) in the first and the second experimental year.
Seed protein concentrations, yield and yield components
The pod length and number of pods per plant were not influenced by the farming system in the 1st EY (). The faba bean variety had a strong impact on both these parameters as well as on total fresh pod yield, which interacted with the farming system. However, the number of pods per plant in ‘Aquadulce’ and ‘AUAANDROSfb001’ was about one third the number of pods harvested from ‘AUALEFKADAfb001’ and ‘AUAMANIfb001’. The length and the total yield of green pods harvested from Aquadulce’ and ‘AUAANDROSfb001’ were significantly lower in the organic than in the conventional plots. In contrast, the length and the total yield of pods harvested from ‘AUALEFKADAfb001’ and ‘AUAMANIfb001’ was not influenced by the cropping system. Finally, the total yield of ‘Aquadulce’ was significantly lower than that of ‘AUAANDROSfb001’ in the organic plots, while it was similar in the conventional plots. Nevertheless, the yield of ‘Aquadulce’ and ‘AUAANDROSfb001’ was significantly higher than that obtained from the two landraces that produce small pods, i.e. ‘AUALEFKADAfb001’ and ‘AUAMANIfb001’. Similar differences in total yield and yield components between ‘Aquadulce’ and ‘AUALEFKADAfb001’ were observed also in the 2nd EY.
Table 5. Influence of the farming system (organic vs. conventional) and faba bean variety (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) on pod number, pod length, and fresh pod yield of faba bean crop in the first and the second experimental year.
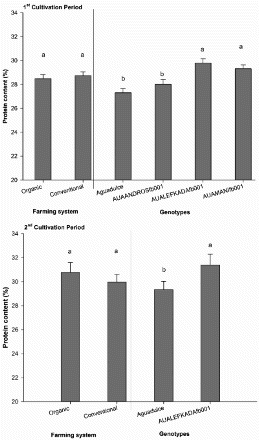
The protein concentration in dry seeds of the four different faba bean varieties ranged from 27.3% to 29.8%, in the 1st EY and from 29.3% to 31.4% in the 2nd EY, without any significant impact of the farming system (). However, the faba bean variety had a significant impact on seed protein concentration. In particular the protein concentrations in dry seeds was significantly higher in ‘AUALEFKADAfb001’ and ‘AUAMANIfb001’ than in ‘Aquadulce’ and ‘AUAANDROSfb001’ in the 1st EY, and in ‘AUALEFKADAfb001’ than in ‘Aquadulce’ in the 2nd EY.
Figure 3. Influence of farming system (organic system vs. conventional system) and faba bean variety (‘Aquadulce’, ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, ‘AUAMANIfb001’) on protein content (%) in faba bean crop in both experimental years. For each factor, bars followed by different letters indicate significant differences according to the Duncan's multiple range test (P < 0.05). Vertical bars indicate the standard errors of the means.
Discussion
Rhizobial colonisation and nodulation in the roots of legumes may be negatively affected by inorganic nitrogen fertilisation (Lim et al. Citation2014). However, in the present study, the application of an inorganic N fertiliser in the conventionally treated plots had no negative effect on root nodulation in any of the evaluated varieties as shown in . The root biomass was significantly influenced by the faba bean variety. The highest root dry weight was recorded in ‘AUALEFKADAfb001’under both farming systems as shown in . Moreover, the application of an inorganic N fertiliser increased the percentage of Ndfa and the total amount of biologically-fixed N per cultivated area unit in some varieties. One explanation for the lack of any negative impact of inorganic N fertilisation on BNF is the low rate of inorganic-N supply. However, Mohamed and Babiker (Citation2012) found that the application of nitrogen fertilisation before sowing at a rate of 20 kg ha−1 enhanced the nodulation process in faba bean plants. Presumably, different cultivars respond differently to a particular inorganic-N application rate, as indicated by the interaction between the farming system and the variety for BNF. Indeed, ‘Aquadulce’ responded positively to the presence of some inorganic N in the root zone immediately after plant emergence in the conventional plots, as indicated by the higher values of dry biomass and BNF compared to the organic plots. In contrast, the Ndfa and the shoot biomass accumulation were not influenced by the farming system in ‘AUAANDROSfb001’, ‘AUALEFKADAfb001’, and ‘AUAMANIfb001’ as shown in . These results suggest that the three evaluated landraces can better adapt to low inorganic N rates during crop establishment that are commonly found in organically treated fields, without any negative repercussions to their final BNF performance. Nevertheless, the fresh pod yield obtained from ‘AUAANDROSfb001’ was lower in the organic than in the conventional treatments, despite the similar BNF performance in the two farming systems. The lower yield performance of ‘AUAANDROSfb001’ was presumably associated with the higher weed density in the organic plots.
Winter legumes such as faba bean, field pea and vetch can improve the soil fertility through their ability to fix atmospheric N2. Among these species, faba bean has been reported to possess the highest nitrogen fixation ability (Bilalis et al. Citation2012). The current study showed that faba bean is a legume with high capacity for N2 fixation since more than 75% of the tissue N content was found to derive from the atmosphere, thereby rendering a net atmospheric N2 input of 119 to 194 kg ha−1, depending on the combination of cropping system and variety. In a recent study conducted in Eastern Austria, Neugschwandtner et al. (Citation2015) also found a high nitrogen fixation capacity by faba bean which ranged from 63 to 219 kg N ha−1 in different years. Similarly, Amanuel et al. (Citation2000) reported input levels of biologically fixed nitrogen ranging from 139 to 210 kg N ha−1 in an experiment with faba bean conducted in Ethiopia.
With respect to the contribution of biological N2-fixation to the total N needs of the crop, the % Ndfa fluctuated between 76.7% and 91% in the present study. Amanuel et al. (Citation2000) found a lower contribution of BNF to faba bean nutrition, with % Ndfa values ranging from 58% to 74%. The varieties used and the different pedoclimatic conditions in the study of Amanuel et al. (Citation2000), particularly precipitation, soil moisture, and temperature levels, in comparison with those prevailing during our study may be responsible for these differences. Indeed, as reported by Collino et al. (Citation2015), the efficiency of BNF is influenced by environmental factors. In another study, Neugschwandtner et al. (Citation2015) found that drought restricted symbiotic N2 fixation.
Drought during flowering and pod formation of faba bean plants constrains its production (Neugschwandtner et al. Citation2015). In most regions around the Mediterranean basin, due to the climate change, drought occurs more frequently (Loizidou et al. Citation2016). For these reasons, it is extremely important to develop faba bean varieties that flower early in spring when grown under the Mediterranean semi-arid conditions. In particular, ‘AUAANDROSfb001’ required 4 and 15 days less to 50% flowering than the commercial variety and the landrace AUALEFKADAfb001, respectively. Additionally, the landraces AUAANDROSfb001 and AUAMANIfb001 required 9 days less to full ripening than the commercial variety as shown in . Especially, ‘AUALEFKADAfb001’, which exhibited also a relatively high BNF capacity, seems a promising landrace that might be exploited in breeding programmes to develop early flowering varieties without compromising yield.
Faba bean is a crop vulnerable to weed competition which can reduce yield up to 50% (Frenda et al. Citation2013). Consequently, effective weed management is crucial in faba bean crops to obtain high yield. Organic farming systems also require crop varieties with high competitive ability against weeds. Currently, published information on competitive ability of faba bean varieties is scarce. In the current study, no significant differences among the evaluated faba bean varieties were found with respect to weed competition as shown in . In a recent study conducted in Egypt, Spain and Tunisia by Rubiales et al. (Citation2014), several accessions and the variety Baraca proved to be the most resistant to Orobanche crenata, a weed species that severely infests faba bean crops. Several studies have shown significant differences in the competitive ability between different varieties in other legumes such as field pea and lentil (Tepe et al. Citation2005; Bilalis et al. Citation2015). In the present study, the lowest total weed density was also recorded under the conventional farming system, which indicates that pendimethalin provided adequate weed control.
Protein content, pod length and number of pods per plant were not significantly affected by the farming system as shown in and . Seed protein concentration in two out of the three evaluated landraces, particularly ‘AUALEFKADAfb001’ and ‘AUAMANIfb001’, was significantly higher than in the commercial variety. This finding indicates that these landraces have a potential to be used in breeding programmes aiming at increasing the protein production by faba bean. In a recent study conducted in Finland by Lizarazo et al. (Citation2015), differences in protein content between faba bean varieties and a significant year × variety interaction were found. According to these authors, these differences may be due to genotypic differences in nitrogen metabolism since most varieties showed lower protein concentration in the cooler than in the warmer years.
In conclusion, root biomass, %Ndfa, protein content, pod length, and total yield were significantly influenced by the faba bean varieties evaluated in the present study. The inorganic N fertilisation at the low rates applied to faba bean in the conventionally treated plots had no negative effect on root nodulation and biological N2 fixation. In contrast, the low availability of inorganic N during the initial developmental stage in the organic plots restricted the BNF performance in the commercial variety ‘Aquadulce’. In summary, the faba bean landraces evaluated in the present study proved to be interesting candidates for exploitation in breeding programmes due to their: earliness, higher adaptability to organic farming systems and the inherent low inorganic N availability in the soil at the initial cropping stage in these systems, high protein content (‘AUALEFKADAfb001’ and ‘AUAMANIfb001’), enhanced nitrogen fixation performance (‘AUALEFKADAfb001’) and high productivity (‘AUAANDROSfb001’) similar to that of the commercial variety.
Acknowledgements
We thank the technician A. Dimitriou for his support in preparing the experimental field and Prof. S.G. Alexandris from AUA for providing the climatic data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes on contributors
Dr. Georgia Ntatsi, was awarded a Diploma in Agriculture from the Agricultural University of Athens (AUA), a MSc Diploma from the University of Ioannina and a Ph.D. degree from the Laboratory of Vegetable Production, Agricultural University of Athens (AUA). She worked as Post-Doctorate Researcher (2013–2017) at the Laboratory of Vegetable Production of AUA and since the beginning of 2018 she is a researcher at the Institute of Plant Breeding and Genetic Resources-ELGO DIMITRA. She is specialized on hydroponics, vegetable grafting, greenhouse environment and its impact on vegetable production and quality, greenhouse gas emissions and biological nitrogen fixation. She has extensive experience in determination of inorganic nutrients in plant tissues and soil samples and quality parameters, greenhouse gases, biological nitrogen fixation, antioxidant parameters of vegetables, molecular biology techniques, metabolomics and bioinformatics tools. She has published 36 papers in refereed international scientific journals, 12 papers in congress proceedings (Acta Hort.), two book chapters and more than 60 abstracts in international conferences with more than 242 citations and an h-index 9. In the last 7 years Dr. Ntatsi has been involved as researcher and Project Manager in two European research projects (LEGUME FUTURES, EUROLEGUME), one LEONARDO Project related to plant irrigation and hydroponics (AGRICOM). She was also MC Member and Editorial Coordinator of the FA1204 COST Action. Since 2017 she is participating in two HORIZON2020 research projects, particularly TRUE, as WP Leader and Project Manager and TOMRES as Project Manager and one Project funded by the Greek Secretariat for Research and Technology (Plant-Up Upgrading the plant capital).
Dr. Anestis Karkanis is an Assistant Professor in Weed Science at the University of Thessaly. He graduated from the Agricultural University of Athens, while he received his Ph.D. from the same University in 2007. He has extensive experience in the weed biology, crop-weed interactions, weed management in field crops and vegetables, integrated weed management, pharmaceutical and nutritional value of weeds, new field crops, herbicide-resistant weeds and their management. He has participated and participates as scientifically responsible or associate in six funded research projects (etc. FP7, Horizon 2020) and has developed scientific collaborations with research teams at the University of Thessaly and at Agricultural University of Athens. He is supervisor of several BSc dissertations and MSc thesis, while is member of advisory and evaluation committee in several BSc dissertations, MSc thesis and one Ph.D thesis. He has taught undergraduate and postgraduate thematic units in Weed Science, Agricultural Pharmacology, Systematic Botany, Weed management, and Protocols & Programs in Plant Protection. He has authored 52 articles in peer-reviewed journals, 38 conference papers and 1 book chapter, with more than 370 citations and an h-index 10.
Mr. Dionisios Yfantopoulos (M.Sc.) is a PhD Student in Laboratory of Vegetable Production, Agricultural University of Athens. He was awarded a Diploma in Technological Educational Institute of Messolonghi - Department of Greenhouse Crops and Floriculture and an MSc Diploma from the Laboratory of Vegetable Production, Agricultural University of Athens (AUA). His main research interests include issues related to vegetable nutrition and fertilization, cultivation systems (greenhouse, field), crop rotation schemes, greenhouse gas emissions, biological nitrogen fixation and issues that relate to traditional varieties of vegetables. He has participated in three European research projects.
Dr. Margit Olle – senior researcher, Dr. Margit Olle graduated Estonian Agricultural University in 1993 (cum laude). She undertook her doctoral studies in 1995–1999 in Norway at the Agricultural University of Norway and defended there her Doctor Scientiarum degree. Since 2008, she has worked as a senior researcher at the Estonian Crop Research Institute (former Jogeva Plant Breeding Institute). She has published numerous review articles in addition to research articles in her field. She has published four books, out of them three in English and one in Estonian. She has been invited to speak at several organizations globally. Margit has received several awards, e.g. her biography is included in Who is Who in the World 32nd and 33rd editions, her CV is in the 2000 outstanding intellectuals of the 21st Century, 9th Edition and many others.
Dr. Travlos Ilias is an Assistant Professor of Agronomy & Weed Science of the Agricultural University of Athens. He graduated from the Agricultural University of Athens, while he received honored scholarships (2 years) from State Scholarships Foundation for his B.Sc. achievements. He received his Ph.D. from Agricultural University of Athens in 2005. He has also received three scholarships from Erasmus+ (Staff Mobility for Teaching). He is a member of the Organizing and Scientific Committees in national and international Scientific Symposia related with weeds. He is also a member of the board of European Weed Research Society (EWRS), Weed Science Society of America (WSSA), European Plant Science Organization (EPSO), Agronomy Society of America (ASA) and the president of Weed Science Society of Greece. He is the National Representative of Greece in EWRS. He has participated as a member of several scientific committees of WSSA and several working groups of EWRS. He has reviewed more than 100 papers and is/was an Associate Editor of several scientific journals. He has published more than 90 papers, including more than 50 papers in refereed international scientific journals, book chapters (invited) and many other papers in congress proceedings. Invited speaker in International Conferences and Invited Professor in European Universities. In the last 10 years, Dr. Travlos has been involved in five EU funded (among them EU INCO, LIFE 07, LIFE 09 and FP7) and many national and cooperative projects. Dr. Travlos specializes in integrated weed management systems, weed surveys, weed biology and reproduction, herbicide resistance, field crops and crop-weed interactions.
Dr. Ricos Thanopoulos is an agronomist working now at the Farm Unit of Agricultural University of Athens. He has graduated from the AUA Crop Science Department and worked initially as an extension adviser and then at the EU agro-environmental programmes of Ministry of Rural Development and Food. In parallel he has been involved in different research areas, such as agricultural entomology, pastures’ management, a field that he received his PhD, biological N-fixation, botany and recently genetic resources with emphasis on landraces. He has published referred articles and participated in national and international congresses.
Professor Dimitrios J. Bilalis, Agricultural University of Athens, is qualified with Ph.D., M.Sc., and B.Sc. His business address is: 75, Iera Odos str., GR 11855, Athens. His research and professional experience includes more than 22 years as an agronomist, Organic Agriculture, Innovative feed Crops, Weed management and Certification in Agriculture. His professional appointments include Professor of Agronomy and Organic Agriculture. The Honors received by him include Dr. Honoris Causa USAMV Cluj. The indicative publications of Professor Bilalis during the last 3 years are the following:
1. Tani E., Chachalis, D., Travlos, I.S., & Bilalis, D. (2016). Environmental conditions influence induction of key ABC-transporter genes affecting glyphosate resistance mechanism in Conyza canadensis. International Journal of Molecular Sciences 17 (4), DOI:10.3390/ijms17040342.
2. Travlos, I.S., Gkotsi, T., Roussis, I., Kontopoulou, C.K., Kakabouki, I., & Bilalis, D.J. (2017). Effects of the herbicides benfluralin, metribuzin and propyzamide on the survival and weight of earthworms (Octodrilus complanatus). Plant, Soil & Environment, 3, 117-124.
3. Travlos, I., Cheimona, N., & Bilalis, D. (2017). Glyphosate efficacy of different salt formulations and adjuvant additives on various weeds. Agronomy, 7(3), 60.
4. Bilalis, D., Tabaxi, I., Zervas, G., Tsiplakou E., Travlos I., Kakabouki, I., Tsioros, S. (2016). Chia (Salvia hispanica) Fodder Yield and Quality as Affected by Sowing Rates and Organic Fertilization . Communications in Soil Science and Plant Analysis. 47(15), pp. 1764-1770.
5. Vellios, E., Karkanis, A., Bilalis, D. (2017). Powdery mildew (Erysiphe cruciferarum) infection on camelina (Camelina sativa) under Mediterranean conditions and the role of wild mustard (Sinapis arvensis) as alternative host of this pathogen. Emirates Journal of Food and Agriculture 29(8), pp. 639-642.
Professor Penelope Bebeli is a Professor at the Laboratory of Plant Breeding and Biometry, Department of Crop Science, Agricultural University of Athens. Her work includes the assessment of genetic inter- and intra-accession diversity using morphological/agronomical traits and molecular markers and the evaluation and exploitation of the performance of these landraces under low input agriculture. She has been working on collection, characterization, evaluation, conservation of plant genetic resources, mainly crop landraces and crop wild relatives. She has participated in many European and National research projects particularly on plant genetic resources.
Professor Dimitrios Savvas is the Director of the Laboratory of Vegetable Production at the Agricultural University of Athens. He was awarded a Diploma in Agriculture from the Agricultural University of Athens (AUA) in 1985 and a Ph.D. degree from the University of Bonn, Germany in 1992. He specializes in plant nutrition with emphasis on soil nitrogen, biological N2-fixation, and soilless culture, abiotic stress physiology, and water management. He has published over 80 papers in refereed international scientific journals (with IF) and many other papers in congress proceedings (Acta Hort.) and as book chapters. He has an h-index of 19 in Scopus and 963 hetero-citations of his publications (updated Feb. 2018). He is a member of the Editorial Board in four international scientific journals with impact factor, particularly Scientia Horticulturae (I.F. 1.624 in 2016), Environmental & Experimental Botany (I.F. 4.369 in 2016), Agricultural Water Management (I.F. 2.848 in 2016) and European Journal of Horticultural Science (I.F. 0.446 in 2016). He has been involved in many EU research projects, either as coordinator or as a member of the research team. In the last 5 years, in addition to several national research projects, Professor Savvas has been involved in the European research projects LEGUME FUTURES (FP7, coordinator for AUA), SIRRIMED (FP7, member of the research team), EUROLEGUME (FP7, coordinator for AUA), TOMRES (HORIZON2020, coordinator for AUA) and TRUE (HORIZON2020, coordinator for AUA). A detailed CV of Professor Dimitrios Savvas is accessible at: http://www.ekk.aua.gr/index.php?sec=members&item=4&doc=2).
Additional information
Funding
References
- Abd-Alla MH, El-Enany AE, Hamada AM, Abdel Wahab AM. 2001. Element distribution in faba bean root nodules under salinity and its effects on growth, nodulation and nitrogen fixation. Rostl Výroba. 47(9):399–404.
- Amanuel G, Kühne RF, Tanner DG, Vlek PLG. 2000. Biological nitrogen fixation in faba bean (Vicia faba L.) in the Ethiopian highlands as affected by P fertilization and inoculation. Biol Fertil Soils. 32:353–359. doi: 10.1007/s003740000258
- Anderson JM, Ingram JSI. 1993. Tropical soil biology and fertility: a handbook of methods. Wallingford: C.A.B. International.
- Bedard-Haughn A, Van Groenigen JW, Van Kessel C. 2003. Tracing 15N through landscapes: potential uses and precautions. J Hydrol. 272:175–190. doi: 10.1016/S0022-1694(02)00263-9
- Bilalis D, Karkanis A, Papastylianou P, Patsiali S, Athanasopoulou M, Barla G, Kakabouki I. 2010. Response of organic linseed (Linum usitatissimum L.) to the combination of tillage systems, (minimum, conventional and no-tillage) and fertilization practices: Seed and oil yield production. Aust J Crop Sci. 4:700–705.
- Bilalis D, Karkanis A, Sidiras N, Travlos I, Efthimiadou A, Thomopoulos P, Kakabouki I. 2012. Maize and legumes root growth and yield as influenced by organic fertilization, under Mediterranean environmental conditions. Romanian Agric Res. 29:211–217.
- Bilalis D, Karkanis A, Travlos I, Antoniadis A, Ntatsi G, Bebeli P, Savvas D. 2015. Wild mustard (Sinapis arvensis L.) and corn poppy (Papaver rhoeas L.) competition with four pea varieties cultivated following conventional or organic farming practices. Bull UASVM Hortic. 72:443–444.
- Bouraoui M, Abbes Z, Rouissi M, Abdi N, Hemissi I, Kouki S, Sifi B. 2016. Effect of rhizobia inoculation, N and P supply on Orobanche foetida parasitising faba bean (Vicia faba minor) under field conditions. Biocontrol Sci Technol. 26:776–791. doi: 10.1080/09583157.2016.1157137
- Bouyoucos GJ. 1962. Hydrometer method improved for making particle size analysis of soils. Agron J. 54:464–465. doi: 10.2134/agronj1962.00021962005400050028x
- Bremer JM. 1960. Determination of nitrogen in soil by Kjedahl method. J Agric Sci. 55:1–23. doi: 10.1017/S0021859600021560
- Cazzato E, Tufarelli V, Ceci E, Stellacci AM, Laudadio V. 2012. Quality, yield and nitrogen fixation of faba bean seeds as affected by sulphur fertilization. Acta Agric Scand Sect. B-Soil Plant Sci. 62:732–738.
- Collino DJ, Salvagiotti F, Perticari A, Piccinetti C, Ovando G, Urquiaga S, Racca RW. 2015. Biological nitrogen fixation in soybean in Argentina: relationships with crop, soil, and meteorological factors. Plant Soil. 392:239–252. doi: 10.1007/s11104-015-2459-8
- Di Paolo E, Garofalo P, Rinaldi M. 2015. Irrigation and nitrogen fertilization treatments on productive and qualitative traits of broad bean (Vicia faba var. minor L.) in a Mediterranean environment. Legum Res. 38:209–218. doi: 10.5958/0976-0571.2015.00069.7
- Dubova L, Šenberga A, Alsina I. 2015. The effect of double inoculation on the broad beans (Vicia faba L.) yield quality. Res Rural Dev. 1:34–39.
- Frenda AS, Ruisi P, Saia S, Frangipane B, Di Miceli G, Amato G, Giambalvo D. 2013. The critical period of weed control in faba bean and chickpea in Mediterranean areas. Weed Sci. 61:452–459. doi: 10.1614/WS-D-12-00137.1
- Habtemichial KH, Singh BR, Aune JB. 2007. Wheat response to N2 fixed by faba bean (Vicia faba L.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid northern Ethiopia. J Nutr Soil Sci. 170:412–418. doi: 10.1002/jpln.200625006
- Hauggaard-Nielsen H, Mundus S, Jensen ES. 2009. Nitrogen dynamics following grain legumes and subsequent catch crops and the effects on succeeding cereal crops. Nutr Cycl Agroecosyst. 84:281–291. doi: 10.1007/s10705-008-9242-7
- Keeney DR, Nelson DW. 1982. Nitrogen-inorganic forms. In: AL Page, RH Miller, Keeney DR, editor. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Agronomy Monograph. 2nd ed. Madison (WI): ASA, SSSA; p. 643–698.
- Kontopoulou CK, Bilalis D, Pappa VA, Rees RM, Savvas D. 2015. Impact of organic farming practices and salinity on yield and greenhouse gas emissions from a common bean crop grown in a Mediterranean environment. Sci. Hortic. 183:48–57. doi: 10.1016/j.scienta.2014.12.012
- Köpke U. 1995. Nutrient management in organic farming systems: The case of nitrogen. Biol Agric Hortic. 11:15–29. doi: 10.1080/01448765.1995.9754690
- Lammerts van Bueren ET, Myers JR. 2011. Organic crop breeding: Integrating organic agricultural approaches and traditional and modern plant breeding methods. In: E.T. Lammerts van Bueren, J.R. Myers, editor. Organic Crop Breeding. Oxford: Wiley-Blackwell; p. 1–13.
- Lavania D, Siddiqui MH, Al-Whaibi MH, Singh AK, Kumar R, Grover A. 2015. Genetic approaches for breeding heat stress tolerance in faba bean (Vicia faba L.). Acta Physiol Plant. 37:1737. doi: 10.1007/s11738-014-1737-z
- Li L, Yang T, Liu R, Redden B, Maalouf F, Zong X. 2017. Food legume production in China. Crop J. 5(2):115–126. doi: 10.1016/j.cj.2016.06.001
- Lim CW, Lee YW, Lee SC, Hwang CH. 2014. Nitrate inhibits soybean nodulation by regulating expression of CLE genes. Plant Sci. 229:1–9. doi: 10.1016/j.plantsci.2014.08.014
- Link W, Ghaouti L. 2012. Faba Bean: Breeding for organic farming systems, in organic crop breeding. In: E.T. Lammerts van Bueren, J.R. Myers, editor. Organic Crop Breeding. Oxford: Wiley-Blackwell; p. 215–225.
- Lizarazo CI, Lampi AM, Liu J, Sontag-Strohm T, Piironen V, Stoddard FL. 2015. Nutritive quality and protein production from grain legumes in a boreal climate. J Sci Food Agric. 95:2053–2064. doi: 10.1002/jsfa.6920
- Loizidou M, Giannakopoulos C, Bindi M, Moustakas K. 2016. Climate change impacts and adaptation options in the Mediterranean basin. Reg Environ Chang. 16:1859–1861. doi: 10.1007/s10113-016-1037-9
- Maalouf F, Nachit M, Ghanem ME, Singh M. 2015. Evaluation of faba bean breeding lines for spectral indices, yield traits and yield stability under diverse environments. Crop Pasture Sci. 66:1012–1023. doi: 10.1071/CP14226
- Mohamed SSE, Babiker HM. 2012. Effects of Rhizobium inoculation and urea fertilization on faba bean (Vicia faba L.) production in a semi-desert zone. Adv Environ Biol. 6:824–830.
- Nelson DW, Sommers LE. 1982. Total carbon, organic carbon and organic matter. In: Page A.L., editor. Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties Second Edition. Madison, (WI, USA): American Society of Agronomy, Inc., Soil Science Society of America, Inc.; p. 539–579.
- Neugschwandtner R, Ziegler K, Kriegner S, Wagentristl H, Kaul HP. 2015. Nitrogen yield and nitrogen fixation of winter faba beans. Acta Agric Scand Sect B-Soil Plant Sci. 65:658–666.
- Rubiales D, Flores F, Emeran AA, Kharrat M, Amri M, Rojas–Molina MM, Sillero JC. 2014. Identification and multi-environment validation of resistance against broomrapes (Orobanche crenata and Orobanche foetida) in faba bean (Vicia faba). Field Crops Res. 166:58–65. doi: 10.1016/j.fcr.2014.06.010
- Sulas L, Roggero PP, Canu S, Seddaiu G. 2013. Potential nitrogen source from field bean for rainfed Mediterranean cropping systems. Agron J. 105:1735–1742. doi: 10.2134/agronj2013.0030
- Tampakaki AP, Fotiadis CT, Ntatsi G, Savvas D. 2017. Phylogenetic multilocus sequence analysis of indigenous slow-growing rhizobia nodulating cowpea (Vigna unguiculata L.) in Greece. Syst Appl Microbiol. 40:179–189. doi: 10.1016/j.syapm.2017.01.001
- Tepe I, Erman M, Yazlik A, Levent R, Ipek K. 2005. Comparison of some winter lentil cultivars in weed–crop competition. Crop Prot. 24:585–589. doi: 10.1016/j.cropro.2004.10.006
- Unkovich M, Herridge D, Peoples M, Cadish G, Boddey R, Giller K, Alves B, Chalk P. 2008. Measuring plant associated nitrogen fixation in agricultural systems. The website of the ACIAR http://aciar.gov.au/files/node/10169/mn136_measuring_plant_associated_nitrogen_fixation_19979.pdf.
- Van Zwieten L, Rose T, Herridge D, Kimber S, Rust J, Cowie A, Morris S. 2015. Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: dissection of causal mechanisms. Plant Soil. 375:7–20. doi: 10.1007/s11104-015-2427-3