ABSTRACT
The use of saline water for plant production will become increasingly necessary over future decades. In some cases, fruit quality such as in tomato, can be improved by irrigation with saline water. The influence of different salt concentrations on physiological responses and the expression of some selected genes of cherry tomato (Solanum lycopersicum L), cv. West Virginia 106, was examined. Tomato plants were grown in peatmoss substrate and irrigated with 0, 25, 50, 75, 100 or 150 mM sodium chloride (NaCl) in a glasshouse. The NaCl treatments of 75, 100 and 150 mM salt resulted in shorter plants, decreased stem width, a lower plant dry weight, fewer flowers, and smaller leaf area, while yield was reduced by treatment with concentrations of 50 mM NaCl and above. Average fruit weight and fruit number were also negatively affected by treatment with 50 mM salt and above. Salinity treatment led to increased fruit total soluble solids, titratable acidity and firmness and improved the taste index. Salt-responsive marker genes identified in Moneymaker were also induced in cherry tomato but not at the highest salt concentrations. Our results indicated that cherry tomato treated with 25 mM NaCl produced fruit with improved quality in comparison with non-salinized control plants without compromising yield, while at 50 and 75 mM the improved fruit quality was accompanied by a reduction in yield.
Introduction
Soil and water salinity have severe effects on crop growth and, in most cases, are associated with yield reduction. Plant growth is inhibited by salinity either through ion toxicity or disruption of osmotic functions (Boari et al. Citation2016). High concentrations of Na+ and Cl− ions in soil or water reduce water potential which reduces water uptake by plant roots and consequently net photosynthesis (Psarras et al. Citation2008). Tomato (Solanum lycopersicum L.) Requires adequate irrigation and nutrients for optimum growth and production. Salinity can have a negative effect on tomato yield via decreases in fruit weight (Cuartero and Fernández-Muñoz Citation1998) or number of fruit and marketable yield (Pengfei et al. Citation2017). Salinity also reduces fresh and dry shoot and root weights of tomato (Chaichi et al. Citation2017) and plant height, stem diameter and leaf number (Bao and Li Citation2010). Conversely, a beneficial effect of salinity on tomato fruit quality has been documented (Cuartero and Fernández-Muñoz Citation1998): some levels of salt improve quality by enhancing fruit flavour and levels of fruit sugar, total soluble solids and acidity (Del Amor et al. Citation2001; Campos et al. Citation2006), without affecting shelf life (Cuartero and Fernández-Muñoz Citation1998). Mizrahi (Citation1982) indicated that fruit shelf life and fruit firmness decrease at salinity levels above 100 mM NaCl while at 50 mM NaCl both traits remain unchanged (Cuartero et al. Citation1996). Plant salt tolerance is a complex trait involving various biochemical and physiological mechanisms. Identification of multiple genes whose expression allows plants to adapt to, or tolerate, some level of salt is essential for breeding improved varieties (Hurkman Citation1992). Wild, salt-tolerant tomato can be a source of alleles for improving cultivated tomato varieties: wild species such as Solanum pimpinellifolium are less sensitive to salt and show adaptations in terms of salt accumulation and expression of genes involved in sodium ion transport, salicylic acid signalling and detoxification (Sun et al. Citation2010). Very little has been reported on the influence of NaCl on growth, quality and postharvest behaviour of cherry tomato. Therefore, the objective of the study was to determine the influence of differing salinity levels on yield, fruit quality, storability and saline marker gene expression of glasshouse-grown cherry tomatoes to establish an estimate for the concentration which becomes limiting for growth and fruit quality before and after harvest and to identify usable physiological, or molecular markers, for effects of salinity in cherry tomato.
Materials and methods
Plant material, growth conditions and treatment
The study used cherry tomato, ‘West Virginia 106’ (‘WVa106’). Plants were grown in potting compost from October 2014 to March 2015 in a glasshouse in Avignon (southern France, 44°N). Seed were germinated in polystyrene trays containing commercial substrate (Seedling media, Klasmann-Deilmann GmbH, Geeste, Germany) on 10th October 2014. Seedlings were transplanted into pots (0.5 L volume) on 20th October 2014. Pots were arranged in a completely randomised block design. Five replicates for each treatment were used. Each replicate consisted of 3 plants. From the 21st October 2014, plants were irrigated with 0, 25, 50, 75, 100 or 150 mM NaCl (0.12, 0.26, 0.54, 0.81, 1.07, 1.58 Sm−1 respectively), with applications of 200 mL 3 times a week for each plant. On 24th November 2014, 15 young plants per treatment were randomly selected for morphological and genetic analysis and measurement of biomass. On 25th November 2014, the remaining tomato plants (15 plants per treatment) were transplanted into 4 L pots containing commercial growing substrate (Substrate 4, Klasmann-Deilmann GmbH). Flowers were mechanically pollinated 3 times a week. All plant side shoots were removed as they appeared. The electrical conductivity (EC) of the soil was measured weekly using an electrical conductivity metre (Grodan, Model Sensor 300, Baud, Netherlands).
Evaluation of biomass and growth parameters
Plant height, stem diameter, number of leaves, fresh weight, dry weight, number of flowers and total leaf area per plant were measured 24 d after the start of salt treatment. Plant height was measured from the main stem base to the apical growing tip, and stem diameter was measured at the internode above the cotyledons with digital callipers (Minimax Mauser digital, Nancy, France). The plants were cut above the surface of the substrate and plant fresh weight was determined. To determine the dry weight, leaves and stems were dried in a forced air oven at 60°C until a constant mass was reached. The total number of flowers per plant was counted. Total leaf area was measured by digital image analysis with the ImageJ software as described by O’Neal et al. (Citation2002). Individual leaves were scanned and saved in digital format using an HP Scan jet G4010 desktop scanner. The preliminary image was converted from colour to greyscale. The highlight and shadow levels within the exposure adjustment were manipulated to create a black image on a white background. A scale of known dimensions was included within the image for calibrating pixel conversion.
Chlorophyll and fluorescence measurements
Chlorophyll content was measured at 30, 37, 43 and 60 d from the start of salt treatment. Measurements were made with a Minolta Chlorophyll metre (SPAD-502, Minolta Camera, Co. Ltd., Osaka, Japan). Ten different positions on a single plant were randomly measured. The mean value was calculated directly by the SPAD metre. Chlorophyll fluorescence measurements on the upper surface of leaves of intact plants were performed 30, 37, 43 and 60 d from the start of salt treatment using a Handy Plant Efficiency Analyser (PEA) (Hansatech Instruments Ltd, Kings Lynn, Norfolk, UK). The variable to maximum fluorescence ratio (Fv/Fm) was calculated. This ratio, measured in the non-energized state after 40 min of adaptation to darkness, is a reliable measure of the maximal (potential) efficiency of excitation capture by open PSII in dark-adapted conditions. The Fv/Fm is used to estimate the functional state of the photosynthetic apparatus in a given environmental situation (Krause and Weis Citation1991). A decrease in Fv/Fm indicates photo-inhibition of PSII.
Ascorbic acid determination
Measurement of total and reduced ascorbic acid content was carried out on leaves at 54 d after the start of treatment as described by Stevens et al. (Citation2006) on material conserved at −80°C.
Gene expression
The expression of potential salt marker genes was carried out on young leaves. Total RNA was isolated from ground powder conserved at −80°C from young mature leaves (200 mg) 2 weeks after the start of salt treatment using phenol–chloroform extraction (Garchery et al. Citation2013). Samples were homogenised with 1 mL of TRI Reagent Solution (Euromedex, Souffel Weyersheim, France) and processed as per the manufacturer’s instructions. Pellets were dissolved in 50 µL of RNAse-free water and stored at −20°C before reverse transcription. To eliminate DNA contamination, 10 µg of total RNA was treated with RQ1 RNAse-free DNAse (Promega, Charbonnieres, France). Reverse transcription was performed with 1 µg of DNA-free RNA, and treated with Rnasin (Promega) to inhibit RNAse activity, using 1 µL of 10 µM oligo (dT)21 and Super ScriptII Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. The cDNA obtained was diluted 5-fold in water and 2 µL aliquots were used. Quantitative real-time PCR (RT-qPCR) analyses were performed with a StratageneMx3005P thermocycler (Stratagene, Cedar Creek, TX) using the Gotaqq PCR Master Mix (Promega) according to the manufacturer’s instructions in a reaction volume of 15 µL. Relative gene expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen Citation2001) using gene-specific primers (). The results were calculated as relative expression compared to a given reference gene.
Table 1. Marker and reference gene primers for RT-qPCR.
Yield and fruit quality measurements
For measurements related to fruit quality, 20 fruit with full red colour were collected randomly from each replicate (plant) and from each cluster to determine fruit weight and diameter. Total yield was determined by calculating the total fruit weight from all clusters.
Evaluation of postharvest quality
About 200 g (per replicate) of fully ripe fruit free of defects and diseases were stored at 20 ± 2°C for 7 d in plastic boxes and several characteristics were measured. Fruit were weighed at the beginning and end of the storage period to determine the weight lost compared to the initial weight. Fruit skin colour was measured with a Minolta (Model CR-400, Konica Minolta, Tokyo, Japan) on 20 fruit per treatment, using the CIELAB colour parameters lightness (L*), a*, b*, chroma (C*) and hue angle (h°). Each measurement was taken at 3 locations for each fruit. A standard white calibration plate was employed to calibrate the colorimeter. The internal quality attributes measured were: total soluble solids content (TSS), titratable acidity (TA), and pH of tomato juice. The TSS of the juice was measured using a digital refractometer (model PR101, Atago (0–45%) Palette Co. Ltd., Tokyo, Japan). The TSS of the first and fourth clusters was measured separately. For the fourth cluster, the TA of the tomato juice was measured using a Compact Titrator (Crison, Barcelona, Spain) and determined by titrating 3 g (diluted with 50 mL distilled water) of juice with 0.1 mol L−1 of sodium hydroxide to an end point of pH 8.1 and expressed in percentage (%). The pH of the juice was determined using a pH-metre (Five easy, TM FE20 mettler, Toledo AG, Schwerzenbach, Switzerland). For each treatment, 2 juice samples from 10 fruit were prepared and 3 readings taken. Fruit internal quality was determined before and after storage. Taste and maturity indices were calculated using the equation described by Pezzarossa et al. (Citation2014) using percentage of TSS and the titratable acidity in the fourth cluster. Fruit firmness was analysed by using a Texture Analyzer (Agra Technologie, Apollinaire Technologie, Serqueux, France) with a 5 mm diameter flat probe. Firmness values, from 5 fruit per replicate, were measured at 3 points on the equatorial region. Total yield per plant was determined from 5 separate harvests, each harvest being carried out at the full red stage.
Statistical analysis
The data were subjected to analysis of variance using SPSS for Windows (ver. 16.0, SPSS Inc., Chicago, IL, USA). Means were separated using Tukey’s multiple range test.
Results
Salt affects biomass and physiological traits
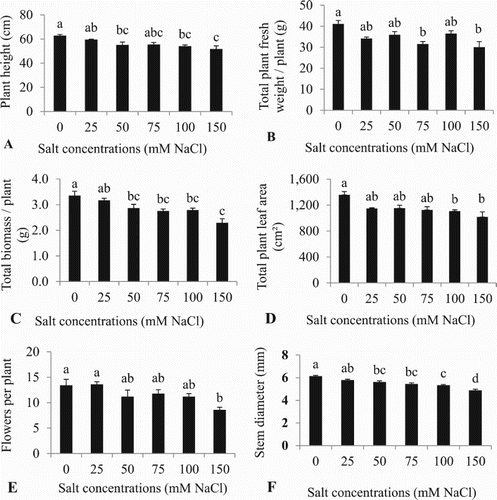
Plant height and stem diameter were traits most sensitive to salt with symptoms appearing at 50 mM NaCl with reductions of 12.1% in plant height and 8.1% of stem diameter compared to the control ((A) and (F)). At the 75 mM salt concentration, negative effects occurred in plant fresh and biomass ((B) and (C)). Total leaf area and flowers number were significantly decreased at higher salt concentrations (100mM and 150 mM) ((D) and (E)) compared to controls. Number of leaves, SPAD reading and chlorophyll fluorescence were not affected by salt treatment (data not shown).
Figure 1. Effect of different NaCl concentrations on: (A) plant height, (B) total plant fresh weight, (C) total biomass, (D) total plant leaf area (E), flowers per plant, and (F) stem diameter of cherry tomato plants at 24 d from start of treatment. Data are mean ± SE of 5 replicates. Different letters indicate significant differences (Tukey Test, P < 0.05%).
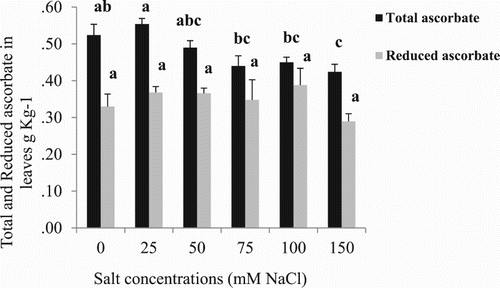
Ascorbic acid levels in leaves
Increasing salinity levels caused a reduction in total ascorbic acid while reduced ascorbic acid was not affected, indicating the differences must be due to differences in dehydroascorbate content (). The reduction between 0 and 150 mM NaCl was 19.4%. At 75, 100 and 150 mM salt levels, decreases in ascorbate occurred compared with 25 mM NaCl.
Effects of salt on yield and fruit quality characteristics
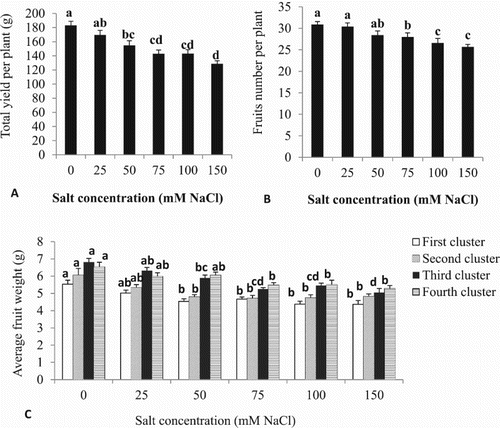
Fruit yield per plant was reduced as salt concentrations increased compared to controls ((A)). Fruit yield decreased beginning with the 50 mM treatment. The salinity concentrations of 75, 100 or 150 mM NaCl caused decreases in total yield per plant of 21.9, 21.8 or 30.1%, respectively, compared to the control. No differences were apparent when the highest salt concentrations were compared. Mean fruit weight and number of fruits were affected by salinity levels ((B) and (C)). Fruit from the first and second clusters weighed less than fruit from the third and fourth clusters. Average fruit weight was affected at 50 mM NaCl, and above compared to the control ((C)). Fruit diameter was constant in all clusters under different salt treatments and ranged from 20–23 mm (data not shown). The interaction between the effects of salinity and cluster positions on fruit number was significant (F = 1.95, p = 0.02).
Figure 3. Effect of different NaCl concentrations on (A) total yield/plant, (B) fruits number/plant, and (C) average fruit weight of cherry tomato. Data are mean ± SE of 10 replicates. Different letters indicate significant differences (Tukey Test, P < 0.05%).
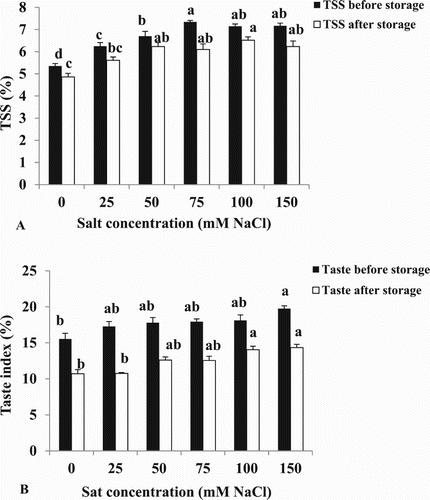
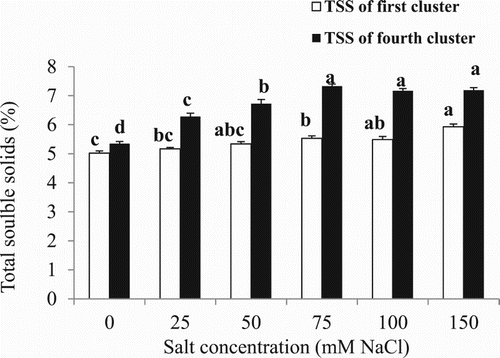
The total soluble solids (TSS) of fully ripe red fruit of saline treated plants was higher than that of fruit of control plants from the 25 mM salt treatment for the fourth cluster and higher from 75 mM for the first and fourth clusters (). A two-way ANOVA was conducted to examine effects of salinity levels and cluster positions on TSS. There was a significant interaction between effects of salinity levels and cluster positions on TSS (F = 16.60, p = 0.00). The TSS of the fruit of the fourth cluster was higher than that of the fruit from the first cluster ().
Figure 4. Effect of different NaCl concentrations on total soluble solids of cherry tomato fruit of the first and fourth cluster. Data are mean ± SE of 10 replicates. Different letters indicate significant differences (Tukey Test, P < 0.05%).
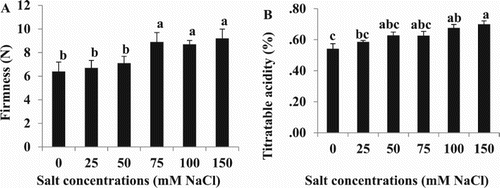
Firmness was increased at salinity levels of 75 mM NaCl and above, compared to the control or salt concentrations of 25 or 50 mM ((A); results from fourth cluster). Fruit from the fourth cluster of salt treated plants showed increases in titratable acidity (TA) at 100 mM salt and above ((B)).
Effect of salt on postharvest quality of cherry tomato
The effects of salinity on fruit quality were measured before and after storage. TSS values increased at 25 mM NaCl before storage and at 50 mM NaCl after storage ((A)). There were no differences between 75, 100 or 150 mM NaCl on TSS. The taste index was increased as salt concentrations increased before or after storage at 150 mM NaCl ((B)). Weight loss, percentage of fruit showing signs of decay, colour and the TSS: acidity ratio were not affected by salinity (data not shown).
Gene expression
With the aim of identifying one or several marker genes for salt stress, we interrogated the Genevestigator database (www.genevestigator.com) and searched for salt-responsive and non-responsive (control) genes from transcriptome data from a 200 mM salt-stress experiment carried out on the Moneymaker variety. For this we used the ‘RefGenes’ function, genes were chosen based on the stability (Q403, Q409 and Q410, used as reference genes), or difference (Q404, Q405, Q406, Q407, Q408), of their expression under control and salt conditions. High, low and medium-expressed genes were selected. shows the primer pairs used and the function of each gene.
Table 2. Encoded function of genes used in RT-qPCR.
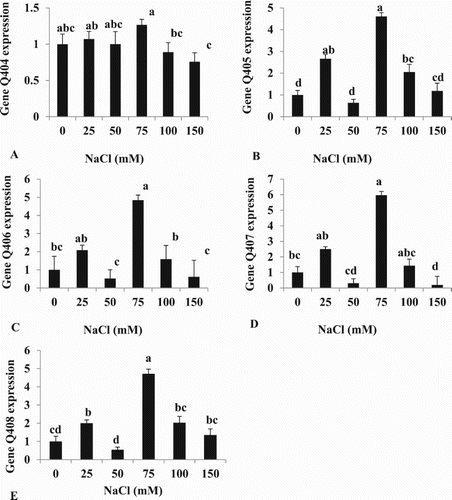
Under our experimental conditions, of the 5 genes responding to salt in Moneymaker that were chosen, gene expression in leaves was higher compared to controls at 75 mM salt, but did not continue to increase at higher salt levels (). All the genes showed a highly similar profile, except for gene Q404 which distinguished the different salt concentrations less than the other genes under test. The salt concentrations of 25 and 100 mM NaCl gave higher expression for certain genes (Q405 and Q408) compared to control conditions (). The gene expression at 50 or 150 mM NaCl was not different for the salt marker genes chosen, except for Q407 which decreased compared to controls at 150 mM. No gene discriminated the control condition from salt conditions in our experiment.
Figure 7. mRNA levels for genes coding for: (A) Q404, (B) Q405, (C) Q406, (D) Q407 and (E) Q408 (salt-activated genes) in young cherry tomato leaves under different NaCl levels. The mean of Q403, Q409 and Q410 non-responsive salt genes were used as reference genes. Total RNA was extracted from tomato leaves, converted to cDNA and subjected to comparative real-time RT–PCR quantification. Relative expression levels were calculated by 2-ΔΔCT (ddCt) method. Bars show mean + SE (n = 5). Different letters indicate significant differences (Tukey Test, P < 0.05%).
Discussion
Previous studies have examined effects of saline irrigation on tomato plant growth and productivity, and reported that salinity detrimentally affects yield, quality and postharvest behaviour of tomato (Bao and Li Citation2010). Most of these studies have been on standard size tomato. In this experiment, we studied the effects of a range of salt concentrations on physiological and post-harvest characteristics and genetic responses of cherry tomato fruit grown under glasshouse conditions because previous studies on cherry tomatoes are limited.
Our results indicated that most of the vegetative growth traits were negatively affected by salt treatment. Our results shown in (A, B, and C) agree with Assimakopoulou et al. (Citation2015), Zhang et al. (Citation2017), and Pengfei et al. (Citation2017) who found that plant height, fresh weight, and biomass in cherry tomato decreased with increasing salt levels. We have also shown in this study ((D)) that total plant leaf area decreased significantly at 100 and 150 mM NaCl. This result is in agreement with the suggestion by Bacha et al. (Citation2017), that leaf area was decreased significantly at 150 mM NaCl in tomato due to the reduction of leaf gas exchange under salinity stress conditions. Our results also showed that a high level of salinity (150 mM NaCl) reduced the number of flowers per plant ((E)). Flower loss or drop in salty conditions may be due to the restriction of water supply before, and during, inflorescence initiation (Saito and Ito Citation1967), or a reduction in potassium levels (Besford and Maw Citation1975) and phosphorus uptake (Menary and Stalen Citation1976). As observed in (F), the stem diameter trait was affected negatively by saline conditions, this could be explained by the occurrence of ionic toxicity and nutritional imbalance due to the extreme accumulation of certain ions such as Cl∼ in plant tissues (Munns and Tester Citation2008). The negative effects of salinity on vegetative growth could also be due to ion toxicity, such as Na+ and Cl−, and creation of an ionic imbalance (Chaichi et al. Citation2017), restriction of root cell growth (Cuartero and Fernández-Muñoz Citation1998), a reduction in photosynthesis (Psarras et al. Citation2008), and restriction of plant growth (Zhu Citation2002).
Ascorbic acid is considered to be one of the most important components of plant antioxidant systems. Our results in support our hypothesis that ascorbic acid content in leaves could be an indicator for salt stress. Azuma et al. (Citation2010) and the present study indicated that the ascorbic acid content decreased at the highest NaCl level (100 mM NaCl in their study and 150 mM in our study) compared to the lower NaCl level. Also, reduced ascorbic acid content in leaves was not affected by our salt treatment so by deduction it is the dehydroascorbic acid that was affected, particularly at the highest salt concentration of 150 mM compared to 25 mM indicating at high salt concentrations the plant is under oxidative stress. Other studies in tomato have shown correlations between the ascorbate pool and stress conditions (Stevens et al. Citation2008). However, Gong et al. (Citation2013) found that ascorbic acid in tomato leaves was increased with increasing salt stress from 0 to 50 mM NaCl and became almost constant at 75 and 100 mM NaCl after 5 and 10 days of treatment. The differences with our results may be due to the fact that our ascorbic acid measurement was carried out after 54 days of salt treatment. Thus, the decline of ascorbic acid was observed during a long exposure to salinity stress.
In this study, the highest yield per plant was obtained with the control treatment, while the lowest values were obtained with the highest salt treatments. The results in (A) support our hypothesis that under greenhouse conditions, it is possible to irrigate tomato plants with saline water at a concentration of 25 mM NaCl without significant yield reduction as well as improving fruit quality. Similar results have been recorded by Del Amor et al. (Citation2001). In another study carried out by Mitchell et al. (Citation1991), it was found to be possible to irrigate tomato plants with saline water (8 dS m−1) with a minor loss of yield. The difference from our findings might be due to different sensitivity of some tomato cultivars compared to others. Our results in (A, B, and C) indicated that the reduction in cherry tomato total yield was due to lower fruit number per plant combined with lower fruit weight under saline conditions (Psarras et al. Citation2008). The same results have been obtained in previous studies such as Zhang et al. (Citation2017) in tomato and Huang et al. (Citation2016) and Pengfei et al. (Citation2017) in cherry tomato. Tomato exhibits yield reduction at salinity levels from 25 to 75 mM NaCl (Reina-Sánchez et al. Citation2005 and results from this study). Our results also agree with Bao and Li (Citation2010) who reported that yield loss was most apparent in the flowering- and fruit-bearing stages, and the expansion stage of first cluster fruit. Tomato fruit yield can be reduced by a decrease in fruit weight, and/or, the number of fruit produced per plant (Cuartero and Fernández-Muñoz Citation1998) or a reduction in flower number (Magan et al. Citation2008). In Bao and Li (Citation2010) and our study, individual fruit weight and fruit number/plant ((B) and (C)) were decreased under higher salt levels compared with the non-saline control treatment. These results could be explained by that fact that high salt levels decrease water potential in plants which reduces water flow into fruit and limits the rate of fruit expansion (Johnson et al. Citation1992; Al-Ismaily et al. Citation2014). In addition, decreased mean fruit weight due to high salt levels could be also explained by an accumulation of Na in plant tissue (Incrocci et al. Citation2006).
As in this study, previous studies have shown that fruit quality is affected in fruit from tomato plants irrigated with saline water: this includes fruit total soluble solids (TSS), pH and titratable acidity (Cuartero and Fernández-Muñoz Citation1998). Tomato fruit quality therefore increased under saline irrigation treatments (Bao and Li Citation2010).
Our results in confirmed that TSS increased by irrigation with saline water. This finding is supported by previous studies (Del Amor et al. Citation2001; Wu et al. Citation2004; Psarras et al. Citation2008; Ruiz et al. Citation2015; Huang et al. Citation2016). Previous studies mention that increasing TSS might be due to a reduction in fruit water content by irrigation with saline water (Del Amor et al. Citation2001; Al-Ismaily et al. Citation2014). On the other hand, Krauss et al. (Citation2006) suggested that increasing TSS could be explained by increases in osmotically effective metabolites with increasing salt levels. Previous studies also mentioned that increasing TSS plays a role in adaptation of plants to salinity. Our present study suggested that the TSS of the fourth cluster was higher than that of first cluster fruits and this result could be due to increasing salinity stress with a prolonged stress period which forces the plant to accumulate more TSS in tissues to adapt to the salinity level (Krauss et al. Citation2006).
Our findings in (A) showed that cherry tomato firmness increased with increasing water salinity at concentrations of 75 mM or above. The increase in firmness, in our study, may be due to salinity strengthening tomato skin and increasing its thickness (Ruiz et al. Citation2015). Petersen et al. (Citation1998) found that the pericarp of tomatoes grown at increased salinity consisted of smaller cells with thicker walls. This could explain the increase in fruit firmness related to irrigation by saline water and supports our hypothesis.
Our results in (B) supported the hypothesis that titratable acidity increased with high salinity levels (100 and 150 mM NaCl in our study). The same trend was reported by Campos et al. (Citation2006) and Segura et al. (Citation2009). Increases in titratable acidity of tomato fruit juice could be due to higher Na+ and/or Cl− contents in the juice which was increased by salt treatments (Del Amor et al. Citation2001).
There was no effect of salinity on percent weight loss after harvest (data not shown). In our study as shown in (A) and (B), the TSS and taste index decreased after storage; this observation may be explained by the utilisation of sugars in fruit respiration (Pelayo et al. Citation2003).
The final part of our study aimed at identifying a gene whose expression was correlated with observed phenotypes and could be used as a marker for salt stress (). The genes chosen were identified in a previous experiment on tomato as being induced by 200 mM salt in Moneymaker. In our experiment the expression of these genes peaked at 75 mM salt concentrations before decreasing back to control levels at higher concentrations. No gene distinguished salt conditions from control conditions. The two most responsive genes, Q405 and Q408 ( and ) encoded a circadian clock coupling factor (Q405) and an ethylene responsive transcription factor 1a (Q408). Therefore the selection of these genes in Moneymaker based on a single salt concentration was not relevant for a range of salt concentrations and therefore their expression was not directly correlated to external salt concentrations. Therefore to fulfil our original quest we would need to carry out further expression analysis using different datasets and/or on larger number of genes. Some candidates could include genes involved in the salt over sensitive pathway which is more active in wild, salt-tolerant tomato than cultivated varieties such as Moneymaker (Sun et al. Citation2010). Other genes induced in salt tolerant tomato in this study included lactolylglutathione lyases and a salicylic acid binding protein.
In conclusion, salinity treatment negatively affects cherry tomato plant growth and yield, but improves tomato fruit quality. Cherry tomato plants can be irrigated with saline water at 0.26 S m−1 (25 mM) without affecting yield and this concentration leads to improvement in fruit quality. Furthermore, use of saline water enhanced tomato fruit quality without affecting storage ability. The most sensitive markers for salt stress are stem width and plant height and the expression of certain genes could be used as biomarkers for resistance to some salt levels but this objective needs further study.
Acknowledgements
The French Institute, Science and Technology Development Fund (Egypt, IMHOTEP Project 33134RF), Institut National de la Recherche Agronomique (INRA, France) and the Faculty of Agriculture, Cairo University are acknowledged for supporting this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mohamed M. El-Mogy is an Associate Professor at the Department of Vegetable Crops, Faculty of Agriculture, Cairo University, Giza, Egypt. Her research interests are production, breeding, and post-harvest technology of most vegetable crops (leafy crops, root crops, legumes, onions, garlic, strawberry, melon, watermelon, pepper, cucumber, etc).
Cecile Garchery is an Assistant Engineer at GAFL Unit, Genetics and Breeding of fruit and Vegetables, INRA, Avignon, France. Her research interests are genetic and molecular basis of fruit quality, functional characterization of plant–pathogen interactions and sustainable resistance management, integration of disease resistance and quality fruit in cultivars.
Rebecca Stevens is a researcher at GAFL Unit, Genetics and Breeding of fruit and Vegetables, INRA, Avignon, France. Her research interests are genetic and molecular basis of fruit quality, functional characterization of plant–pathogen interactions and sustainable resistance management, integration of disease resistance and quality fruit in cultivars.
ORCID
Mohamed M. El-Mogy http://orcid.org/0000-0001-7598-7557
Additional information
Funding
References
- Al-Ismaily SS, Al-Yahyai RA, Al-Rawahy SA. 2014. Mixed fertilizer can improve fruit yield and quality of field-grown tomatoes irrigated with saline water. J Plant Nutr. 37:1981–1996. doi: 10.1080/01904167.2014.920364
- Assimakopoulou A, Nifakos K, Salmas I, Kalogeropoulos P. 2015. Growth, ion uptake, and yield responses of three indigenous small-sized Greek tomato (Lycopersicon esculentum L.) Cultivars and four hybrids of cherry tomato under NaCl salinity stress. Commun Soil Sci Plant Anal. 46:2357–2377. doi: 10.1080/00103624.2015.1081924
- Azuma R, Ito N, Nakayama N, Suwa R, Nguyen NT, Larrinaga-Mayoral JA, Esaka M, Fujiyama H, Saneoka H. 2010. Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annuum L.). Sci Hortic. 125:171–178. doi: 10.1016/j.scienta.2010.04.006
- Bacha H, Tekaya M, Drine S, Guasmi F, Touil L, Enneb H, Triki T, Cheour F, Ferchichi A. 2017. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. S Afr J Bot. 108:364–369. doi: 10.1016/j.sajb.2016.08.018
- Bao H, Li YX. 2010. Effect of stage-specific saline irrigation on greenhouse tomato production. Irrig Sci. 28:421–430. doi:10.1007/s00271-009-0204-x.
- Besford RT, Maw GA. 1975. Effect of potassium nutrition on tomato plant growth and fruit development. Plant Soil. 42:395–412. doi:10.1007/BF00010015.
- Boari F, Donadio A, Pace B, Schiattone MI, Cantore V. 2016. Kaolin improves salinity tolerance, water use efficiency and quality of tomato. Agric Water Manag. 167:29–37. doi:10.1016/j.agwat.2015.12.021.
- Campos CAB, Fernandes PD, Gheyi HR, Blanco FF, Campos SAF. 2006. Yield and fruit quality of industrial tomato under saline irrigation. Sci Agric. 63:146–152. doi:10.1590/S0103-90162006000200006.
- Chaichi MR, Keshavarz-Afshar R, Lu B, Rostamza M. 2017. Growth and nutrient uptake of tomato in response to application of saline water, biological fertilizer, and surfactant. J Plant Nutr. 40(4):457–466. doi: 10.1080/01904167.2016.1246567
- Cuartero J, Baena J, Soria T, Fernandez-Munoz R. 1996. Evolucion de la dureza del fruto del tomate, como un componente de la calidad, en cultivares de larga duracion y normales cultivados en concentraciones salinas. (Evolution of the hardness of the fruit of the tomato, as a component of the quality, in cultivars of long duration and normal cultivated in saline concentrations). Actas de Horticultura. 13:59–65.
- Cuartero J, Fernández-Muñoz R. 1998. Tomato and salinity. Sci Hortic. 78:83–125. doi:10.1016/S0304-4238(98)00191-5.
- Del Amor FM, Martinez V, Cerda A. 2001. Salt tolerance of tomato plants as affected by stage of plant development. HortScience. 36:1260–1263.
- Garchery C, Gest N, Do PT, Alhagdow M, Baldet P, Rothan C, Massot C, Gautier H, Aarrouf J, Fernie AR, Stevens R. 2013. A diminution in ascorbate oxidase activity affects carbon allocation and improves yield in Tomato under water deficit. Plant, Cell Environ. 36:159–175. doi: 10.1111/j.1365-3040.2012.02564.x
- Gong B, Wena D, VandenLangenberg K, Wei M, Yang F, Shia Q, Wang X. 2013. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic. 157:1–12. doi: 10.1016/j.scienta.2013.03.032
- Huang C, Peng F, You Q, Xue X, Wang T, Liao J. 2016. Growth, yield and fruit quality of cherry tomato irrigated with saline water at different developmental stages. Acta Agr Scand B S P Sci. 66(4):317–324.
- Hurkman WJ. 1992. Effect of salt stress on plant gene expression: a review. Plant Soil. 146:145–151. doi:10.1007/BF00012007.
- Incrocci L, Malorgio F, Bartola AD, Pardossi A. 2006. The influence of drip irrigation or subirrigation on tomato grown in closed-loop substrate culture with saline water. Sci Hortic. 107:365–372. doi: 10.1016/j.scienta.2005.12.001
- Johnson RW, Dixon MA, Lee DR. 1992. Water relations of the tomato during fruit growth. Plant, Cell Environ. 15:947–953. doi:10.1111/j.1365-3040.1992.tb01027.x.
- Krause GH, Weis E. 1991. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 42:313–349. doi:10.1146/annurev.pp.42.060191. 001525 doi: 10.1146/annurev.pp.42.060191.001525
- Krauss S, Schnitzler WH, Grassmann J, Woitke M. 2006. The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J Agric Food Chem. 54:441–448. doi: 10.1021/jf051930a
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using RealTime quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi:10.1006/meth.2001. 1262 doi: 10.1006/meth.2001.1262
- Magan JJ, Gallardo M, Thompson RB, Lorenzo P. 2008. Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in greenhouses in Mediterranean climatic conditions. Agric Water Manag. 95:1041–1055. doi:10.1016/j.agwat.2008.03.011.
- Menary RC, Staden JV. 1976. Effects of phosphorus nutrition and cytokinins on flowering in the tomato, Lycopersicon esculentum Mill. Aust J Plant Physiol. 3:201–205. doi: 10.1071/PP9760201.
- Mitchell JP, Shennan C, Grattan SR, May DM. 1991. Tomato fruits yields and quality under water deficit and salinity. J Amer Soc Hort Sci. 116:215–221.
- Mizrahi Y. 1982. Effect of salinity on tomato fruit ripening. Plant Physiol. 69:966–970. doi:10.1104/pp.69.4.966.
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911
- O’Neal ME, Landis DA, Isaacs R. 2002. An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Econ Entomol. 95:1190–1194. doi:10.1603/0022-0493-95.6.1190.
- Pelayo C, Ebeler SE, Kader AA. 2003. Postharvest life and flavor quality of three strawberry cultivars kept at 5°C in air or air + 20 kPa CO2. Postharvest Biol Technol. 27:171–183. doi:10.1016/S0925-5214(02)00059-5.
- Pengfei Z, Yanyan D, Masateru S, Natsumi M, Kengo I. 2017. Interactions of salinity stress and flower thinning on tomato growth, yield, and water use efficiency. Commun Soil Sci Plant Anal. 48(22):2601–2611.
- Petersen KK, Willumsen J, Kaack K. 1998. Composition and taste of tomatoes as affected by increased salinity and different salinity sources. J Hortic Sci Biotechnol. 73(2):205–215. doi:10.1080/14620316.1998.11510966.
- Pezzarossa B, Rosellini I, Borghesi E, Tonutti P, Malorgio F. 2014. Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci Hortic. 165:106–110. doi:10.1016/j.scienta.2013.10. 029 doi: 10.1016/j.scienta.2013.10.029
- Psarras G, Bertaki M, Chartzoulakis K. 2008. Response of greenhouse tomato to salt stress and K+ supplement. Plant Biosyst. 142:149–153. doi:10.1080/11263500701872903.
- Reina-Sánchez A, Romero-Aranda R, Cuartero J. 2005. Plant water uptake and water use efficiency of greenhouse tomato cultivars irrigated with saline water. Agric Water Manag. 78:54–66. doi:10.1016/j.agwat.2005.04.021.
- Ruiz MS, Yasuor H, Ben-Gal A, Yermiyahu U, Saranga Y, Elbaum R. 2015. Salinity induced fruit hypodermis thickening alters the texture of tomato (Solanum lycopersicum Mill) fruit. Sci Hortic. 192:244–249. doi:10.1016/j.scienta.2015.06.002.
- Saito T, Ito H. 1967. Studies on the growth and fruiting in tomato X. Effects of early environmental conditions and cultural treatments on the morphological and physiological development of flower and flower drop 2. Effect of watering, defoliation and application of gibberellin. J Jpn Soc Hortic Sci. 36:281–289. doi: 10.2503/jjshs.36.281
- Segura ML, Contreras JI, Salinas R, Lao MT. 2009. Influence of salinity and fertilization level on greenhouse tomato yield and quality. Commun Soil Sci Plant Anal. 40:485–497. doi: 10.1080/00103620802697764
- Stevens R, Buret M, Garchery C, Carretero Y, Causse M. 2006. Technique for rapid, small-scale analysis of Vitamin C levels in fruit and application to a Tomato mutant collection. J Agric Food Chem. 54:6159–6165. doi:10.1021/jf061241e.
- Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M. 2008. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 31(8):1086–1096. doi:10.1111/j.1365-3040.2008.01824.x.
- Sun W, Xu X, Zhu H, Liu A, Liu L, Li J, Hua X. 2010. Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol. 51(6):997–1006. doi: 10.1093/pcp/pcq056
- Wu M, Buck JS, Kubota C. 2004. Effects of nutrient solution EC, plant microclimate and cultivars on fruit quality and yield of hydroponic tomatoes (Lycopersicum esculentum L.). Acta Hortic. 659:541–547. doi: 10.17660/ActaHortic.2004.659.70
- Zhang P, Senge M, Dai Y. 2017. Effects of salinity stress at different growth stages on tomato growth, yield, and water-use efficiency. Commun Soil Sci Plant Anal. 48(6):624–634. doi: 10.1080/00103624.2016.1269803
- Zhu J. 2002. Salt and drought stress signal transduction in plants. J Plant Biol. 14:267–273.