ABSTRACT
Fusarium wilt is one of the major soil-borne diseases of tomato crop globally. The study aimed to evaluate the efficacy of medicinal plants in the control of Fusarium wilt in tomato. Methanolic extracts of Monsonia burkena and Moringa oleifera were assessed in vitro and under greenhouse conditions. The in vitro experiments evaluated the effect of both extracts on Fusarium oxysporum f. sp lycopersici growth and response to varying concentrations. In greenhouse experiment, tomato seedlings cv. HTX14 were inoculated with conidial suspension of F. oxysporum and transplanted into pasteurised growth media amended with plant extract. Seedlings were treated with aqueous extracts at varying concentrations with an interval of 7 days between applications. Control treatments were treated with sterile distilled water. Both plant extracts significantly reduced pathogen growth in vitro and reduced wilt severity under greenhouse conditions. The highest mycelial growth suppression was observed in Mon. burkeana treatments. Under greenhouse conditions, both plant extracts significantly (P ≤ 0.05) reduced Fusarium wilt severity and had a positive effect on plant growth parameters. A significant increase in soil-pH was also recorded in extract treated soil resulting in reduction in disease severity. The results further provide new scientific information on how their effect on soil pH can be beneficial in the control of Fusarium wilt.
Introduction
Tomato (Lycopersicum esculentum L.) is the most economically important vegetable crop in Limpopo province of South Africa, where it is produced both for export and consumption. However, the tomato crop is susceptible to numerous diseases, including those caused by soil-borne pathogens (Afroz et al. Citation2010). Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici one of the most-wide spread and economically important soil-borne disease of tomato worldwide (Hanaa et al. Citation2011). The pathogen colonises plant's roots and vascular systems resulting in wilting (Harari Citation2016).
Recently, Fusarium wilt caused by F. oxysporum f.sp. lycopersici race 3 was reported to cause serious damage in the northern parts of Limpopo Province, South Africa (Jacobs et al. Citation2013). There are currently no tolerant or resistant tomato cultivars to this race and other control measures have proven to be unsuccessful in its management. Fusarium wilt is normally managed through application of crop rotation, and soil fumigation with fungicides and planting of resistant cultivars have been suggested (Kekuda et al. Citation2016). However, these methods have proved to be ineffective and unsustainable. For example, application of soil fumigants has detrimental effects on the environment and humans which has resulted in their removal from the market, whilst cultivar resistance is unreliable due to the presence of different pathogen races (El-Mohamedy et al. Citation2013; Harari, Citation2016). Seed treatment with protective fungicides is only effective during seedling stage and the plants become highly susceptible to the disease during flowering stage (Hanaa et al. Citation2011). In recent years, more attention has been given in identifying alternative methods in the management of plant diseases.
Naturally occurring compounds derived from plants have been successfully tested against F. oxysporum and other plant pathogens (Kekuda et al. Citation2016; Rongai et al. Citation2017). For example, crude extracts of pomegranate peel significantly reduced F. oxysporum population and wilt severity in naturally infected soils (Rongai et al. Citation2017). Disease suppression was found to be due to induced systemic resistance through the accumulation of pathogenesis-related proteins (McGovern, Citation2015). Metabolic changes such as induction of phenol biosynthesis enzymes, anti-oxidant defensive enzymes and phenolic accumulation also increases plant resistance (Akladious et al. Citation2015). Both Mon burkeana and Mor. oleifera have been shown to suppress fungal induced plant diseases in numerous studies (Gopalakrishnan et al. Citation2016; Kena Citation2016; Choudhury et al. Citation2017). Therefore, the objective of this study was to determine the effect of methanolic extracts of Mor. oleifera and Mon. burkeana on the growth of F. oxysporum f. sp. lycopersici and wilt severity under laboratory and greenhouse conditions respectively.
Material and methods
Study site
Laboratory and greenhouse experiments were carried-out at the University of Limpopo Plant Pathology Laboratory and Green Biotechnology Research Centre greenhouse respectively during April 2016 to July 2017.
Fusarium wilt pathogen
F. oxysporum f. sp. lycopersici race 3 (PPRI 5457) was isolated from a diseased tomato plant collected in 2013 from Venda. The fungus was further identified and confirmed by the Mycology unit at the Bio-systematic division of Agricultural Research Council-Plant Protection Research Institute (ARC-PPRI), Pretoria, South Africa. The isolate was further tested on healthy tomato plant to confirm its pathogenicity. A pure culture of the fungal pathogen was kept at 4°C until further use.
Plant extracts preparation
Monsonia burkeana (whole plant) and Mor. oleifera (fresh, healthy leaves) were gently washed under slow running tap water, patted with a paper towel to remove excess water and shade dried for approximately 21 days. Dry plant materials were ground in a Wiley mill (Prestige: Model FZ-102) and powdered using waring blender (Aldrich sigma: Model Z272191). One hundred grams of Mon. burkeana and Mor. oleifera powder were added separately to 700 ml of methanol and placed on a rotary shaker for 24 h. Methanol was then evaporated on a rotary evaporator under reduced pressure at 64°C. The obtained extract was oven dried for 21 days at 35°C to constant weight, yielding green solid suspension. Prepared plant extracts were kept at 4°C until further use.
For the greenhouse experiment, aqueous extracts were prepared by adding selected concentrations of Mon. burkeana (4, 6, 8 g/ml) and Mor. oleifera (2, 4 and 6 g/ml) in 100 ml of distilled water. The mixture was decocted at 100°C for 15 min then left to cool before filtering, thereafter, applied as a treatment in the greenhouse experiment.
In vitro experiment
Effects of plant extracts on mycelial growth of F. oxysporum f. sp. lycopersici
An amount of 0, 2, 4, 6, 8 and 10 g of each plant extract powder was weighed then separately dissolved in 10 ml sterile distilled water and thoroughly mixed before being added to 200 ml bottles containing sterilised Potato Dextrose Agar (PDA) before autoclaving at 121°C. The experiment was laid out in a completely randomised design (CRD) with six treatments and four replicates. Six concentrations (0, 2, 4, 6, 8 and 10 g/ml) of each plant extracts (Mon. burkeana and Mor. oleifera) were used as treatments. The extract amended medium was transferred equally into 48 Petri plates and then allowed to solidify overnight.
Seven days old F. oxysporum f. sp. lycopersici culture plugs of 5 mm in diameter was cut from the edge of actively growing culture and was placed at the centre of extract amended Petri plates. Inoculated plates were then incubated at ± 25°C under aseptic condition for seven days. Non-amended Potato Dextrose Agar (PDA) medium plates served as control treatments. Pathogen colony growth was measured using a transparent ruler after ± 7 days. Mycelial growth inhibition was calculated using the formula (RI = (C-T/C) × 100). Where: C = average diameter of fungal colony in control plates and T = average diameter of fungal colony in extracts amended plates.Increased Relative Impact (RI) was depicted through positive (+) and decreased RI through negative (−) sign. Mean suppression level (y-axis) and Mon. burkeana or Mor. oleifera concentration level (x-axis) were further subjected to the lines of the best fit using excel 2016 to determine pathogen response. The responses of mean suppression to increasing Mon. burkeana or Mor. oleifera concentration level were modelled by the regression curve estimations resulting to a quadratic equation: Y = b2 x2 + b1 x + a, where Y = Mean suppression levels; x = Mon. burkeana or Mor. oleifera concentration level with −b1 ∕ 2b2 = x value for the saturation point for each extract.
Greenhouse experiment
Inoculum preparation
Pathogen inoculum was prepared by culturing F. oxysporum f. sp. lycopersici for 7 days in darkness at ± 25°C on potato dextrose agar (PDA). Microconidia was then harvested by flooding the plates with 10 ml sterile distilled water followed by gentle scrapping of the mycelium with a sterile inoculation needle to dislodge spores. The collected conidia were transferred into 250 ml media bottles containing 100 ml sterile distilled water bottles and incubated for 24 h. This was further filtered through three layers of fleece filter and conidial suspension was further adjusted to a final concentration of 1 × 106 conidia/ml under a light microscope using a hyemocytometer.
Plant inoculation and treatment description
Healthy, four weeks old tomato seedlings of cultivar HTX14 were uprooted and roots were gently washed under slow running tap water to remove peat debris. Roots were then dipped in conidia suspension of F. oxysporum f. sp. lycopersici for 30 min. Seedlings were transferred into 25 cm plastic pots containing a mixture of pasteurised sand and hygromix in a ratio 3:1 and left to stand for seven days. One seedling was planted in each pot. After a seven days period, growth mixture was treated by incorporating Mon. burkeana and Mor. oleifera plant extracts treatments at the rate of 250 ml/plant. In control treatments, seedlings were dipped in sterile distilled water and received the same amount of sterile water thereafter the subsequent applications of water and plant extracts were done at seven-day intervals until termination after forty days.
Both Mon. burkeana and Mor. oleifera extract concentrations applied in the greenhouse were selected from in vitro experiments based on low, moderate and high inhibition ability. A total of five treatments were used in the study. 0+: Control, sterile soil pathogen inoculated and not amended with any plant extract, 0-: Control, sterile soil not inoculated and treated with sterile water, Pathogen inoculated, soil treated with either 4 g/ml Mon. burkeana or 2 g/ml Mor. oleifera, Pathogen inoculated, soil treated with either 6 g/ml Mon. burkeana or 4 g/ml Mor. oleifera, and Pathogen inoculated, soil treated with either 8 g/ml Mon. burkeana or 6 g/ml Mor. oleifera. The experiment was arranged in a Randomised Complete Block Design (RCBD) and each treatment was replicated six times.
Effect of plant extracts on tomato growth parameters and Fusarium wilt severity under greenhouse conditions
Before plants were uprooted, growth parameters such as leaf discolouration, plant height, chlorophyll content, soil-pH, fresh root weight and leaf litter were recorded to determine treatment effect on plant growth. Fusarium wilt severity was recorded after 45 days of planting by examining shoot, stem and root symptoms. Below and aboveground plant parts were assessed using a scale of 0–5 (Shazia et al. Citation2015). Shoot symptoms was assessed on a severity scale of 0–5 where: 0 = healthy; 1 = Moderate leaf yellowing 10%; 2 = Moderate wilting 30%; 3 = Stunted plant with yellowing 50%; 4 = Severe stunting with majority of leaves wilted 70%; 5 = Dead plant 100%. Stem and root symptoms was assessed on the severity scale of 0–5 scale where: (0 = Healthy; 1 = no internal browning, discrete superficial lesions on taproot or stem base 2 = Superficial taproot lesion, slight internal vascular discolouration (30%); 3 = Entire taproot brownish and moderate vascular discolouration (50%); 4 = severe vascular discolouration (70%); 5 = dead plants (100%). Disease severity was calculated using the formula: (Percentage disease severity = area of plant part affected / total area × 100).
All the experiments were repeated at least twice.
Statistical analysis
Data was subjected to SAS statistical programme to generate one-way partial ANOVA. Severity Percent data were transformed to arcsine before generating ANOVA. Original data was then recorded for mean separation. Mean separation was achieved by using Fisher's least significant difference (LSD) test to determine the differences within treatments at the probability level of 5%.
Results
Effects of plant extracts on mycelial growth of F. oxysporum f. sp. lycopersici in vitro
Results showing the effect of both Mon. burkeana and Mor. oleifera plant extracts on the mycelial growth of F. oxysporum f. sp. lycopersici are presented in . Both plant extracts significantly (P ≤ 0.05) reduced mycelial growth of the pathogen in all treatments. There was however, variation in relation to the type of extract and the level of pathogen growth suppression at different concentration.
Table 1. Effect of Mon. burkeana and Mor. oleifera extracts on mycelial growth of F. oxysporum f. sp. lycopersici.
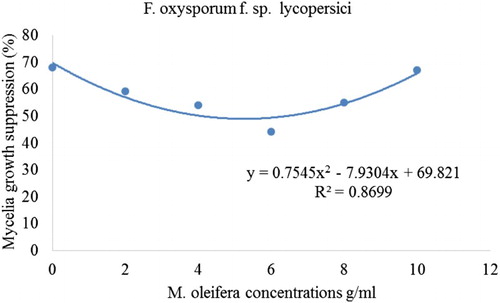
In all cases where PDA was amended with Mon. burkeana at varying concentrations, there was a significant reduction in F. oxysporum f. sp. lycopersici mycelial growth as compared to control. The effect of this extract was more prominent at highest concentrations where the highest mycelial growth inhibition was recorded at 8 g/ml (61%) and 1 g/ml (76%). There was however, no significant difference in pathogen growth inhibition in PDA amended with 2 and 6 g/ml. Pathogen inhibition percentage was also observed in Mor. oleifera treatments against the test pathogen. A significant growth inhibition of F. oxysporum f. sp. lycopersici (35%) was recorded where PDA was amended with 4 and 6 g/ml. Highest mycelial growth inhibition was recorded at 6 g/ml. Mycelial growth suppression also displayed quadratic relationships when regressed against plant extracts of Mon. burkeana and Mor. oleifera ( and ). The treatments levels of Mon. burkeana contributed 76% to the total treatment variation (TTV) in mean mycelial growth suppression of F. oxysporum f. sp. lycopersici (). Moringa oleifera contributed 87% (F. oxysporum f. sp. lycopersici) towards the TTV in mean mycelial growth suppression ().
Figure 1. Quadratic relationship between mycelial growth of Fusarium oxysporum f.sp. lycopersici and Monsonia burkeana plant extract concentrations.
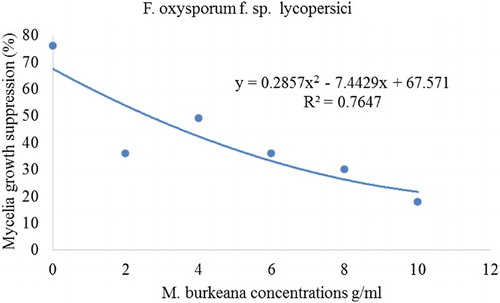
Figure 2. Relationship between percentage mycelial growth of Fusarium oxysporum f. sp. lycopersici and Moringa oleifera plant extract concentrations.
Although methanol extract of both Mon. burkeana and Mor. oleifera significantly (P ≤ 0.05) reduced mycelial growth of F. oxysporum f. sp. lycopersici at different concentrations, the level of inhibition varied significantly (P ≤ 0.05) between plants. For example, Mon. burkeana showed the highest suppression at concentration 1 g/ml (76%) while in Mor. oleifera treatments, 35% was found to be the highest as shown on .
Effect of Mon. burkeana and Mor. oleifera extracts on tomato growth parameters under greenhouse conditions
Treatments of tomato plants with both extracts of Mon. burkeana and Mor. oleifera had varying effect on measured plant parameters. The results of soil amended with different concentrations of Mon. burkeana and Mor. oleifera on growth parameters of tomato were presented in . Infected soil amended with Mon. burkeana extract significantly (P ≤ 0. 05) reduced leaf litter resulting in the highest mean of 0.1 in 6 g/ml amendment. Application of Mon. burkeana (8 g/ml) improved the chlorophyll content resulting in a mean of 12.4 which can be concluded that Mon. burkeana extracts had fertiliser effect on tomato plants treated. However, plant height, soil-pH and fresh root weight were not significant at P ≤ 0. 05.
Table 2. Effect of Mon. burkeana and Mor. oleifera extracts on tomato growth parameters and soil pH in soil inoculated with F. oxysporum f. sp. lycopersici.
Amending the infected soil with Mor. oleifera extracts, had a significant (P ≤ 0. 05) effect on leaf litter on all the treatments recording the highest mean of 3 on 6 g/ml. Increasing extracts concentration stimulated soil-pH and fresh root weight on 6 g/ml resulting to a mean of 7.9 and 58.7 respectively which lead to an increase in plant height on 4 g/ml (1.9 mean). Moringa oleifera significantly (P ≤ 0.05) increased chlorophyll content with a mean of 21.6. However, plant height was not significantly affected at P ≤ 0. 05.
Effect of Mon. burkeana and Mor. oleifera extracts on Fusarium wilt severity under greenhouse conditions
The results of Fusarium wilt severity in soil amended with different concentrations of Mon. burkeana are presented in . There was a clear variation on the effect of each plant extract on plant wilt and stem and root discolouration. For example, amending the F. oxysporum f sp lycopersici infected soil with 6 g/ml of Mon. burkeana extract significantly (P ≤ 0.05) reduced wilting intensity in tomato plants with a mean of 0.6. However, the same concentration did not reduce stem and root discolouration, resulting in no significant differences between treated and non-treated inoculated plants. The highest reduction disease severity mean of 0.5 in stem and root discolouration for this plant extract was observed where pathogen infected soil was amended with 8 g/ml of plant extract. Under the same concentration, the number of wilted plants was the same as in control. Stem and root discolouration in soils amended with 4 and 6 g/ml were not significantly (P ≤ 0.05) different from inoculated, non-amended control.
Table 3. Effect of Mon. burkeana and Mor. oleifera extracts on fusarium wilt severity under greenhouse conditions.
In Mor. oleifera treatments, there was a significant reduction in a number of plants showing wilt and stem and root discolouration symptoms in all concentrations (). The highest reduction in both wilt intensity and stem and root discolouration was observed where infected soil was amended with 4 g/ml of Mor. oleifera extract. However, this reduction was not significantly from 2 to 6 g/ml treatments at P ≤ 0.05. The highest wilt and vascular discolouration was recorded in inoculated non-amended control. It was further observed that increase in concentrations for Mor. oleifera plant extracts did not influence the intensity of Fusarium wilt severity in infected tomato plants.
Discussion
The results of this study indicate that extracts of both tested plants can be effective in the management of Fusarium wilt of tomato. Application of both Mon. burkeana and Mor. oleifera reduced mycelial growth of F. oxysporum, f. sp. lycopersici Fusarium wilt severity. Although the effect of both plants on F. oxysporum f. sp lycopersici is not documented, In both the results of this study confirm their antifungal activities. The results are also consistent with previous reports where the two plants were successful in controlling other plant diseases (Adandonon et al. Citation2006; Kena, Citation2016). The use of plant extracts in the management of plant diseases is seen as an alternative measure (Shervin et al. Citation2011; Sales et al. Citation2016). The antifungal activities of both Mon. burkeana and Mor. oleifera have been tested against other pathogens including Penicillium digitatum and Sclerotium rolfsii (Abandonon et al. Citation2006). In both studies, methanol extracts of the two plants significantly reduced pathogen growth in vitro and citrus grey mould and cowpea damping-off and stem rot respectively. Other studies (Adandonon et al. Citation2006; Mohammed Citation2015) have reported on the efficacy of leaf extracts of Mor. oleifera on soil-borne fungal pathogens namely Rhizoctonia and Pythium spp. Although information is lacking on the antifungal activities of Mon. burkeana and Mor. oleifera against F. oxysporum, other studies have reported on its sensitivity towards other plant extracts. Thymus vulgaris extract was also found to be highly inhibitory towards mycelial growth of F. oxysporum under laboratory conditions (Al-Rahmah et al. Citation2013; Torre et al. Citation2016). Fusarium oxysporum was also found to be highly sensitive to different types of essential in reduced conidial germination and Fusarium wilt severity under field conditions (Torre et al. Citation2016).
An observation of concentration dependent inhibitory effect was made where mycelial growth inhibition decreased with an increase in plant extract concentration () and this was further explained in and . Pathogen growth in Mor. oleifera treatments () clearly shows loss of sensitivity at higher concentrations. However, this phenomenon was not observed in Mon. burkeana (), where increasing extract concentrations from 8 g/ml resulted in increased mycelial growth of F. oxysporum f sp lycopersici. Loss of sensitivity at higher concentrations has been reported by Mboto et al. (Citation2009) and they attributed this to a number of factors including reduced enzymatic activities in treated plants which affects plant defence mechanisms. Another suggested factor might be loss of stability by the active ingredient during incubation period (Mboto et al. Citation2009). From the tested treatments, an increase of the extracts concentration resulted in the increase of pathogen mycelia suppression to a point where the suppression reached a threshold, after which increase in treatment concentrations resulted to stimulated growth of the pathogens. These findings are in line with Salisbury and Ross (Citation1992) saturation and limiting factor model and Liebig, (Citation1841) law of the minimum. Other researchers such as Srivastava et al. (Citation2010) used the model and reported that aqueous leaf extracts of some medicinal plants were least effective against F. oxysporum f. sp. lycopersici at higher concentrations.
Table 4. Monsonia burkeana and Moringa oleifera concentrations for optimal mycelia growth suppression of Fusarium oxysporum f. sp. lycopersici in vitro.
Most medicinal plant including Mon. burkeana and Mor. oleifera contain a number of phytochemicals that exhibit antimicrobial activity (Mboto et al. Citation2009; Mamphiswana et al. Citation2011; Waing et al. Citation2015). Most of these phytochemicals include secondary metabolites and compound such as flavonoids and tannins (Mamphiswana et al. Citation2011; Shafighi et al. Citation2012), which are the main antifungal components associated with disease suppression and plant growth stimulation. Although this study did not look into the chemical constituents on both tested plants, it can be postulated that their inhibitory effect was due to the antibiotics responsible for pathogen growth suppression. This was based on the results reported by a number of authors who have found the antibiotic effects of medicinal plants as their main approach in disease control (Jamil et al. Citation2010; Mohamed et al. Citation2010; Moyo et al. Citation2012). Also, biological differences in fungal pathogens can result in varying responses when treated with different extracts. Moyo et al. (Citation2012) further argued that such variations can also be due to the ability of different extracts to also supress conidial germination resulting in reduced ability to infect plants. The ability of conidia and chlamydospores to germinate has a significant effect on disease severity (Joseph et al. Citation2008; Kena and Swart Citation2011).
In addition to suppressing pathogen growth and disease severity, both plant extracts significantly increased pH in amended soil which correlated with a reduction in disease severity. Soil pH has long been associated with the plant susceptibility towards various diseases including Fusarium wilt and has been found to significantly influence the level of disease severity in susceptible cultivars (Fang et al. Citation2012). Previous reports (Ghorbani et al. Citation2008; Fang et al. Citation2012) have shown that application of calcium hydroxide in highly infected soils resulted in reduced incidence and development of Fusarium wilt in tomato and this was attributed to an increase in soil pH (7.5–8.0). Increase in soil pH is believed to decrease availability of microelements such as iron, manganese and zinc, essential in the development of Fusarium wilt causal pathogen (Ghorbani et al. Citation2008). In the current study, extracts were found to stimulate soil pH which lead to high chlorophyll content, plant height, fresh root weight reducing incidence of leaf litter and leaf discolouration as a results of the presence of Fusarium wilt inoculum. This was probably due to the high concentration of the growth promoting substances (secondary metabolites) contained in this treatment (Mamphiswana et al. Citation2011; Waing et al. Citation2015).
In conclusion, from the results obtained in the current study, it could be concluded that both extracts of Mor. oleifera and Mon. burkeana were effective in reducing Fusarium wilt of tomato under greenhouse conditions and reduced pathogen growth under laboratory conditions. The main positive effect of both extracts was on improved plant growth which might suggest their ability to induce resistance in diseased plants. The study further discovered the ability of these plants to inhibit pathogen growth and reduce disease severity in infected plants through an increased soil pH levels which has been found to Fusarium wilt development. Further studies on their mode of action are therefore necessary for their inclusion in integrated disease management programme.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
Financial assistance to carry-out this study was provided by National Research Foundation, South Africa (Grant Number 75909).
Notes on contributors
Ms Mapula Tshepo Pertunia Hlokwe has completed her MSc in Plant Production at UL in 2018 and he is currently working as an intern in the Plant pathology laboratory. Her main role includes assisting undergraduate students with plant collection and isolation of plant pathogens.
Dr Mapotso Anna Kena is plant pathologist with more than 15 years of experience in higher learning teaching and supervision of postgraduate students. Her main research focus is on using alternative disease management strategies such as improved plant health through farming systems and application of soil amendments to reduce soil-borne disease severity in infected fields.
Mr Ndivhuwo David Mamphiswana is a Lecturer and PhD candidate in the department of plant production and his main duties include teaching undergraduate modules and co-supervising honors and MSc research projects related to his field.
ORCID
Mapotso Anna Kena http://orcid.org/0000-0002-8553-2596
Additional information
Funding
References
- Adandonon A, Aveling TAS, Labuschagne N, Tamo M. 2006. Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur J Plant Pathol. 23:97–127.
- Afroz A, Khan MR, Komatsu S. 2010. Proteomic analysis of tomato cultivars treated with growth elicitor's jasmonic acid and salicylic acid. Prot Pept Lett. 17:836–846. doi: 10.2174/092986610791306634
- Akladious SA, Isaac GS, Abu-Tahon MA. 2015. Induction and resistance against Fusarium wilt disease of tomato by using sweet basil (Ocinum basilicum L) extract. Can J Plant Sci. 95:689–701. doi: 10.4141/cjps-2014-127
- Al-Rahmah AN, Mostafa AA, Abdel-Megeed A, Yakout SM, Hussein SA. 2013. Fungicidal activities of certain methanol plant extracts against tomato phytopathogenic fungi. Afr J Microbiol Res. 7:517–524.
- Choudhury D, Anand YR, Kundu S, Nath R, Kole RK, Saha J. 2017. Effect of plant extracts against sheath blight of rice caused by Rhizoctonia solani. J Pharmacogn Phytochem. 6:399–404.
- El-Mohamedy RSR, Abdel-Kader MM, Abd-El-Kareem F, El-Mougy NS. 2013. Essential oils, inorganic acid and potassium salts as control measures against the growth of tomato root rot pathogens in vitro. JAT. 9:1507–1520.
- Fang X, You MP, Barbetti MJ. 2012. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil-pH, soil organic amendments and crop rotation. Eur J Plant Pathol. 134:619–629. doi: 10.1007/s10658-012-0042-1
- Ghorbani R, Wilcockson S, Koocheki A, Leifert C. 2008. Soil management for sustainable crop disease control: a review. Environ Chem Lett. 6:149–162. doi: 10.1007/s10311-008-0147-0
- Gopalakrishnan L, Doriya K, Kumar DS. 2016. Moringa oleifera: a review on nutritive importance and its medicinal application. FSHW. 5:49–56.
- Hanaa RMF, Abdou ZA, Salama DA, Ibrahim AR, Sror HAM. 2011. Effect of neem and willow aqueous extracts on fusarium wilt disease in tomato seedlings: induction of antioxidant defensive enzymes. Ann J Agr Sci. 56:1–13. doi: 10.2298/JAS1101001B
- Harari H. 2016. Bio-control of tomato Fusarium wilt by Trichoderma species under in vitro and in vivo conditions. Cercet Agron Mold. 1:91–98.
- Jacobs A, Govender R, van Heerden SW. 2013. Fusarium oxysporum f. sp. lycopersici race 3 causing tomato wilt in South Africa. Austral Plant Dis Notes. 8:145–147. doi: 10.1007/s13314-013-0118-6
- Jamil M, Iqbal W, Bangash A, Rehman S, Imran QM, Rha ES. 2010. Constitutive expression of OSC3H33, OSC3H50 and OSC3H37 genes in rice under salt stress. Pak J Bot. 42:4003–4009.
- Joseph B, Dar MA, Kumar V. 2008. Bio-efficacy of plant extracts to control Fusarium solani f. sp. melongenae incident of Brinjal wilt. Glob J Biochem. 3:56–59.
- Kekuda PTR, Akarsh S, Nawaz SAN, Ranjitha MC, Darshini SM, Vidya P. 2016. In vitro antifungal activity of some plants against Bipolarissarokiniana (Sacc.) Shoem. Int J Curr Microbiol Appl Sci. 5:331–37. doi: 10.20546/ijcmas.2016.506.037
- Kena MA. 2016. Antifungal activities of Monsonia burkeana and Euphorbia ingens extracts against Penicillium digitatum, the causal agent of citrus green mould. Afr Plant Prot. 19:1–3.
- Kena MA, Swart WJ. 2011. Effect of plant extracts on in vitro suppression of fungal pathogens and control of damping-off of vegetable seedlings in the greenhouse. Botswan J Agri Appl Sci. 2:36–44.
- Liebig J. 1841. Organic chemistry in its applications to agriculture and physiology. Cambridge: John Owen. First American m.
- Mamphiswana ND, Mashele PW, Mdee LK. 2011. Distribution of selected essential nutrient elements and secondary metabolites in Monsonia burkeana. Afr J Agric Res. 18:2570–2575.
- Mboto CI, Eja ME, Adegoke AA, Iwatt GD, Asikong BE, Takon I, Udo SM, Akeh M. 2009. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medically important microorganisms. Afr J Microbiol Res. 3:557–559.
- McGovern RJ. 2015. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 73:78–92. doi: 10.1016/j.cropro.2015.02.021
- Mohamed M, Sirajudeen KNS, Swamy M, Yaacob NS, Sulaiman SA. 2010. Studies on the antioxidant properties of Tualang honey of Malaysia. Afr J Tradit Complement Altern Med. 7:59–63.
- Mohammed FAA. 2015. Antioxidants composition of moringa (Moringa 'oleifera Lam) in different plant organs [Masters thesis] ; p. 18–62.
- Moyo B, Masika PJ, Muchenje V. 2012. Antimicrobial activities of Moringa oleifera Lam leaf extracts. Afr J Biotechnol. 11:2797–2802. doi: 10.5897/AJB10.686
- Rongai D, Pulcini P, Pesce B, Milano F. 2017. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur J Plant Pathol. 147:229–238. doi: 10.1007/s10658-016-0994-7
- Sales MDC, Costa HB, Fernandes PMB, Ventura JA, Meira DD. 2016. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac J Trop Biomed. 6:26–31. doi: 10.1016/j.apjtb.2015.09.026
- Salisbury FB, Ross CW. 1992. Plant physiology, 4th ed. Belmont, CA: Wadsworth. p. 682.
- Shafighi M, Amjad L, Madaniin M. 2012. Antifungal activity of methanolic extract of various parts of Punica granatum L. IJSER. 3:1–4.
- Shazia S, Muhammad A, Sobiya S. 2015. Management of Fusarium oxysporum f. sp. Capsici by leaf extract of Eucalyptus citriodora. Pak J Bot. 47:1177–1182.
- Shervin H, Kamran R, Salar J, Ali E. 2011. Comparing neem extract with chemical control on Fusarium oxysporum and Meloidogyne incognita complex of tomato. Adv Environ Biol. 21:77–431.
- Srivastava S, Singh VP, Kumar R, Srivastava M, Sinh A, Simon S. 2010. In vitro evaluation of Carbendazim 50% WP, antagonists and botanicals against Fusarium oxysporum f. sp. psidii associated with rhizosphere soil of Guava. Asian J Plant Pathol. 20:1–8.
- Torre A, Caradonia F, Matere A, Battaglia V. 2016. Using plant essential oils to control fusarium wilt in tomato plants. Eur J Plant Pathol. 144:487–496. doi: 10.1007/s10658-015-0789-2
- Waing KGD, Abella EA, Kalaw SP, Waing FP, Galvez CT. 2015. Antagonistic interactions among different species of leaf litter fungi of Central Luzon State University. Plant Pathol Quar. 5:122–130. doi: 10.5943/ppq/5/2/9
