 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose: Floating hydroponic system for lettuce greenhouse production consists of plants inserted in styrofoam platform floating in a 15 cm-tall nutrient solution, which is aerated by some growers while others maintain the solution static. The present investigation had the objective of determining the effect of -N and K in Romaine lettuce cultivated in floating hydroponic system and the impact of nutrient solution aeration. Materials and methods: Plants were transplanted into 34 L rigid plastic containers with aerated or non-aerated solutions containing either 15/7, 12/8.5 or 12/2.85 meq L−1 of
/K concentration. Results: Aeration of the nutrient solution promoted plant growth, leaf K, P, and Mg, and water uptake, however, photosynthesis and transpiration rates were enhanced under non-aeration conditions. Decreasing N did not affect leaf N uptake and accumulation; however, decreasing K markedly decreased leaf P and K in aerated plants. Despite the higher transpiration and stomatic conductance in non-aerated plants, water uptake was higher in aerated plants. Non-aerated plants exhibited decreased leaf K accumulation. Conclusions: non-aeration or reduction of K concentration of the nutrient solution may be a strategy to reduce leaf K in lettuce; leaf N concentration was unaffected by aeration.
Introduction
Hydroponic leafy greens cultivation is an actively growing industry that has attracted greenhouse growers due to the consumer’s interest for healthier nutrition (Taban and Halkman Citation2011), the higher product quality obtained in hydroponics (Luna et al. Citation2013), and the feasibility for year round production. Lettuce (Lactuca sativa L.), arugula (Eruca sativa Mill), kale (Brassica oleracea L. convar. Acephala (DC.) Alef.), mustard (Brassica rapa subsp oleifera L.) and spinach (Spinacia oleracea. L.) are among the most demanded species, however, Romaine type lettuce is one of the main species grown by small to medium growers (Barickman et al. Citation2016).
Soilless growing systems are recommended for lettuce production because of the improved control of mineral nutrition required to standardise the quality characteristics for fresh-cut products (Luna et al. Citation2013). In México, the floating system is the most used hydroponic system for lettuce production, while the nutrient film technique is ranked second. The floating system, also known as the floating raft, or pond culture method, consists of plants inserted in styrofoam platform that are floating in a nutrient solution stored on a 15 cm-tall pond.
Some growers recirculate the nutrient solution with a water pump, however, most growers maintain the solution static with no recirculation nor aeration or oxygen injection, as described by Mahlangu et al. (Citation2016). Static, non-aerated or oxygenated solutions lead to hypoxic conditions, causing decreased root permeability to water and impairment in nutrient uptake rate (Bramley and Tyerman Citation2010).
Nonetheless, growers claim that there are no detrimental effects on lettuce growth or quality in spite of the reports indicating that roots with adequate air supply are more efficient in nutrient uptake in other species (Morard et al. Citation2000). Zheng et al. (Citation2007) indicated that dissolved oxygen in the nutrient solution as low as 8.5 mg L−1 allowed maximum growth of tomato grown in rockwool cubes, whereas Ehret et al. (Citation2010) reported that, in general, dissolved oxygen at 4–5 mg L−1 does not affect yield in cucumber (Cucumis sativus L.) or pepper (Capsicum annuum L.) plants. In contrast, oxygen at 0.6 mg L−1 caused aerenchyma formation in soybean (Glycine max (L.) Merrill) whereas roots grown at 6.6–6.7 mg L−1 oxygen exhibited marginal root porosity (Thomas et al. Citation2005). In lettuce, hypoxia conditions at 2.2 and 0.9 mg L−1 oxygen resulted in decreased growth and total antioxidant activity when compared to control plants at 9.2 mg L−1 oxygen (Tang et al. Citation2015).
Nitrogen (N) and potassium (K) are among the most demanded nutrients by crops (Hawkesford et al. Citation2012). Leafy vegetables, such as lettuce, are known as high accumulators, especially under low light conditions (Blom-Zandstra Citation1989), however, high leaf
concentrations are undesirable as
metabolites such as nitrite, nitroso compounds and nitric oxide may cause human health issues (Parks et al. Citation2008) such as methemoglobinemia or cancer (Blom-Zandstra Citation1989). The European Commission has established a threshold of
for lettuce in 3000–5000 mg kg−1 of fresh weight (Colonna et al. Citation2016).
In addition to human health issues caused by high intake, high levels of K accumulated in plant tissues may also affect chronic kidney disease patients as they are unable to completely eliminate it, resulting in K accumulation in blood, which in turn provokes hyperkalemia, a life threatening disease (Asaduzzaman et al. Citation2018).
Efforts by plant scientists have been settled to reduce and K by developing cultivation methods that reduce the concentration of such ions in the edible parts of plants, with minimum deleterious effects on plant growth and yield (Ogawa et al. Citation2012). Reports indicate that nutrient solutions with N from 7.1–8.6 meq L−1 (Mahlangu et al. Citation2016) and K at 3.0 meq L−1 K (Sublett et al. Citation2018), are adequate for optimum yield and quality and may result in reduced content of both ions in hydroponic lettuce. However, in hydroponic systems, there is little research as to the effect of
-N and K concentration and if aeration of the nutrient solution may affect the accumulation of both ions. The present investigation has the objective of determining the effect of
-N and K in Romaine lettuce cultivated in floating hydroponic system and the impact of nutrient solution aeration on plant growth, nutrient status, and gas exchange parameters.
Material and methods
Cultural conditions
The experiment was carried out in a greenhouse located at the Universidad Autónoma Agraria Antonio Narro in northern Mexico (25° 30’ N latitude, 101° 03’ W longitude, 1610 masl). The average minimum and maximum temperatures recorded during the study was 12.6°C and 34.7°C, respectively (average 19.9°C). The relative humidity ranged from 39% to 94% (average 75%). The incident photosynthetically active radiation (PAR) during the period of highest insolation (12:00pm–2:00pm h) was on average 323 μmol m−2 s−1.
Treatments and nutrient solutions preparation
Romaine type lettuce plants (Lactuca sativa L. var. longifolia) cv Lulú were transplanted into 34 L rigid plastic containers (69 × 39 × 16 cm; length, width, and height), 5 plants per container, in a deep water floating system. Electric conductivity and pH of the nutrient solution was maintained at 2.2−2.5 dS m−1 and 5.8−6.2, respectively.
The experiment study consisted of two factors: (1) the and K concentration (), and (2) the nutrient solution in the containers was aerated with an air pump or non-aerated (). In aerated solutions, an air pump (EC PLUS model 728459, Intertek, China) pumping 3566 GPH and with a diaphragm and filter to avoid the entrance of dust into the solution was used. Aeration was maintained 24 h a day throughout experiment duration and allowed a dissolved oxygen concentration of 6.2 mg L−1 (Microprocessor Dissolved Oxygen meter model HI 9146, HANNA Instruments, Woonsocket RI, USA). In non-aerated solutions, the solution remained stranded in the containers and had an initial oxygen concentration of 6.08 mg L−1; oxygen concentration decreased gradually to 1.9 mg L−1 after 9 days of cultivation.
Table 1. Treatments applied to lettuce plants grown in a floating hydroponic system to assess the effects of aereation of the nutrient solution and the 
 /K concentration.
/K concentration.
The nutrient solution was completed with (meq L−1): 1 , 5.5
, 8 Ca2+, 3 Mg2+, and micronutrients (µeq L−1): 44.8 Fe, 0.3 Cu, 0.9 Zn, 12.0 Mn, 2.5 Mo, provided as Fe−EDTA, Cu−EDTA, Zn−EDTA, Mn−EDTA and (NH4)6MoO2·2H2O, respectively. Boron was supplied at 0.25 mg L−1 as H3BO3 as it is an uncharged molecule at pH < 7.0.
Assessment of plant responses
At experiment termination, 36 days after transplant, root length, root volume (water displacement in a graduated cylinder method), and leaves and root fresh and dry weights were measured on four plants from each experimental unit. The fresh weight of leaves and roots was measured separately; then, these plant parts were washed with distilled water, dried in an oven at 62°C for 72 h and weighed. Leaf firmness was measured with an 8 mm plunger Effigi penetrometer (Mc Cormic Fruit Co., Yakima, WA); two measurements per plant, three plants per replication, were taken at both sides of the middle vein of the external leaf. Nutrient solution consumed by the plants was replenished weekly with distilled water and measured to determine water consumption at experiment termination.
Leaf nutrient analysis
Leaf dry tissues were ground to pass through a 40 mesh screen (A-10, TEKMAR, IKA Labortechnik, Germany); a 0.25 g sample of ground leaf tissue was digested in 2 mL of a 2:1 mixture of H2SO4 and HClO4, and 1 mL of 30% H2O2. The digest was taken to 25 mL with distilled water and filtered prior measuring P, K, Ca and Mg concentration by inductively coupled plasma atomic emission spectrometer (Model Liberty, VARIAN, Santa Clara, CA) (Soltanpour et al. Citation1996). Nitrogen was determined by the semi-micro Kjeldahl procedure (Bremner Citation1996). Total N and K accumulated in leaf tissues on a fresh weigh basis were calculated using both nutrient concentrations and the shoot fresh weight.
Gas exchange parameters
Net photosynthesis rate, transpiration rate, stomatic conductance and leaf temperature were measured with an Infra-Red Gas Analyser (IRGA) (LI-COR, Inc., LI-6200, Lincoln, NE, USA) at noon 24 days after planting on one young fully developed leaf per plant, three plants per replication. The IRGA was set at PAR 400 µmol m–2 s–1, 355 ppm CO2 and 25.5°C.
Statistical design and analysis
The experiment was distributed with a randomised block design with a 3 × 2 factorial arrangement and four replications (containers with 5 plants) per treatment. Data were analysed with SAS (v. 9.0) to obtain an ANOVA; when significance was detected, a mean comparison with Duncan’s multiple range test was conducted.
Results
Aeration of the nutrient solution resulted in lettuce plants with greater root fresh weight, root volume and root length compared to those of non-aerated solutions (). Similarly, higher water consumption was detected in plants under aerated conditions (). Root fresh weight was also affected by the /K concentration as plants treated with 12/8.5 and 15/7 meq L−1 exhibited the higher root fresh weight (). The interaction resulted non-significant for any of the root growth parameters and root dry weight was not significantly affected ().
Table 2. Main effects of aeration and the 
 /K concentration in the nutrient solution on root growth parameters and water consumption in lettuce plants grown in a floating hydroponic culture.
/K concentration in the nutrient solution on root growth parameters and water consumption in lettuce plants grown in a floating hydroponic culture.
Leaf fresh and dry weight and leaf length were greater in plants grown in aerated solutions, although leaf firmness was unaffected (). Neither the and K concentration nor the interaction with the aeration of the nutrient solution significantly affected leaf growth or firmness.
Table 3. Main effects of aeration and the 
 /K concentration in the nutrient solution on leaf growth parameters and firmness in lettuce plants grown in a floating hydroponic culture.
/K concentration in the nutrient solution on leaf growth parameters and firmness in lettuce plants grown in a floating hydroponic culture.
Leaf N concentration was not affected (), however, leaf K significantly decreased in plants with non-aerated solutions and when the solutions had the lowest K concentration (). The significant interaction for leaf K indicates that the decrease was more marked in plants under aerated conditions compared to that of non-aerated plants ().
Figure 1. Effect of the /K concentration and aeration/non aeration of the nutrient solution en leaf potassium concentration (Aeration: p = 0.001;
/K: p = 0.001; Interaction: p = 0.050) and accumulation (Aeration: p = 0.001;
/K: p = 0.001; Interaction: p = 0.001) in lettuce plants grown in a floating hydroponic system. FW = fresh weight. Bars represent the standard error of the mean (n = 4).
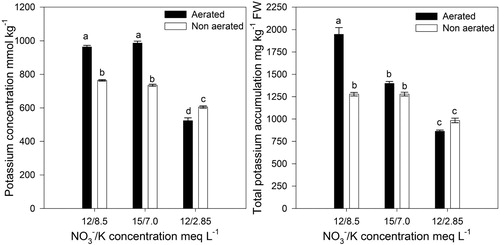
Table 4. Main effects of aeration and the 
 /K concentration in the nutrient solution on leaf nitrogen (N), total N accumulation, calcium (Ca) and magnesium (Mg) concentration in lettuce plants grown in a floating hydroponic culture. Nitrogen, Ca and Mg are on a dry weight basis.
/K concentration in the nutrient solution on leaf nitrogen (N), total N accumulation, calcium (Ca) and magnesium (Mg) concentration in lettuce plants grown in a floating hydroponic culture. Nitrogen, Ca and Mg are on a dry weight basis.
In terms of total N accumulation per kilogram of fresh weight, N was not affected by aeration nor the and K concentration (), however, total K accumulation per kilogram of fresh weight was significantly decreased in non-aerated plants when K concentration in the nutrient solution was high (); at low K concentrations there was no aeration effect ().
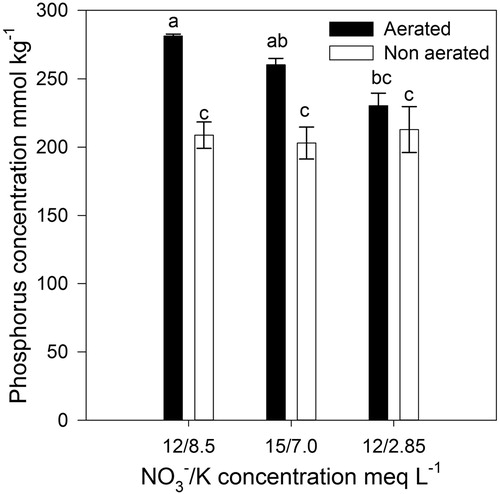
Leaf P concentration was affected by the interaction between aeration of the nutrient solution and the /K concentration (); plants under non-aerated conditions exhibited no effect on leaf P, however, aerated plants showed the highest leaf P concentration when the
/K concentration was 12/8.5 and 15/7 meq L−1 (). Leaf Mg was higher in aerated plants than in non-aerated plants (), and Ca and Mg, increased when K in the nutrient solution was decreased ().
Figure 2. Effect of the /K concentration and aeration/non aeration of the nutrient solution on leaf phosphorus concentration in lettuce plants grown in a floating hydroponic system (Aeration: p = 0.001;
/K: p = 0.050; Interaction: p = 0.024). Bars represent the standard error of the mean (n = 4).
Photosynthesis rate, stomatic conductance and transpiration rate were higher in non-aerated plants (). Photosynthesis rate was not affected by the /K concentration, however, stomatic conductance and transpiration rate increased as K in the nutrient solution was decreased from 8.5–2.85 meq L−1 (). Leaf temperature and the interactions were not significantly affected ().
Table 5. Main effect of aeration and the 
 /K concentration in the nutrient solution on gas exchange parameters and leaf temperature in lettuce plants grown in a floating hydroponic culture.
/K concentration in the nutrient solution on gas exchange parameters and leaf temperature in lettuce plants grown in a floating hydroponic culture.
Discussion
Effect of nutrient solution aeration
Aeration of the nutrient solution promoted a higher leaf and root growth in lettuce plants. Similar reports have been published in lettuce (Tesi et al. Citation2003) and purslane (Portulaca oleracea L.) (Lara et al. Citation2011), however, in contrast, Conesa et al. (Citation2015) indicated that aeration did not affect yield in baby leaf lettuce, although dry weight was higher in highly aerated plants.
Leaf firmness is a quality trait highly appreciated by consumers as it is associated with a crunchy texture and freshness (Fillion and Kilcast Citation2002). In the present study, we are reporting no effects of aeration on leaf firmness, probably due to the no effect of aeration on leaf Ca concentration, as this element is reported to affect lettuce texture (Yosoff et al. Citation2015). Simonne et al. (Citation2001) reported that providing with Ca as the accompanying ion is recommended over sources with K to increase crunchiness of lettuce (Simonne et al. Citation2001).
Lettuce plants cultivated in hydroponics tend to contain higher N or (Yosoff et al. Citation2015), which is considered detrimental as excess
intake may cause health issues in humans (Nicola et al. Citation2012), such as metahemoglobinemia or gastric cancer (Yosoff et al. Citation2015) due to the conversion of
to nitrite or nitrosamines, respectively (Blom-Zandstra Citation1989). In the present study, aeration of the nutrient solution did not affect leaf N concentration (dry weight basis) or total content (fresh weight basis), which is in agreement with the results reported by Conesa et al. (Citation2015) for baby leaf lettuce in that leaf
-N was comparable in both aerated and non-aerated plants, and by Tang et al. (Citation2015) when oxygen concentration in the nutrient solution was at 2.2 mg L−1, nonetheless, Tang et al. (Citation2015) reported that more severe hypoxic conditions (0.9 mg L−1) caused a significant reduction in leaf N concentration in lettuce at ambient pressure.
The fact that non-aerated plants exhibited similar leaf N concentration or content to that of aerated plants is somewhat unexpected as the decrease in root respiration, that has been reported under hypoxia conditions (Chun and Takakura Citation1994; Bar-Yosef and Lieth Citation2013), renders reduced ATP synthesis and thus less energy available for uptake as it has to actively enter to the symplast through carriers or channels at the plasma membrane (Li et al. Citation2013).
The unaffected uptake observed despite the low air supply may be associated with the formation of air spaces in the root cortex and aerenchyma in the lower parts of the leaves, as reported under low oxygen conditions for head lettuce (cv Great Lakes) plants (Wagatsuma et al. Citation1990), enabling root respiration and hypoxia tolerance. Another possibility is that
demands for non-aerated plants were adjusted so that a lower uptake met the lower growth rate, resulting in comparable N concentrations.
The impact of low oxygen supply under non-aerated conditions was observed for leaf P, K and Mg as, compared to aerated plants, they decreased up to 22%, 25% and 31%, respectively. Tang et al. (Citation2015) reported similar results in lettuce at ambient pressure as K was decreased by 24% and 49% when oxygen concentration was at 2.2 and 0.9 ppm. Morard and Silvestre (Citation1996) indicated that the depressive effects of low root oxygen supply affects nutrient and water uptake in the following order: K > N>P > H2O > Mg = Ca; however, in our study, the order for plants fed with solutions containing 15 and 7 meq L−1 of and K, respectively, was: Mg > K>P > H2O > N = Ca.
The decrease in total K concentration, in mmol kg−1 of dry weight, observed in the present study was of 21% in non-aerated plants fed with solutions containing 12 and 8.5 meq L−1 of and K, respectively, whereas the reduction in total K content, in mg kg−1 of fresh weight, was of 34%. In addition, reducing K concentration in the nutrient solution from 8.5–2.85 meq L−1 resulted in a 46% reduction in K concentration and 56% reduction in total K content, which is comparable to reports by Ogawa et al. (Citation2012). Thus, as the consumption of low K foods is beneficial for people with chronic kidney disease and dialysis patients as they are unable to excrete it to maintain K within normal range in the human body (Choi and Ha Citation2013), both K reduction and non-aeration of the in the nutrient solution may be of interest as alternatives to reduce leaf K in lettuce.
Several researchers have designed strategies to produce low K plants for patients with such issues; for example, Ogawa et al. (Citation2012) fed lettuce plants with no K for the second half of the growing period, obtaining a 70% reduction in K content with no effect on plant growth. In other studies, K restriction strategies have been investigated to produce low K tomato (Solanum lycopersicon L.) (Tsukagoshi et al. Citation2017) and melon (Cucumis melo L.) fruits (Asaduzzaman et al. Citation2018), as well as in microgreens of lettuce and chicory (Cichorium intybus L.) (Renna et al. Citation2018).
Our results suggest that, non-aeration of the nutrient solution may also be a strategy to reduce K in lettuce tissues for patients with kidney diseases, although this was accompanied by a reduction in fresh weight and leaf P. Nonetheless, this fresh weight reduction was of only 17% when the nutrient solution contained 12 and 8.5 meq L−1 of and K, respectively.
Our results showed that water uptake was reduced by 16% in non-aerated plants. Water uptake decrease has been ascribed to hypoxia conditions (Morard and Silvestre Citation1996) due to reduced root hydraulic conductance and permeability of aquaporines (Colmer and Voesenek Citation2009). In the present study, our results showed that water uptake was associated with the increased root growth observed in plants fed with 15 meq L−1
. Reports indicate that hypoxia conditions imposed during 120 h leads to reduction in photosynthesis and transpiration rate in barley (Hordeum vulgare L.) (Yordanova et al. Citation2003) and maize (Zea mays L.) (Yordanova and Popova Citation2007). Our results are in contrast to those reports as higher photosynthesis and transpiration rates were observed in non-aerated plants.
Bar-Yosef and Lieth (Citation2013) reported that roses (Rosa hybrida L.) transplanted on November and receiving aeration from 0% to 100% exhibited unaffected evapotranspiration until March. In April, plants under 0%, 12.5% and 25% aeration showed higher evapotranspiration than those receiving higher aeration rates; however, later on the season, the trend reversed as plants under high aereation showed higher evapotranspiration than those with lower aeration. These results suggest that the gas exchange parameters observed in our study may be because younger plants have sufficient oxygen with low aeration rates while older plants demand higher oxygen supply and thus higher aereation rates (Bar-Yosef and Lieth Citation2013). Thus, the inconsistency between our gas exchange results and those reported for other species under hypoxia conditions may be due to the fact that Romaine lettuce plants are harvested in a very early stage and thus they have lower oxygen requirements.
Effect of the nutrient solution composition
In the present study, nutrient solution composition had marginal effects on plant growth; nonetheless, plants treated with solutions of low K resulted in low leaf K and P, whereas leaf Ca and Mg were increased. The increase in Ca and Mg may be associated to the antagonistic relations among the three cations, as increasing K results in depletion of Ca and Mg uptake. Reports by Ogawa et al. (Citation2012) are in line with our findings as restrictions in K supply to hydroponic lettuce resulted in a significant increase in Mg accumulation in plant tissues. Zhang et al. (Citation2017) reported no effect on transpiration rate and stomatic conductance in Romaine lettuce when K in the nutrient solution was decreased from 4 to 1 meq L−1, while photosyntesis rate was not affected even when K is supplied at 2 meq L−1; this is in contrast to our results as we observed that the severe leaf K reduction (−47% in aerated plants and −21% in non-aerated plants) when low K was supplied was associated with an increase in stomatic conductance and transpiration rate, however, photosynthesis was unaffected.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes on contributors
Daniela Alvarado-Camarillo is a junior full time researcher and professor in the Departamento de Ciencias del Suelo at Universidad Autónoma Agraria Antonio Narro, Saltillo, Coah., México. She received her Doctor of Science in protected agriculture in 2017 and is currently working on hydroponic cultivation of vegetable species, plant nutrition and cultivation of vegetables in plant factory systems.
Dr Luis A. Valdez-Aguilar is a senior full time researcher and professor at Universidad Autónoma Agraria Antonio Narro. His research is mainly focused on hydroponic cultivation of greenhouse ornamental and vegetable species, nutrient interactions, and design of fertilisation programmes for ornamentals and hydroponic systems. He received his PhD in horticulture from Texas A&M University in 2004 and has published 100 papers.
José Antonio González-Fuentes is a senior full time researcher and professor at Universidad Autónoma Agraria Antonio Narro. His research is mainly focused on hydroponic cultivation of greenhouse ornamental species and berries. He received his PhD in horticulture from University of California at Davis in 2013 and has published 20 papers.
Emilio Rascón-Alvarado is a senior full time researcher and professor in the Departamento de Ciencias del Suelo at Universidad Autónoma Agraria Antonio Narro, Saltillo, Coah., México. His research is focused on the organic agriculture, development of vermicoposts and the study of the effect of humic and fulvic acids to improve soil physicochemical traits. He received his Doctor of Science in Agricultural Systems Engineering at Universidad Autónoma Agraria Antonio Narro, México in 2006.
Fidel M. Peña-Ramos is a senior full time researcher and professor in the Departamento de Ciencias del Suelo at Universidad Autónoma Agraria Antonio Narro, Saltillo, Coah., México. His research is focused on the statistical methods applied to agricultural sciences, modelling plant responses to environmental conditions and the use of humic and fulvic acids to improve soil physicochemical traits. He received his Master’s degree at Universidad Autónoma Agraria Antonio Narro in 2011.
ORCID
Luis Alonso Valdez-Aguilar http://orcid.org/0000-0002-2510-1962
References
- Asaduzzaman M, Raihan Talukder M, Tanaka H, Ueno M, Kawaguchi M, Yano S, Ban T, Asao T. 2018. Production of low-potassium content melon through hydroponic nutrient management using perlite substrate. Front Plant Sci. 9:1382. doi: 10.3389/fpls.2018.01382
- Barickman TC, Horgan TE, Wheeler JR, Sams CE. 2016. Elevated levels of potassium in greenhouse-grown red romaine lettuce impacts mineral nutrient and soluble sugar concentrations. HortScience. 51:504–509. doi: 10.21273/HORTSCI.51.5.504
- Bar-Yosef B, Lieth JH. 2013. Effects of oxygen concentration in solution and uptake rate by roots on cut roses yield, and nutrients and sugars content in leaves. Sci Hortic. 155:49–55. doi: 10.1016/j.scienta.2013.03.002
- Blom-Zandstra M. 1989. Nitrate accumulation in vegetables and its relationship to quality. Ann Appl Biol. 115:553–561. doi: 10.1111/j.1744-7348.1989.tb06577.x
- Bramley H, Tyerman SD. 2010. Root water transport under waterlogged conditions and the roles of aquaporins. In: S. Mancuso, S Shabala, editors. Waterlogging signalling and tolerance in plants. Berlin: Springer; p. 151–180.
- Bremner JM. 1996. Total nitrogen. In: DL Sparks, editor. Methods of soil analysis. Part II. Chemical methods. Madison, WI: American Society of Agronomy. Soil Science Society of America; p. 1085–1086.
- Choi HY, Ha SK. 2013. Potassium balances in maintenance hemodialysis. Electrolytes Blood Press. 11:9–16. doi: 10.5049/EBP.2013.11.1.9
- Chun C, Takakura T. 1994. Rate of root respiration of lettuce under various dissolved oxygen concentrations in hydroponics. Environ Control Biol. 32:125–135. doi: 10.2525/ecb1963.32.125
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol. 36:665–681. doi: 10.1071/FP09144
- Colonna E, Rouphael Y, Barbieri G, De Pascale S. 2016. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 199:702–710. doi: 10.1016/j.foodchem.2015.12.068
- Conesa E, Fernández JA, Niñirola D, Egea-Gilabert C. 2015. Nutrient solution aeration and growing cycles affect quality and yield of fresh-cut baby leaf red lettuce. Agric Food Sci. 24:313–322. doi: 10.23986/afsci.52792
- Ehret DL, Edwards D, Helmer T, Lin W, Jones G, Dorais M, Papadopoulos AP. 2010. Effects of oxygen-enriched nutrient solution on greenhouse cucumber and pepper production. Sci Hortic. 125:602–607. doi: 10.1016/j.scienta.2010.05.009
- Fillion L, Kilcast D. 2002. Consumer perception of crispiness and crunchiness in fruits and vegetables. Food Quality. 13:23–29. doi: 10.1016/S0950-3293(01)00053-2
- Hawkesford M, Horst W, Kichey TMR, Schjørring JK, Møller IS, White P. 2012. Functions of macronutrients. In: P. Marschner, editor. Marschner's mineral nutrition of higher plants. Walthman, USA: Elsevier Science; p. 135–189.
- Lara LJ, Egea-Gilabert C, Niñirola D, Conesa E, Fernández JA. 2011. Effect of aeration of the nutrient solution on the growth and quality of purslane (Portulaca oleracea). J Hortic Sci Biotechnol. 86:603–610. doi: 10.1080/14620316.2011.11512810
- Li SX, Wang ZH, Stewart BA. 2013. Responses of crop plants to ammonium and nitrate N. Adv Agron. 118:205–397. doi: 10.1016/B978-0-12-405942-9.00005-0
- Luna MC, Martínez-Sánchez A, Selma MV, Tudela JA, Baixauli C, Gil MI. 2013. Influence of nutrient solutions in an open-field soilless system on the quality characteristics and shelf life of fresh-cut red and green lettuces (Lactuca sativa L.) in different seasons. J Sci Food Agric. 93:415–421. doi: 10.1002/jsfa.5777
- Mahlangu RIS, Maboko MM, Sivakumar D, Soundy P, Jifon J. 2016. Lettuce (Lactuca sativa L.) growth, yield and quality response to nitrogen fertilization in a non-circulating hydroponic system. J Plant Nutr. 39:1766–1775. doi: 10.1080/01904167.2016.1187739
- Morard P, Silvestre J. 1996. Plant injury due to oxygen deficiency in the root environment of soilless culture: a review. Plant Soil. 184:243–254. doi: 10.1007/BF00010453
- Morard P, Lacoste L, Silvestre J. 2000. Effect of oxygen deficiency on uptake of water and mineral nutrients by tomato plants in soilless culture. J Plant Nutr. 23:1063–1078. doi: 10.1080/01904160009382082
- Nicola S, Egea-Gilabert C, Niñirola D, Conesa E, Pignata G, Fontana E, Fernández JA. 2012. Nitrogen and aeration levels of the nutrient solution in soilless cultivation systems as important growing conditions affecting inherent quality of baby leaf vegetables: a review. Acta Hortic. 1099:167–177.
- Ogawa A, Eguchi T, Toyofuku K. 2012. Cultivation methods for leafy vegetables and tomatoes with low potassium content for dialysis patients. Environ Control Biol. 50:407–414. doi: 10.2525/ecb.50.407
- Parks SE, Huett DO, Campbell LC, Spohr LJ. 2008. Nitrate and nitrite in Australian leafy vegetables. Aust J Agric Res. 59:632–638. doi: 10.1071/AR07198
- Renna M, Castellino M, Leoni B, Paradiso V, Santamaria P. 2018. Microgreens production with low potassium content for patients with impaired kidney function. Nutrients. 10:675. doi: 10.3390/nu10060675
- Simonne E, Simonne A, Wells L. 2001. Nitrogen source affects crunchiness, but not lettuce yield. J Plant Nutr. 24:743–751. doi: 10.1081/PLN-100103667
- Soltanpour PN, Johnson GW, Workman SM, Jones JB, Miller RO. 1996. Inductively coupled plasma emission spectrometry and inductively coupled plasma mass spectrometry. In: DL Sparks, editor. Methods of soil analysis. Part 3. Chemical methods. Madison, WI: Soil Science Society of North America; p. 91–139.
- Sublett W, Barickman T, Sams C. 2018. Effects of elevated temperature and potassium on biomass and quality of dark red ‘lollo Rosso’ lettuce. Horticulturae. 4:11. doi: 10.3390/horticulturae4020011
- Thomas AL, Guerreiro SMC, Sodek L L. 2005. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann Bot. 96:1191–1198. doi: 10.1093/aob/mci272
- Wagatsuma T, Nakashima T, Tawaraya K, Watanabe S, Kamio A, Ueki A. 1990. Role of plant aerenchyma in wet tolerance of and methane emission from plants. In: ML Beusichem, editor. Plant nutrition – physiology and applications. Dordrecht: Springer; p. 455–461.
- Taban BM, Halkman AK. 2011. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe. 17:286–287. doi: 10.1016/j.anaerobe.2011.04.004
- Tang Y, Gao F, Guo S, Li F. 2015. The morphology, physiology and nutritional quality of lettuce grown under hypobaria and hypoxia. Acta Astronaut. 112:29–36. doi: 10.1016/j.actaastro.2015.03.005
- Tesi R, Lenzi A, Lombardi P. 2003. Effect of salinity and oxygen level on lettuce grown in a floating system. Acta Hortic. 609:383–387. doi: 10.17660/ActaHortic.2003.609.58
- Tsukagoshi S, Yamazoe H, Hohjo M, Shinohara Y, Ikegami F, Johkan M, Maruo T. 2017. Production of low-potassium tomato fruit for dialysis patients by NFT or by rockwool culture. Acta Hortic. 1206:233–238.
- Yordanova RY, Popova LP. 2007. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plant. 29:535–541. doi: 10.1007/s11738-007-0064-z
- Yordanova RY, Alexieva VS, Popova LP. 2003. Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants. Russ J Plant Physiol. 50:163–167. doi: 10.1023/A:1022908811211
- Yosoff SF, Mohamed MTM, Parvez A, Ahmad SH, Ghazali FM, Hassan H. 2015. Production system and harvesting stage influence on nitrate content and quality of butterhead lettuce. Bragantia. 74:322–330. doi: 10.1590/1678-4499.0453
- Zhang G, Johkan M, Hohjo M, Tsukagoshi S, Maruo T. 2017. Plant growth and photosynthesis response to low potassium conditions in three lettuce (Lactuca sativa) types. Hortic J. 86:229–237. doi: 10.2503/hortj.OKD-008
- Zheng Y, Wang L, Dixon M. 2007. An upper limit for elevated root zone dissolved oxygen concentration for tomato. Sci Hortic. 113:162–165. doi: 10.1016/j.scienta.2007.03.011
