?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nitrogen fertiliser application can effectively increase the net primary productivity (NPP) of agriculture and grassland ecosystems. There is some long-term retention of nitrogen applied to soils, but much is lost to runoff, to in situ denitrification and to gaseous forms of nitrogen (such as NH3 and NOX). So it is particularly important to improve nitrogen-use efficiency through various ways. Therefore, the effects of the urea with urease inhibitor N-(n-butyl)thiophosphoric triamide (NBPT) or/and nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on the NPP, NH3 and 15N recovery were researched on the alpine grassland ecosystem. The results showed that the addition NBPT to urea can significantly reduce the cumulative NH3 emission of urea-N compared with urea alone. The NBPT or DMPP addition to urea significantly increased the above-ground NPP and the economic efficiency. Urea added to NBPT or NBPT+DMPP could increase the 15N recovery rate of plant and soil (0-40 cm) by nearly 20%. The results indicated that urea combined with DMPP may be a good way to nitrogen fertiliser retention and reduction of NH3 emission for the alpine grassland of the northeast of the Qinghai-Tibet Plateau.
Main text introduction
The Qinghai-Tibet Plateau (QTP) is one of the main animal husbandry bases in China. The grassland area is about 1.5 million ha, accounting for 37.6% of the total grassland area of China. There are 2.26 × 105 ha of sown pastures and 3.3 × 103 ha planted to forage crops, including oats and alfalfa, in Tongde, Xinghai and Zeku counties of QTP. Almost all these planted areas are on former alpine steppe, and some of which is underlain with permafrost. The soil fertility level in alpine meadow is variable with available N and P as the main limiting factors for the productivity.
Nitrogen fertiliser is widely used to maintain the productivity of artificial sown pastures and fodder crops. However, ammonia volatilisation is one of the main factors to reduce the utilisation rate of nitrogen fertiliser in the cropping and pasture lands (Gurgel et al. Citation2016). The long-term application of large amounts of nitrogen fertiliser not only causes a huge loss in energy and resource but also negatively affects the ecological environment (Zaman et al. Citation2008; Singh et al. Citation2013). Research has shown that average NH3 losses worldwide are of the order of 14% (10–19%) of the N fertilisers used (Ferm Citation1998) and are higher in the warm climates (Liyanage et al. Citation2014).
The utilisation rate of nitrogen fertiliser is affected by various factors, such as fertilisation level, fertilisation method, soil traits, meteorological conditions and vegetation types, and the average N utilisation rate is only 30−35% (Raun et al. Citation2002; Cong et al. Citation2017; Cjp et al. Citation2012). Reducing ammonia volatilisation and nitrification of the nitrogen fertiliser are the main ways to improve the nitrogen utilisation rate, reduce environmental pollution and realise the sustainable development of crop and pasture lands (Keshavarz et al. Citation2018).
In the past, the way to reduce volatilisation and increase the utilisation rate of nitrogen fertiliser was mainly to apply fertiliser several times, reducing the amount of nitrogen applied each time (Watson et al. Citation2010; Klikocka et al. Citation2017; Li et al. Citation2018) or with better management of water and fertiliser (Khalid et al. Citation2018). The planted areas on former alpine meadow receive (NH4)2HPO4 as the base fertiliser and the CO(NH2)2 as the additional fertiliser. The amount of the (NH4)2HPO4 is 75–375 kg hm−2 and CO(NH2)2 is 75–300 kg hm−2 (Zhang et al. Citation2003; Li et al. Citation2004).
Urease inhibitors can delay the hydrolysis of urea in the soil. Thereby, indirectly delaying the oxidation of to
, while the nitrification inhibitor directly inhibits the oxidation of
to
, and also reduces
leaching and gas loss such as N2 and N2O (Martins et al. Citation2017; Li et al. Citation2018). The most widely used urease inhibitors in an agricultural ecosystem are hydroquinone (HQ) and triammonium butyl thiophosphate (NBPT, C4H14N3PS) (Hagenkampkorth et al. Citation2015). NBPT is currently one of the most effective soil urease inhibitors. Under non-acidic soils and good aeration conditions, NBPT can effectively weaken the formation of
. It increased the utilisation rate of urea. NBPT will achieve the best effect combined with urea. NBPT with generally 0.5–1% of nitrogen application is more effective in alkaline soil (Watson et al. Citation2010). Some related experiments also proved that the inhibition of HQ on urease is time-dependent, and the inhibition gradually weakens with time (Ma et al. Citation2019).
There are many types of nitrification inhibitors, mainly 2-chloro-6-(trichloromethyl)pyridine (CP), dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP) and amidinothiourea (ASU) (Slagen and Kerkhoff Citation1984; Trenkel Citation2010; Soares et al. Citation2012). The combination of both inhibitors can potentially result in the reduction of NH3 volatilisation and NO3 leaching losses, which may have economic and environmental impacts (Soares et al. Citation2012). DMPP has many advantages compared with the currently widely used nitrification inhibitors on the market, such as non-toxic to plants, non-polluting to the soil and non-irritating to humans. The nitrification inhibition efficiency is high. Because DMPP is easily adsorbed by soil colloids, it migrates slowly in the soil and is not easily washed by rain. Moreover, DMPP only needs to add 1% of urea to obtain a well nitrification inhibitory effect (Trenkel Citation2010).
Published studies show that urea with urease inhibitor or nitrification inhibitor can slow down ammonia volatilisation loss and improve the utilisation rate of nitrogen fertiliser (Ni et al. Citation2014). Guided by these findings, we sought to assess the effectiveness of such inhibitors in high-altitude areas of NE QTP.
We need to know how to increase the nitrogen utilisation rate on sown pasturelands. Therefore, we studied the effects of urea combined with NBPT or/and DMPP on the NH3 volatilisation, the above-ground and underground NPP and nitrogen recovery rate based on the use of a nitrogen isotope labelling technique.
Materials and methods
Overview of the research area
The experiment was conducted at the forage breeding field at Tongde County, Qinghai Province (Wang et al. Citation2016). The site lies 35°15′20″N and 100°39′21″E with an altitude of 3280 m. The annual average precipitation is 429.8 mm, and the annual average temperature is 0.2°C. The active accumulated temperature of more than 0°C is 1503.0°C, and the active accumulated temperature is 1309.0°C in the forage growing season.
There is no absolute frost-free period. The soil type is classified as kastanozems (IUSS WG WRB Citation2015). Parent material is a wide range of unconsolidated materials. Soil profile development was studied by Hu et al. (Citation1991). The soil chemical and physical properties are shown in . Soil analysis (0–20 cm) was performed using the methods of Dewis and Freitas (Citation1970) and Nakatani et al. (Citation2011).
Table 1. Chemical and physical properties of the soil samples.
As the main forage species of artificial grassland on the QTP, oats grow in the alpine regions with an altitude of 3000–4000 m. Oats not only have the biological characteristics of salt–alkali tolerance, drought and cold tolerance and high yield but also have rich nutrition and good palatability. It is an important forage species for artificial grassland in alpine regions. According to statistics, oats have the largest cultivation area, accounting for about 70% of the artificial grassland area of the QTP (Zhao and Shi Citation2011). Oat forage is mainly used in winter to provide additional forage for cattle and sheep and plays an important role in maintaining the balance of grass and livestock in alpine grasslands. Therefore, the result of this study represented grasslands and is widely represented in the QTP.
Experimental design
We conducted this experiment on 1200 m2 within the oat field. Oats is an annual unicast artificial grassland. It is sown at the beginning of May. (NH4)2HPO4 is used as the base fertiliser. The application rate is 50 kg·hm−2. The final output is for local animal husbandry production. There were five treatments: (i) a non-N fertiliser treatment; (ii) a urea application treatment; (iii) a U + urease inhibitors (NBPT) treatment. (iv) a U + nitrification inhibitor (DMPP) treatment and (v) a U + NBPT + DMPP treatment ().
Table 2. Experimental design.
We adopted a random block design and set up five groups of 2 × 3 m treatment sampling plots. Each treatment had 3 repetitions, and a total of 15 treatment sampling plots arranged along a transect at 1 m intervals. Each plot received 55.20 kg N hm−2 in early July 2015. The 15N abundance of the U was 10.2%. The amount of DMPP and NBPT was 1% and 0.5% of the nitrogen amount by the method of Watson et al. (Citation2010) and Pereira et al. (Citation2013).
Determination of ammonia volatilisation
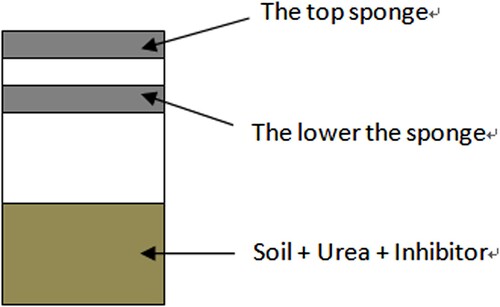
Ammonia volatilisation was determined by the closed chamber method (Wang et al. Citation2002). PVC pipe with a diameter of 19.2 cm and a length of 40 cm was inserted in each treatment plot, which is 20 cm below ground level and 20 cm above ground level. An ammonia recovery unit was installed as shown in . Two sponges with 2 cm thick and 20 cm in diameter were used as ammonia absorption media that were uniformly immersed in 30 mL of glycerol phosphate solution (50 mL of phosphoric acid + 40 mL of glycerol, ultrapure water to 1 L) (Wang et al. Citation2002), then carefully placed in PVC tube. The upper sponge was placed on top of the PVC pipe to prevent ammonia and dust from entering the device; the lower sponge was 10 cm away from the soil surface to absorb the ammonia volatilised from the soil. The ammonia volatilisation rate was measured on the 1, 2, 3, 4, 5, 6, 7, 9 and 11 days after fertilisation. The lower layer sponge was taken out and stored in a refrigerator at −4°C. They were shaken for 1 h on a shaker with a speed of 150 r·min−1 to obtain an extract. To determine the concentration in the extract, we followed the method of Yang et al. (Citation2019) and used the FUTURA equipment (produced by Alliance Instruments, France)
Figure 1. Schematic diagram of ammonia volatilisation absorption device. The lower sponge with 30 ml (50 mL of phosphoric acid + 40 mL of glycerol, then added ultrapure water to 1 L) captures the ammonia volatilised from the soil. The top sponge blocks with the glycerol phosphate solution the ammonia in the atmosphere.
Determination of δ15N values of above-ground tissue, root and soil
We collected above-ground tissue, root and soil samples from each treatment plot at the end of August. The soil sample was collected by three cores (diameter 5 cm) from each plot, divided into 0–10, 10–20, 20–30 and 30–40 cm soil layers. All the soil of same depth in each treatment plot was mixed together. The soil samples were immediately transferred to the laboratory and passed through a 2-mm soil sieve to remove roots. The roots (0–10, 10–20, 20–30 and 30–40 cm) were rinsed with tap water, with 0.5 mmol·L−1 CaCl2 solution rinsed 10 min, then rinsed with distilled water to remove 15N attached to the root surface (Reverchon et al. Citation2014). The above-ground tissue (area 50 × 50 cm) was collected in the plot. Stems, leaves and roots were dried at 80°C, then ground and passed through a 100 mesh sieve (Templer et al. Citation2012). Soil samples were naturally air-dried, then ground and passed through a 100 mesh sieve. The δ15N value and the nitrogen content of each sample were analysed by an elemental analyser continuous flow Isotope ratio mass spectrometer at U.S. Davis Stable Isotope Facility.
Calculations and statistical analyses
Ammonia volatilisation rate
where A is the
content (mg N) measured in the ventilation method; B is the cross-sectional area of the capture device (m2) and C is the time of each continuous capture. The data are the average of three replicate values for each treatment (Wang et al. Citation2002).
Cumulative loss of ammonia volatilisation
where V1 is ammonia absorption in the lower layer sponge for 24 h each fertilisation treatment (mg N); V0 is the ammonia absorption in the lower layer sponge for 24 h CK treatment (mg N).
Nitrogen recovery
We used 15N tracer recoveries based on N mass, amount of 15N applied and 15N enrichments of different pools. We calculated the proportion of 15N tracer recovered (15Nrec) within different pools as follows:
where F is fractional abundance (15N/(14N + 15N)) in the sample, in the non-labelled reference sample or in the tracer; Npool and Ntracer are the masses of N in the different pools and in the tracer applied to that plot, respectively (Providoli et al. Citation2005). We used 15N tracer recoveries as estimates of different pools net N retention of the cohort of N added at specific times.
Statistical analysis
We tested the significance of the difference of all the parameters from different treatments using one-way ANOVA, LSD. All statistical analyses were conducted using Excel 2007 and SPSS 19.0. Use OriginPro 8.0 software drawing app.
Results
Ammonia volatilisation rate
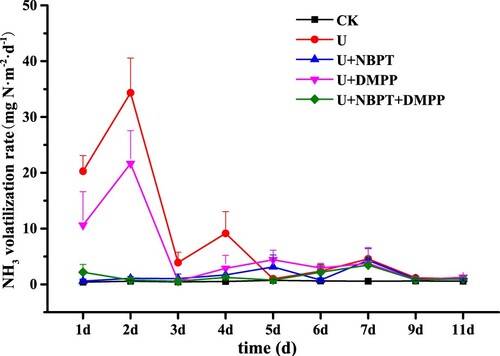
Ammonia volatilisation rate in U combined with NBPT/DMPP is shown in . The rate of ammonia volatilisation was kept at the lowest level in CK treatment, and the range of variation is 0.41–0.71 mg N·m−2·d−1. The ammonia volatilisation rate of the urea alone reached the maximum value of 34.34 mg N·m−2·d−1 on the second day, then it decreased after the third day. The ammonia volatilisation rate of the U + DMPP reached a maximum value of 21.65 mg N·m−2·d−1 on the second day, a second peak of 4.41 mg N·m−2·d−1 appeared on the 5th day, and then decreased slowly with time. The ammonia volatilisation rate of the U + NBPT and U + NBPT + DMPP reached the maximum on the 7th day, which was 4.32 and 3.46 mg N·m−2·d−1. Compared with urea treatment, the time to reach peak ammonia volatilisation rate was delayed in the U+NBPT and U+NBPT+DMPP treatment; the maximum ammonia volatilisation rate decreased by 87.4% and 89.9%, respectively.
Ammonia volatilisation accumulation
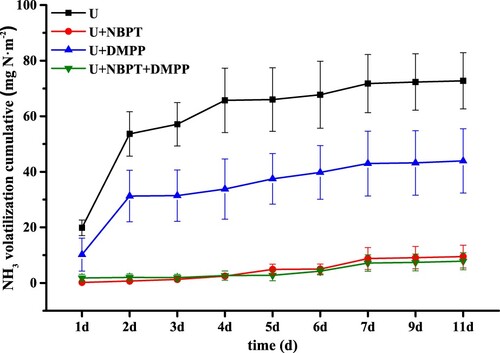
Ammonia volatilisation accumulation under the different treatments is shown in . It can be seen that the accumulative ammonia volatilisation was 72.8 mg N·m−2 for 11 days under the urea alone. The cumulative ammonia volatilisation in U + NBPT, U + DMPP and U + NBPT + DMPP was 9.48, 43.93, 7.82 mg N·m−2, respectively – a decrease of 87.0%, 39.6% and 89.0% compared with urea alone. There was no significant difference in ammonia volatile accumulation loss between U + NBPT and U + NBPT + DMPP, but the U + NBPT, U + DMPP and U + NBPT + DMPP was significantly lower (P < .05) compared with urea alone. Treatments involving inhibitors can reduce significantly the cumulative loss of ammonia volatilisation from urea fertiliser. But the U + DMPP was significantly lower than U + NBPT and U + NBPT + DMPP on ammonia volatilisation.
Net primary productivity
The NPP of stems, leaves and roots is shown in . The NPP at the site ranged from 565.6 g·m−2·y−1 in CK to over 700 g·m−2·y−1. U + DMPP significantly improves NPP compared with single urea treatment. U + DMPP significantly improves root primary productivity than U + NBPT and U + NBPT + DMPP. There are no significant differences for above-ground NPP in U + NBPT, U + DMPP and U + NBPT + DMPP. However, the average above-ground NPP of U + DMPP is higher than U + NBPT and U + NBPT + DMPP. The results show that the addition of NBPT or DMPP with urea can increase NPP by 7% and 5.6% (above-ground biomass), 5.0% and 8.0% (total biomass) compared with urea alone.
Table 3. The cauline leaf and root primary productivity of alpine oat land at different treatments (g·m−2·y−1).
Recovery of 15N in plant and soil
N concentration and δ15N value were analysed under no 15N fertiliser in former alpine grassland (). The results show that N concentration is 30.0 g·kg−1 in plant stems and leaves, 13.8–15.6 g·kg−1 in roots and 2.0–2.4 g·kg−1 in the soils. The δ15N value of plant stems and leaves is 7.7‰, and the root system is 7.1–16.9‰. The soil δ15N values vary from 5.9‰ to 6.6‰ with different depths.
Table 4. The nitrogen concentration and δ15N value under CK treatment.
The recovery rate of 15N in plant stems and leaves, root and soil under the different treatments are shown in . The recovery rate of the total 15N (cauline leaves + roots + soil) was 57.7% in urea alone rising to 79.8% in U + NBPT + DMPP.
Table 5. Recovery rates of 15N urea in plants and soils of alpine artificial grassland (%).
The 15N recovery in plant stems and leaves ranged from 28.5% to 37.6% among treatments with the highest levels in U + NBPT + DMPP. The 15N recovery was 2.3–2.7% in plant roots. The 15N recovery was 25.9–41.6% in 0–40 cm soil layer. Therefore, the15N applied to the soil was mainly distributed in the plant above-ground tissue and soil after 50 days; the 15N distribution in the root was the least. The 15N labelled urea in the soil was mainly distributed in the 0–10 cm layer across all treatments.
The 15N recovery rate in plant stems and leaves was the highest in U + NBPT reaching 37.6%, and the lowest was 28.5% in U + DMPP. The 15N recovery rate of plant roots was not significantly different among treatments, only 2.3–2.7%, while in soil it was up to 41.7% in U + NBPT + DMPP. The rate was 25.9–29.6% in other treatments.
So we can conclude that the addition of NBPT or DMPP could significantly increase the urea recovery rate in former alpine grassland sites. Compared with urea alone, added NBPT + DMPP could increase the 15N recovery rate by 22%, and added NBPT alone could increase the 15N recovery rate by 12%. There was no significant difference between U and U + DMPP. The 15N recovery rate of U + DMPP was significantly lower than that of U + NBPT and U + NBPT + DMPP.
Economic beneficial
In the domestic market, the price of dried oat grass is 244 dollar·ton−1, and the prices of NBPT and DMPP are 13.7 and 16.8 dollar·kg−1, respectively. It can be seen from that the output value of each fertilisation treatment is increased by 169.1–266.69 dollar·hm−2, with an increase of 13.7–21.7%. Compared with a single application of the urea alone, the output value of dry oat grass treated with U + NBPT, U + DMPP and U + NBPT + DMPP is increased by 46.2%, 57.7% and 27.0%, respectively. After deducting the cost of the urea and inhibitors, U + NBPT, U + DMPP and U + NBPT + DMPP are increased by 74.3, 88.3 and 32.6 dollar·hm−2, by 48.5%, 57.6% and 21.3% compared with treatment 2, respectively.
Table 6. The economic benefits of different treatments (dollar·hm−2).
Discussion
Effect of nitrogen fertiliser with added urease/nitrification inhibitor on the ammonia volatilisation rate and accumulated ammonia loss
Ammonia volatilisation was an important nitrogen loss pathway when urea was applied to neutral or alkaline soil (Chien et al. Citation2009). Zhang et al. (Citation2013) found that the addition of the urease inhibitor NBPT did not affect the peak period of ammonia volatilisation rate and all treatments reached the peak on the fourth day after urea application. However, our results showed that the peak time of ammonia volatilisation rate was delayed where inhibitors were added to fertiliser, compared with the single urea treatment, and the peak value of ammonia volatilisation rate was significantly reduced. The main reason was that the soil of the study area was alkaline. After the urea was applied into the soil, it is quickly hydrolysed by soil urease into ammonium nitrogen, providing favourable conditions for ammonia volatilisation. Added NBPT can slow down the hydrolysis of urea-amide nitrogen to ammonium nitrogen and the conversion of ammonium nitrogen to nitrate nitrogen in the soil. The soil content was reduced, which effectively inhibited ammonia volatilisation loss (Turner et al. Citation2010). It may be that the soil microbial carbon source was rich and promoted microbial activity, which caused some nitrogen to be converted from mineralised state to be better retained (Jiang et al. Citation2014).
In addition, Freney et al. (1994) and Soares et al. (Citation2012) considered that the urea + nitrification inhibitor PPDA (phenylphosphoryl diamine) increased the amount of ammonia volatilisation loss. But our study found that urea + DMPP (Treatment 4) can reduce the accumulation loss of ammonia volatilisation compared with a single application of urea alone. It may have some relationships between ammonia volatilisation and the local soil texture, climate and inhibitor concentration (Soares et al. Citation2012). Research shows that adding 1%, 2%, 3%, 4%, 5%, 6% and 7% of different doses DMPP to (NH4)2SO4 has no significant difference in nitrification inhibition to the calcareous soil in Xinjiang. There is also no significant difference in the cumulative loss of ammonia volatilisation (Wang et al. Citation2017). It indicated that the effect of DMPP has nothing to do with the amount of addition. Barth et al. (Citation2001) obtained the same results in the farmland ecosystems. When the amount of DMPP in fertiliser increased by 10 times, its inhibitory effect did not change and there was no significant difference in ammonia volatilisation loss. Therefore, 1% of nitrogen is the optimal dosage of DMPP. If the amount of DMPP is increased, its inhibitory effect will not be much improved (Vilas et al. Citation2019).
This study proved that the ammonia volatilisation accumulation of urea combined with nitrification inhibitor was higher than urea combined with urease inhibitor. It may be that DMPP inhibited the activity of soil nitrifying bacteria and delayed the conversion of ammonium nitrogen to nitrate nitrogen. This led to an increase of the ammonia nitrogen in the soil and promoted the volatilisation of ammonia (Zhang et al. Citation2013).
The effect of nitrogen fertiliser added urease/nitrification inhibitor on nitrogen recovery rate
Tang and Weng (Citation1998) pointed out that the urea added nitrification inhibitor EP (2-cthymyl pyrudine) or urease inhibitor NBPT (N-butyl thiophosphoryl diamine) can increase N efficiency and reduce N loss. While Shoji et al. (Citation2001) indicated that CO(NH2)2 added nitrification inhibitor DCD had no significant influence on N fertiliser utilisation, but increased nitrogen loss. In our study, the utilisation rate of N fertiliser was 31.7% under a single U application Treatment 2, and that was 31.0% in Treatment 4 (U + DMPP). However, the utilisation rates of nitrogen fertiliser in treatments with added inhibitors (Treatments 3, 4 and 5) were 40.1% and 38.1%, which were 8.4% and 7.0% higher than that of single N fertiliser application. The results showed that the addition of two inhibitors (NBPT + DMPP) increased the N utilisation rate and that the addition of DMPP alone has no significant effect on N utilisation rate.
Wang et al. (Citation2016) found that the 15N recovery rates of the plant above-ground tissue, root and soil were 6.6%, 3.3% and 24.9%,when the K15NO3 was used as a tracer through the study of labelled nitrogen in artificial grassland of the QTP. When 15NH4Cl was used as the tracer, the recovery rates were 4.2%, 3.5% and 34.2% in the plant above-ground, root and soil, respectively. In the present study, 15N-urea was used as a tracer and the 15N recovery rates in plant above-ground tissue, roots and soil were 25.4%, 2.3% and 25.9%, respectively. The nitrogen recovery of U alone (Treatment 2) was better than that of the other two N forms, and the 15N utilisation of was higher in stems and leaves.
Templer et al. (Citation2012) reviewed the results of a 15N tracer study and found that retained an average of 62.7% of 15N in terrestrial ecosystem. According to different recovery times, the average total 15N recovery of ecosystems was 59.60% (<1 week), 80.10% (1 week to 1 month), 50.70% (1–3 months), 69.4% (3–18 months) and 61.6% (>18 months). In our study, the 15N recovery rate of plants and soils (0–40 cm) was 57.66–79.80% after 15N tracer applying one growing season of the alpine oat field. Compared with a single application of U, the best measure was fertiliser with two inhibitors, nitrogen recovery rate increased by 22%. The addition of single inhibitor led to a nitrogen recovery rate that increased by 12%. It is indicated that the addition of urease inhibitor NBTP can effectively improve the recovery rate of urea of the dark chestnut soil of QTP.
After applying 15N urea fertiliser alone, the 42.3% N was not recovered in plants and soil. Part of the lost nitrogen disappeared through ammonia volatilisation; another part of entered the atmosphere in the form of N2O and N2 through denitrification, and some
was lost by leaching. These were all ways in which traceable N fertilisers may be lost. These results indicated that reducing gaseous N loss and soil N leaching was an important step in the fertilisation process of Tongde site. In the dark chestnut soil of Tongde, the urea + DMPP had no significant effect on nitrogen fertiliser utilisation. However, fertiliser plus inhibitors can effectively reduce the loss of N and the nitrogen loss is 20.3% and 30.3%, respectively. Our research has shown that NBPT has significant effects on reducing ammonia volatilisation and improving fertiliser utilisation rate in alpine areas above 3000 m a.s.l., Worldwide, such landscapes are vital to the lives and livelihoods of millions of people.
Output and economic benefits
Economic benefit is an important indicator of the artificial grassland ecosystem, and a standard cannot be ignored for herders increasing their income. We should not only pay attention to the environmental effects of urea + inhibitors but also evaluate the ratio of input to output. The results of this study showed that after deducting the cost of urea and inhibitors, U + NBPT, U + DCD, and U + NBPT + DCD treatments increased by 74.3, 88.3 and 32.6 dollar·hm−2, by 48.5%, 57.6% and 21.3% compared with single urea treatment, respectively. In summary, on the one hand, in the practical application of urease inhibitors and nitrification inhibitors, we should pay attention to the reasonable dosage of inhibitors. On the other hand, we should reduce production costs as much as possible to play the largest economic role.
Acknowledgements
This work was supported in parts by the National Key Research and Development Program of China (Grant Nos. 2016YFC0501906-1) and the Natural Science Foundation of China (Grant Nos. 41761107). The manuscript is approved by all authors for publication. Liu Pan would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Liu Pan
Liu Pan is in the School of Life Sciences, Qinghai Normal University, Xining, People's Republic of China.
Wang Wenying
Wang Wenying is in the School of Life Sciences, Qinghai Normal University, Xining, People's Republic of China.
Zhou Huakun
Zhou Huakun is in the Key Laboratory of Restoration Ecology in Cold Region of Qinghai Province, Northwest Plateau Institute of Biology, Chinese Academy of Sciences, Xining, People's Republic of China.
Yang Chong
Yang Chong is in the School of Life Sciences, Qinghai Normal University, Xining, People's Republic of China.
References
- Barth G, Tucher SV, Schmidhalter U. 2001. Influence of soil parameters on the efficiency of the new nitrification inhibitor DMPP. In: Horst WJ, Schenk MK, Bürkert A., editors. Plant nutrition: food security and sustainability of agro-ecosystems through basic and applied research. Dordrecht: Kluwer Academic; p. 756–757.
- Chien SH, Prochnow LI, Cantarella H. 2009. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron. 102:267–322.
- Cjp G, Aarons SR, Powell JM. 2012. Nitrogen use efficiency and manure management practices in contrasting dairy production systems. Agric Ecosyst Environ. 147:73–81.
- Cong XH, Shi FZ, Ruan XM, Luo YX, Ma TC, Luo ZX. 2017. Effects of nitrogen fertilizer application rate on nitrogen use efficiency and grain yield and quality of different rice varieties. Chinese J Appl Ecol. 28(4):162–169.
- Dewis J, Freitas F. 1970. Physical and chemical methods of soil and water analysis. Soils Bulletin. 26(4):119–163.
- Ferm M. 1998. Atmospheric ammonia and ammonium transport in Europe and critical loads: a review. Nutr Cycling Agroecosyst. 51:5–17.
- Gurgel GCD, Ferrari AC, Fontana A, Polidoro JC, Coelho LDM, Zonta E. 2016. Ammonia volatilization from mixed mineral fertilizers containing urea. Pesqu Agropecu Bras. 9:1686–1694.
- Hagenkampkorth F, Haeussermann A, Hartung E. 2015. Effect of urease inhibitor application on urease activity in three different cubicle housing systems under practical conditions. Agr Ecosyst Environ. 202:168–177.
- Hu SX, Xu QZ, Zhang WX, Wu WY. 1991. Historic evolution of chestnut soil in the nort-heastern marginal area of the Qinghai-Tibet plateau. Act Pedologica Sinica. 28:203–210.
- IUSS Working Group WRB. 2015. World reference base for soil resources 2014, update 2015 International Soil Classification System for naming soils and creating legends for soil maps. Roma: FAO. World Soil Resources Reports No. 106; 192 p.
- Jiang X, Cao L, Zhang R, Yan L, Mao Y, Yang Y. 2014. Effects of nitrogen addition and litter properties on litter decomposition and enzyme activities of individual fungi. Appl Soil Ecol. 80:108–115.
- Keshavarz AR, Lin R, Mohammed YA, Chen C. 2018. Agronomic effects of urease and nitrification inhibitors on ammonia volatilization and nitrogen utilization in a dryland farming system: field and laboratory investigation. J Cleaner Prod. 172:4130–4139.
- Khalid HM, Mahmod IF, Barakbah SS. 2018. Impact of water management on fertilizer and tillering dynamics in rice. Int J Agric Biol. 20:37–40.
- Klikocka H, Cybulska M, Nowak A. 2017. Efficiency of fertilization and utilization of nitrogen and sulphur by spring wheat. Pol J Environ Stud. 26:2029–2036.
- Li GD, Schwenke GD, Hayes RC, Hongtao X, Lowrie AJ, Lowrie RJ. 2018. Does 3,4-dimethylpyrazole phosphate or n-(n-butyl) thiophosphorictriamide reduce nitrous oxide emissions from a rain-fed cropping system? Soil Res. 56:296–305.
- Li QY, Shi JJ, Ma YS, Dong QM. 2004. Effects of fertilizer application on sown pasture in the fountain head region of the Yangtze, Yellow and Mekong rivers. Pratacultural Sci. 21:35–38.
- Liyanage LRMC, Jayakody AN, Gunaratne GP. 2014. Ammonia volatilization from frequently applied fertilizers for the low-country tea growing soils of Sri Lanka. Trop Agr Res. 26(1):48–61.
- Ma Q, Wu ZJ, Yu WT, Zhou CR, Ning CC, Yuan HY, Xia ZQ. 2019. Does the incorporation of dicyandiamide and hydroquinone with straw enhance the nitrogen supplying capacity in soil? Appl Soil Ecol. 136:158–162.
- Martins MR, Sant’Anna SAC, Zaman M, Santos RC, Monteiro RC, Alves BJR, Jantalia CP, Boddey RM, Urquiaga S. 2017. Strategies for the use of urease and nitrification inhibitors with urea: impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric Ecosyst Environ. 247:54–62.
- Nakatani AS, Martines AM, Nogueira MA, Fagotti DSL, Oliveira AG, Bini D. 2011. Changes in the genetic structure of bacteria and microbial activity in an agricultural soil amended with tannery sludge. Soil Biol Biochem. 43:106–114.
- Ni K, Pacholski A, Kage H. 2014. Ammonia volatilization after application of urea to winter wheat over 3 years affected by novel urease and nitrification inhibitors. Agric Ecosyst Environ. 197:184–194.
- Pereira J, Barneze AS, Misselbrook TH, Coutinho J, Moreira N, Trindade H. 2013. Effects of a urease inhibitor and aluminium chloride alone or combined with a nitrification inhibitor on gaseous N emissions following soil application of cattle urine. Biosystems Eng. 115:396–407.
- Providoli I, Bugmann H, Siegwolf R, Buchmann N, Schleppi P. 2005. Flow of deposited inorganic N in two gleysol-dominated mountain catchments traced with 15NO3--N and 15NH4+-N. Biogeochemistry. 76:453–475.
- Raun WR, Solie JB, Johnson GV, Stone ML, Mullen RW, Freeman KW, Thomason WE, Lukina EV.2002. Improving nitrogen use efficiency in Cereal Grain Production with optical sensing and variable rate application. Agron J. 94(4):815–820.
- Reverchon F, Flicker RC, Yang H, Yan G, Zhang D. 2014. Changes in δ15N in a soil–plant system under different biochar feedstocks and application rates. Biol Fertil Soils. 50:275–283.
- Shoji S, Delgado J, Mosier A, Miura Y. 2001. Use of controlled release fertilizers and nitrification inhibitors to increase nitrogen use efficiency and to conserve air andwater quality. Commun Soil Sci Plant Anal. 32:1051–1070.
- Singh J, Kunhikrishnan A, Bolan NS, Saggar S. 2013. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Sci Total Environ. 465:56–63.
- Slagen JHG, Kerkhoff H. 1984. Nitrification inhibitors in agriculture and horticulture: a literature review. Fertil Res. 5:1e76.
- Soares JR, Cantarella H, Menegale MLDC. 2012. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol Biochem. 52:82–89.
- Tang JY, Weng BQ. 1998. Discussion on several methods and mechanisms of improving nitrogen use efficiency in paddy fields. J Plant Nutr Fertil. 4:242–248.
- Templer PH, Mack MC, Christenson LM, Compton JE, Crook HD, Currie WS, Curtis CJ, Dail DB, D'Antonio CM. 2012. Sinks for nitrogen inputs in terrestrial ecosystems: a meta-analysis of 15N tracer field studies. Ecology. 93:1816–1829.
- Trenkel ME. 2010. Slow- and controlled-release and stabilized fertilizer: an option for enhancing nutrient efficiency in agriculture. Paris: International Fertilizer Industry Association.
- Turner DA, Edis RB, Chen D, Freney JR, Denmead OT, Christie R. 2010. Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agric Ecosyst Environ. 137:261–266.
- Vilas MP, Verburg K, Thorburn PJ, Probert ME, Bonnett GD. 2019. A framework for analysing nitrification inhibition: a case study on 3,4-dimethylpyrazole phosphate (DMPP). Sci Total Environ. 672:846–854.
- Wang CH, Liu XJ, Ju XT, Zhang FS. 2002. Field in situ determination of ammonia volatilization from soil:venting method. Plant Nutr Fertil Sci. 8:205–209.
- Wang WY, Li WQ, Zhou HK, Kang Q, Ma XL, Liu P, Wang Z. 2016. Dynamics of soil dissolved organic nitrogen and inorganic nitrogen pool in alpine artificial grasslands. Ecol Environ Sci. 25:30–35.
- Wang XW, Liu T, Chu GX. 2017. Inhibition of DCD, DMPP and nitrapyrin on soil nitrification and their appropriate use dosage. J Plant Nutr Fertil. 23:54–61.
- Watson CJ, Akhonzada NA, Hamilton JTG, Matthews DI. 2010. Rate and mode of application of the urease inhibitor n-(n-butyl) thiophosphorictriamide on ammonia volatilization from surface-applied urea. Soil Use Manag. 24:246–253.
- Yang Y, Ni XY, Liu BM, Tao LZ, Yu LX, Wang Q, Yang Y, Liu J, Wu YJ. 2019. Measuring field ammonia emissions and canopy ammonia fluxes in agriculture using portable ammonia detector method. J Cleaner Prod. 216:542–551.
- Zaman M, Nguyen ML, Blennerhassett JD, Quin BF. 2008. Reducing NH3, N2O and NO3-–N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol Fertil Soils. 44:693–705.
- Zhang Y, Wei QI, Zhou C, Ding M, Liu L, Gao J, Bai W, Wang Z, Du Z. 2013. Spatial and temporal variability in the net primary production (NPP) of alpine grassland on Tibetan Plateau from 1982 to 2009. Acta Geographica Sinica. 24:1197–1211.
- Zhang YS, Zhao XQ, Huang DQ. 2003. The study on sustainable using of perennial sowing grassland in the Qinghai-Tibet Plateau pasture. ActaPratacultural Sci. 3:22–27.
- Zhao GQ, Shi SL. 2011. The current situation of oat research and production, problems and strategy in Tibetan plateau. Pratacultural Sci. 21:17–21.