ABSTRACT
Faba bean is susceptible to several leaf diseases caused by pathogenic fungi. With the increasing importance of faba bean in northern European cropping systems, the importance of its leaf diseases is likely to increase. The aim of this study was to discriminate the diseases and to test agronomic strategies for limiting their spread in a five-year experiment. Chocolate spot disease caused by Botrytis spp. and leaf blotches caused by an Alternaria/Stemphylium complex dominated, the severity of rust was lower, and downy mildew occurred in only one year. The severity of the diseases depended on weather conditions and cultivar, but not on sowing rate. Application of fungicides significantly decreased the severity of chocolate spot, leaf blotch and rust, but not downy mildew, and in no case was the disease progress stopped. None of the cultivars showed strong resistance to disease. The Alternaria/Stemphylium leaf blotch is an emerging disease of faba bean in northern Europe. Work continues on the identification of the responsible species in the chocolate spot and leaf blotch complexes and the determination of their relative importance in causing disease.
Introduction
Faba bean (Vicia faba L.) is one of the most important grain legumes, with the highest global average grain yield after soybean (FAOstat Citation2020). Its importance in Europe is increasing (Eurostat Citation2020), partly as a result of efforts to reduce reliance on imported soy protein for feed use (Watson et al. Citation2017).
Faba bean is affected by a wide range of leaf diseases (Stoddard et al. Citation2010; Karkanis et al. Citation2018; Plūduma-Pauniņa et al. Citation2018). Of these, chocolate spot (caused by Botrytis spp.) is the most widely written about (Stoddard et al. Citation2010). According to bibliometric analysis, Ascochyta blight (caused by Didymella fabae) and rust (caused by Uromyces viciae-fabae) are of approximately equal importance. Leaf blights (caused by Alternaria spp. and Stemphylium spp.), Cercospora leaf spot (caused by Cercospora zonata) and downy mildew (caused by Peronospora viciae f. sp. fabae) are also found. Each disease has its environmental optima (Stoddard et al. Citation2010), so the dominant disease varies across regions and years.
Chocolate spot is attributable to at least three species of Botrytis: B. fabae, B. cinerea and B. fabiopsis (Zhang et al. Citation2010), and possibly others (Bankina et al. Citation2017). In some conditions, grey mould caused by B. cinerea has been described as a separate disease (Boligłowa et al. Citation2016), but it is challenging to distinguish these pathogens under field conditions. Leaf blights caused by Alternaria spp. and Stemphylium spp. have become more widely observed in recent years, suggesting an increase in distribution and destructiveness (Sheikh et al. Citation2015; Vasić et al. Citation2019) and they are well established in the Baltic region (Plūduma-Pauniņa et al. Citation2019). Alternaria alternata and A. tenuissima are considered the main causal agents of Alternaria leaf blight, but other species have been found (Rahman et al. Citation2002; Coca-Morante and Mamani-Álvarez Citation2012). Similarly, several species of Stemphylium are associated with the disease on faba bean, including S. botryosum, S. eturmiunum and S. vesicarium (Sheikh et al. Citation2015; Caudillo-Ruiz et al. Citation2017; Vaghefi et al. Citation2020).
Meteorological conditions are one of the main limiting factors that affect disease development. In general, temperatures around 20 °C, free moisture on the leaves and high (<90%) relative humidity are optimal conditions for the development of most leaf blotch diseases (Harrison Citation1988; Stoddard et al. Citation2010; Salam et al. Citation2016; Watson et al. Citation2017). Disease development depends also on agronomic practices, with high crop density increasing the spread of disease by reducing transmission distances and increasing canopy humidity, while intercropping can reduce the spread by intercepting propagules on non-susceptible species (Stoddard et al. Citation2010). Genetic resistance to diseases is the ideal way to control them, but few sources of strong or long-lasting resistance to any disease have been found in faba bean (Stoddard et al. Citation2010; Ijaz et al. Citation2018; Sudheesh et al. Citation2019). Hence, fungicide application is often the only available tool for controlling disease development and azoles, strobilurins and dithiocarbamates are considered effective against several leaf diseases of faba bean (Ahmed et al. Citation2016).
With the increasing importance of faba bean in northern European cropping systems, the importance of its leaf diseases is likely to increase. Hence, we set out to discriminate the diseases from each other, to evaluate their relative importance and to test agronomic strategies for limiting their spread in a five-year experiment.
Materials and methods
Field trials were conducted from 2015 to 2019 at the Pēterlauki Research and Study Farm of the Latvia University of Life Sciences and Technologies (56.54 °N, 23.71 °E). The prevailing soil type is silty loam characterised as an Endocalcaric Abruptic Luvisol. Disease assessment was part of a larger experiment investigating agronomic strategies for optimising yield of faba bean. Data about disease development were obtained from a three-factor experiment: cultivar (‘Isabell’; ‘Boxer’ and ‘Laura’), sowing rate (30, 40 and 50 germinable seeds m−2) and fungicide (combined boscalid, 267 g kg−1 and pyraclostrobin, 67 g kg−1 application or without fungicide), with four replicates in a full factorial, randomised split block. Sowing dates were 26 March 2015, 5 April 2016, 4 April 2017, 29 April 2018 and 15 April 2019. Harvest dates were 27 August 2015, 29 August 2016, 24 September 2017, 13 August 2018, and 29 August 2019. Detailed descriptions of the trial site and agronomic practices have been published (Plūduma-Pauniņa et al. Citation2018; Plūduma-Pauniņa et al. Citation2019).
Diseases were identified by visual evaluation when symptoms were typical and by microscopic evaluation of spores obtained from infected tissues.
Severity of diseases were evaluated on a 10-point scale (0: no disease symptoms, 1: 1–3 spots on the whole plant; 2: 4–6 spots on the plant; 3: up to 10% of the whole plant covered by spots; 4: 11–25% of the whole plant covered by spots; 5: 26–50% of the whole plant covered by spots; 6: 51–75% of the whole plant covered by spots; 7: >75% of the whole plant covered by spots; 8: all leaves covered with spots; 9: plant is dead) every two weeks from the time of appearance of first symptoms until crop maturity. Areas under the disease progress curves (AUDPC) were calculated (Simko and Piepho Citation2012).
Temperature and amount of precipitation were recorded daily by an automatic weather station near the experimental field () and the hydrothermal coefficient (HTC) was calculated (Vlăduţ et al. Citation2017) when the daily average temperature was above 10 °C (). Values of HTC close to 0.9–1.1 are considered as optimal conditions, values above 1.1 as wet, and those below 0.9 as dry.
Table 1. Average temperature (°C), amount of precipitation (mm) and hydrothermal coefficient (HTC) during the growing season at the Pēterlauki Research and Study farm of the Latvia University of Life Sciences and Technologies.
Multifactorial analysis of variance (ANOVA) was used to evaluate the influence of year, cultivar, sowing rate and fungicide use on the development of faba bean diseases using R software. The differences among factor groups were compared using Bonferroni test with an alpha level of 0.05.
Results
Symptoms and disease identification
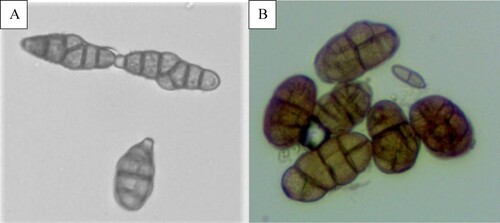
Chocolate spot disease was detected every year. Typical small reddish-brown spots and/or large necrotic blotches with reddish margins were observed on the leaves, stems and pods (). In some cases, small black sclerotia were found, mostly on the pods. In wet conditions, blotches were covered with powdery grey mould that consisted of conidiophores and conidia (.)
Figure 1. Botrytis spp., (A) conidiophores and conidia (magnification × 40) and (B) symptoms of chocolate spot disease on a leaf.
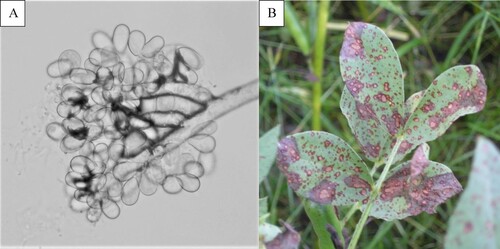
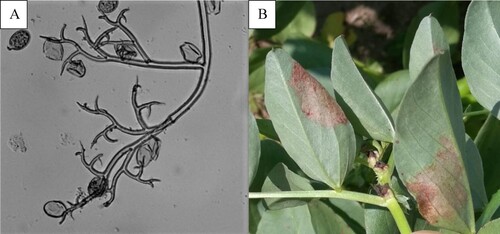
Large, grey to black, necrotic and fused blotches were also observed on leaves every year (). The symptoms were typical of either Alternaria or Stemphylium, so diagnosis via microscopy was necessary. Spores typical of both genera Alternaria and Stemphylium were found, indicating that they were the causal agents (). It was not possible to distinguish the blotches caused by one or the other pathogen under field conditions, and both pathogens were isolated from the same plants and same blotches, so this disease appears to have been caused by a complex of the two pathogens and was called Alternaria/Stemphylium leaf blotch.
Rust infected plants every year, and was identified by its distinctive and bright orange uredo-pustules.
Downy mildew formed blotches with reddish tint on the underside of leaves in 2017 and conidiophores typical of genus Peronospora were found ().
Seasonal progress of diseases
The first symptoms of both chocolate spot and leaf blotch were observed in early June, before the start of flowering. In most years, rapid disease development started in July (end of flowering to development of pods), except in 2016, when the progress of disease started earlier ( and ); in this season, the faba beans developed earlier and ended flowering at the end of June.
Figure 5. Dynamics of development of chocolate spot, caused by Botrytis spp. in untreated plots. Data show means across cultivars and plant densities.
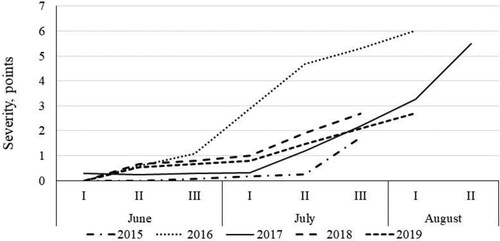
Figure 6. Dynamics of development of leaf blotch (caused by Alternaria/Stemphylium complex) in untreated plots. Data show means across cultivars and plant densities.
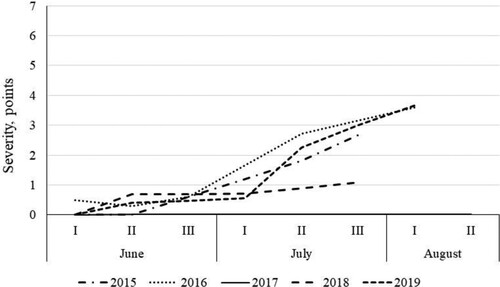
Rust appeared during pod development and its scores remained low, failing to reach 1 point until August. Downy mildew was found only in 2017 and the first symptoms were seen in the third decade of July (development of pods). During pod ripening, disease severity ranged from 1.2 to 2.4 points.
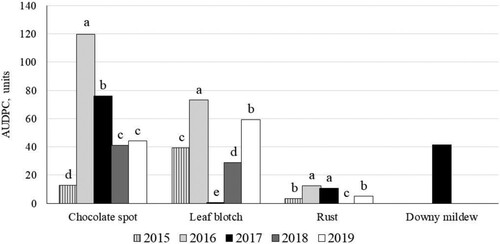
The AUDPC differed widely between years (p < 0.0001) (). The highest values for chocolate spot, leaf blotch and rust were observed in 2016. The lowest severity of chocolate spot was in 2015, only traces of leaf blotch were observed in 2017, and rust was not detected in 2018.
Figure 7. Development of faba bean leaf diseases as AUDPC, depending on year (2015–2019) in untreated plots. Data show means across cultivars and crop densities. Significantly (p < 0.001) different means within a year are labelled with different letters.
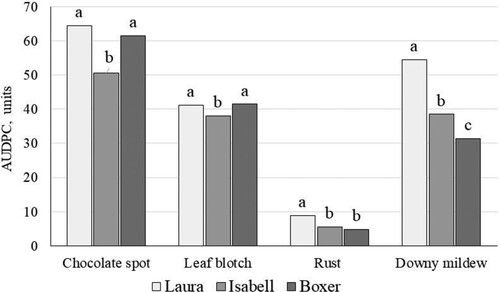
Cultivars showed differences in AUDPC averaged across years (p < 0.001 for leaf blotch and p < 0.0001 for the other diseases), but none could be classified as resistant (). Cultivar ‘Laura’ was the most sensitive to all observed diseases, whereas ‘Boxer’ was least sensitive to downy mildew but equal to ‘Laura’ in sensitivity to Alternaria/Stemphylium leaf blotch and chocolate spot. ‘Isabell’ was least sensitive to chocolate spot.
Figure 8. Development of faba bean leaf diseases as AUDPC, depending on cultivar in untreated plots. Data show means across years and crop densities. Significantly (p < 0.001) different means within a disease are labelled with different letters.
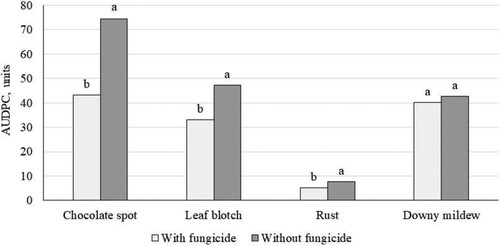
Fungicide application decreased the AUDPC of all diseases (p < 0.0001), except downy mildew (p > 0.05) ().
Figure 9. Development of faba bean leaf diseases depending on fungicide application. Data show means across years, crop densities and cultivars (p > 0.05 for downy mildew; p < 0.0001 for other diseases). Different letters indicate significance of difference between treatments within a disease.
Seed rate did not influence the development of any of the diseases.
Treatment interactions were not statistically significant.
Discussion
During the course of this experiment, chocolate spot was the most prevalent disease, followed by Alternaria/Stemphylium leaf blotch, while rust was minor and downy mildew was found in only one year (). In two of the five years, leaf blotch symptoms were more severe than those of any other disease, indicating the need for improved knowledge about this parasite complex and how it may be controlled.
The ubiquity of chocolate spot disease was according to expectations, as was its moderate impact in three of the five years. It was most severe, with the disease severity approaching 6 (>50% of leaf area covered with spots), in 2016 and 2017. This was associated with a high HTC and heavy rainfall in early July of both years, confirming the importance of leaf wetness for the spread of this disease (Stoddard et al. Citation2010). Furthermore, the cool weather in July and August 2017 was associated with prolonged growth of the crop and availability of green leaves for the disease to spread, as shown by the prolonged disease score curve for that year (). In contrast, the cool and dry weather of 2015 was associated with a low incidence of this disease.
Leaf blotch caused by Alternaria and Stemphylium spp. was observed in every year except 2017. Symptoms of Alternaria infection have been described as slowly increasing brown spots with concentric rings, often water-soaked (Rahman et al. Citation2002; Abd El-Hai Citation2015) while those of Stemphylium infection are large, grey to black spots growing from the leaf margins (Vaghefi et al. Citation2020). In the present work, the leaf spots were large, dark and shapeless as the result of coalescence of smaller spots (), thus showing some of the symptoms of both pathogens. Furthermore, both genera were recovered from the spots. In Australia, although two species of Stemphylium were recovered from leaf spots on faba bean, only some isolates of one (S. eturmiunum) were pathogenic in subsequent tests, and the same may be true here. In Bolivia, several species of Alternaria were mentioned as causal agents of leaf blotch (Coca-Morante and Mamani-Álvarez Citation2012). Both genera have been found to cause significant crop damage, with more than 90% of plants affected by Alternaria in some fields in Japan (Rahman et al. Citation2002) and lethal damage attributed to S. eturmiunum in Australia (Vaghefi et al. Citation2020). Neither genus is identifiable to species level by morphological tools alone, and DNA sequence data has been used for species identification in both Alternaria (Woudenberg et al. Citation2013) and Stemphylium (Vaghefi et al. Citation2020).
In the present study, the leaf blotches were found before flowering, similar to the timing of Stemphylium leaf blotch in Australia (Vaghefi et al. Citation2020) and Alternaria leaf spot in Japan (Rahman et al. Citation2002). Although the maximum severity of Alternaria/Stemphylium leaf blotch was only 3.7 points, in contrast to 6.0 for chocolate spot, the AUDPC of leaf blotch exceeded that of chocolate spot in both 2015 and 2019 ( and ). In both these years, the mean summer temperature was slightly lower and the rainfall was much lower than in 2016, the year that was particularly favourable for chocolate spot. The absence of leaf blotch in 2017 was associated with the strong presence of downy mildew and it is not possible to determine whether the absence was due to poor conditions or to the presence of the other disease. Taken together with results from several countries in different climatic zones, our results indicate that this disease complex is important and tools for its control need to be developed.
Rust disease was found every year, but only after flowering and its severity did not exceed one point. Rust is not considered a devasting disease in northern Europe, but it is an important problem in dry and warm climates (Sillero et al. Citation2006; Ijaz et al. Citation2018).
Downy mildew was found only in 2017, so it is difficult to make any conclusions about occurrence and harmfulness of this disease in the Baltic region. The 2017 growing season had the lowest mean temperature of the five years of experiments, particularly in early July temperature when the rainfall was also plentiful, leading to a combination of relatively low temperature and high humidity that is favourable for the development of this disease (Stegmark, Citation1994; Stoddard et al. Citation2010). Downy mildew is a common problem on spring-sown faba bean in the United Kingdom (Thomas et al. Citation1999).
There were only small differences between cultivars in response to the various diseases (). Genetic resistance to chocolate spot disease is partial at best (Bouhassan et al. Citation2004) and knowledge about cultivar differences in response to Alternaria / Stemphylium leaf blotch is still very limited (Sheikh et al. Citation2015). The fact that both chocolate spot and leaf blotch are caused by several related pathogens with possibly different modes of infection is likely to impede breeding for resistance until more is known about both the pathogens and the host responses.
Seed rate did not influence the development of any diseases in our investigations. Optimal seed rate is often considered as an important tool to reduce the development of leaf diseases, because high density promotes high air humidity and leaf wetness (Stoddard et al. Citation2010), although Doussoulin et al. (Citation2015) did not find correlation between density of plants and severity of diseases. Nevertheless, the relatively low seed rates in the current experiment (30–50 m−−2) may not have been enough to cause problems.
The fungicide combination (boscalid and pyraclostrobin) provided partial protection against chocolate spot, Alternaria/Stemphylium leaf blotch and rust on average (), but efficacy depended on year and cultivar, with greater effectiveness in years when pathogen pressure was greater. These fungicides are not considered effective against oomycetes, so their lack of effect on downy mildew was expected.
In conclusion, these experiments have shown that Alternaria/Stemphylium leaf blotch is an emerging disease of increasing importance on faba bean in northern Europe, as also demonstrated in other countries, and that it is comparable in impact to chocolate spot disease. Each disease complex has its optima of temperature and moisture, so it should be possible to develop forecasts for the timing of disease control interventions such as the use of fungicides. The diseases are easily distinguished by their typical symptoms. Lowering crop density was not an effective tool to reduce disease incidence and fungicide application was only partly successful. Better sources of resistance are needed if the diseases are to be controlled by genetic means. Work continues on the identification of the responsible species in the chocolate spot and leaf blotch complexes and determination of their relative importance in causing disease.
Acknowledgement
The research was supported by ERA-NET SusCrop project ‘LegumeGap: Increasing productivity and sustainability of European plant protein production by closing the grain legume yield gap’ and Latvian Council of Sciences ‘Pathogenicity and diversity of Botrytis spp. – important causal agents of legume diseases’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Biruta Bankina
Biruta Bankina, Dr. biol., professor, works at the Institute of Soil and Plant Sciences, Latvia University of Life Sciences and Technologies. she has worked as a principal researcher in several projects as plant pathologist. She studies cereal and pulses diseases and their causal agents and life cycles of diseases as well as. She has over 70 refeed scientific publications and has supervised 3 completed Doctoral Thesis.
Gunita Bimšteine
Gunita Bimšteine, Dr. agr., associated professor, works at the Institute of Soil and Plant Sciences, Latvia University of Life Sciences and Technologies. She has worked as a senior researcher in several projects as plant pathologist. She studies potato, cereal, faba bean and vegetable diseases and their causal agents. She participates in ERANET SusCrop ‘LegumeGap’ (2019-2022) and in project “Pathogenicity and diversity of Botrytis spp. – important causal agents of legume diseases” (2020–2022) founded by Latvian Council of Sciences. She has over 10 refereed journal articles and over 50 other scientific publications and currently supervising two Doctoral Thesis.
Jānis Kaņeps
Janis Kaneps, Mg. agr., works at the Institute of Soil and Plant Sciences, Latvia University of Life Sciences and Technologies, Latvia. Area of expertise is cereal and pulse crop diseases and their causal agents.
Ieva Plūduma-Pauniņa
Ieva Pluduma-Paunina, Mg. agr., agronomist, works at the Research and Study farm “Peterlauki”, Latvia University of Life Sciences and Technologies. She has worked as researcher in several projects, for example, ERANET SusCrop ‘LegumeGap’ (2019-2022). She is on her way to finish doctoral studies in agronomy, writing her doctoral thesis about faba beans (“Faba beans’ (Vicia faba L.) yield formation depending on growing solutions’).
Zinta Gaile
Zinta Gaile, Dr. agr., professor, works at the Institute of Soil and Plant Sciences, Latvia University of Life Sciences and Technologies. She has worked as a principal researcher in several projects studying crop yield formation and agro-technology of the cereals, oil-seed rape, legumes. She has 66 quotable scientific publications and over 200 other publications, she has supervised 4 completed Doctoral Thesis.
Līga Paura
Liga Paura, Dr.agr., professor and leader researcher at the department of Control systems Latvia University of Life Sciences and Technologies. She has an experience in agricultural data analysis including crop and animal sciences. She has research experience in application of GLM models for genetic parameters and animal breeding values estimation, and data statistical analysis with R and SPSS.
Fred L. Stoddard
Frederick L. Stoddard (PhD (Camb), Docent (Hki), DrHC (LLU)) works at the Department of Agricultural Sciences at the University of Helsinki, Finland. He is one of the Nordic region's leading legume scientists and one of Finland's leading crop scientists. He has 139 refereed journal articles and over 200 other publications, and has supervised or co-supervised 30 completed PhD projects. His research centres on faba bean and includes genomics and genetics, agronomy and cropping systems, environmental impacts, and quality for food and feed. At the EU level, he has been a WP leader in several projects, currently ERANET SusCrop ‘ProFaba’ (2019-2022), and coordinates ERANET SusCrop ‘LegumeGap’ (2019-2022). He participates in Academy of Finland project ‘Leg4Life’ (2019-2025) and H2020 projects ‘Legumes Translated’ (2019-2022) and ‘InnoFoodAfrica’ (2020-2024). He holds the August T Larsson Visiting Fellowship at the Swedish University of Agricultural Sciences, 2020-2022.
References
- Abd El-Hai KM. 2015. Controlling of Alternaria leaf spot disease on faba bean using some growth substances. Asian J Plant Pathol. 9:123–134. https://doi.org/10.3923/ajppaj.2015.124.134.
- Ahmed S, Abang MM, Maalouf F. 2016. Integrated management of Ascochyta blight (Didymella fabae) on faba bean under Mediterranean conditions. Crop Prot. 81:65–69. https://doi.org/10.1016/j.cropro.2015.12.013.
- Bankina B, Bimšteine G, Neusa-Luca I, Roga A, Fridmanis D. 2017. Less known species of Botrytis spp. – the causal agents of faba bean chocolate spot. In: M. Kukwa, editor. Book of abstracts. XX symposium of Baltic mycologists and lichenologists. p. 27.
- Boligłowa E, Gleń-Karolczyk K, Gospodarek J. 2016. Effect of intensity of broad bean protection with biopreparations against fungal diseases. J Res Appl Agricult Eng. 61(3):38–42.
- Bouhassan A, Sadiki M, Tivoli B. 2004. Evaluation of a collection of faba bean (Vicia faba L.) genotypes originating from the Maghreb for resistance to chocolate spot (Botrytis fabae) by assessment in the field and laboratory. Euphytica. 135:55–62. https://doi.org/10.1023/B:EUPH.0000009540.98531.4d.
- Caudillo-Ruiz KB, Bhadauria V, Banniza S. 2017. Aetiology of stemphylium blight on lentil in Canada. Can J Plant Pathol. 39:422–432. https://doi.org/10.1080/07060661.2017.1378728.
- Coca-Morante M, Mamani-Álvarez F. 2012. Control of leaf spot diseases on ecotypes of faba bean (Vicia faba L.) produced in the Andean region of Bolivia. Am J Plant Sci. 03:1150–1158. https://doi.org/10.4236/ajps.2012.38139.
- Doussoulin H, Andrade N, Acuña R. 2015. Influencia de la fecha y densidad de siembra sobre el desarrollo de patógenos presentes en cultivares de haba (Vicia faba L.) de crecimiento determinado. Agro Sur. 43:25–30. https://doi.org/10.4206/agrosur.2015.v43n1-04.
- Eurostat. 2020. Agriculture database [database]. Retrieved from: https://ec.europa.eu/eurostat/data/database, visited 24 November 2020.
- FAOstat. 2020. Crops Database [database] Retrieved from: http://www.fao.org/faostat/en/#data, visited 24 November 2020.
- Harrison JG. 1988. The biology of botrytis spp. on Vicia beans and chocolate spot disease - a review. Plant Pathol. 37:168–201. https://doi.org/10.1111/j.1365-3059.1988.tb02064.x.
- Ijaz U, Adhikari KN, Stoddard FL, Trethowan RM. 2018. Rust resistance in faba bean (Vicia faba L.): status and strategies for improvement. Australas Plant Pathol. 47:71–81. https://doi.org/10.1007/s13313-017-0528-6.
- Karkanis A, Ntatsi G, Lepse L, Fernández JA, Vågen IM, Rewald B, Alsiņa I, Kronberga A, Balliu A, Olle M, et al. 2018. Faba bean cultivation – revealing novel managing practices for more sustainable and competitive European cropping systems. Front Plant Sci. 9:1115. https://doi.org/10.3389/fpls.2018.01115.
- Plūduma-Pauniņa I, Gaile Z, Bankina B, Balodis R. 2018. Field bean (Vicia faba L. yield and quality depending on some agrotechnical aspects. Agron Res. 16:212–220. https://doi.org/10.15159/AR.18.035.
- Plūduma-Pauniņa I, Gaile Z, Bankina B, Balodis R. 2019. Variety, seeding rate and disease control affect faba bean yield components. Agronomy Res. 17:621–634. https://doi.org/10.15159/AR.19.079.
- Rahman MZ, Honda Y, Islam SZ, Muroguchi N, Arase S. 2002. Leaf spot disease of broad bean (Vicia faba L.) caused by Alternaria tenuissima — A new disease in Japan. J Gen Plant Pathol. 68:31–37. https://doi.org/10.1007/PL00013049.
- Salam MU, Day TK, Ahmed AU, Nessa B, Haque AHMM, Subedi S, Malik AI, Rahman MM, Erskine W. 2016. Stempedia: a weather-based model to explore and manage the risk of lentil Stemphylium blight disease. Australas Plant Pathol. 45:499–507. https://doi.org/10.1007/s13313-016-0434-3.
- Sheikh F, Dehghani H, Aghajani MA. 2015. Screening faba bean (Vicia faba L.) genotypes for resistance to Stemphylium blight in Iran. Eur J Plant Pathol. 143:677–689. https://doi.org/10.1007/s10658-015-0718-4.
- Sillero JC, Fondevilla S, Davidson J, Vaz Patto MC, Warkentin TD, Thomas J, Rubiales D. 2006. Screening techniques and sources of resistance to rusts and mildews in grain legumes. Euphytica. 147:255–272. https://doi.org/10.1007/s10681-006-6544-1.
- Simko I, Piepho HP. 2012. The area under the disease progress stairs: calculation, advantage, and application. Phytopathology®. 102:381–389. https://doi.org/10.1094/PHYTO-07-11-0216.
- Stegmark R. 1994. Downy mildew on peas (Peronospora viciae f.sp. pisi). Agronomie. 14:641–647.
- Stoddard FL, Nicholas AH, Rubiales D, Thomas J, Villegas-Fernández AM. 2010. Integrated pest management in faba bean. Field Crops Res. 115:308–318. https://doi.org/10.1016/j.fcr.2009.07.002.
- Sudheesh S, Kimber RBE, Braich S, Forster JW, Paull JG, Kaur S. 2019. Construction of an integrated genetic linkage map and detection of quantitative trait loci for ascochyta blight resistance in faba bean (Vicia faba L.). Euphytica. 215:42.
- Thomas JE, Kenyon DM, Kightley SPJ. 1999. Progress in the exploitation of disease resistance in oilseed rape, field peas and field beans. Aspect Appl Biol. 56:67–74.
- Vaghefi N, Thompson SM, Kimber RBE, Thomas GJ, Kant P, Barbetti MJ, van Leur JAG. 2020. Multilocus phylogeny and pathogenicity of Stemphylium species associated with legumes in Australia. Mycol Prog. 19:381–396. https://doi.org/10.1007/s11557-020-01566-8.
- Vasić T, Živković J, Marković J, Stanojević I, Filipović S, Terzić D. 2019. Phytopathogenic fungi causers fungal diseases of the faba bean (Vicia faba L.) in Serbia. Biologica Nyssana. 10:17–21. https://doi.org/10.5281/zenodo.3463990.
- Vlăduţ A, Nikolova N, Licurici M. 2017. Aridity assessment within southern Romania and northern Bulgaria. Hrvatski Geografski Glasnik/Croatian Geographical Bulletin. 79:5–26. https://doi.org/10.21861/HGG.2017.79.02.01.
- Watson CA, Reckling M, Preissel S, Bachinger J, Bergkvist G, Kuhlman T, Lindström K, Nemecek T, Topp CFE, Vanhatalo A, et al. 2017. Grain legume production and Use in European Agricultural systems. Adv Agron. 144:235–303. https://doi.org/10.1016/bs.agron.2017.03.003.
- Woudenberg JHC, Groenewald JZ, Binder M, Crous PW. 2013. Alternaria redefined. Stud Mycol. 75:171–212. https://doi.org/10.3114/sim0015.
- Zhang J, Wu M-D, Li G-Q, Yang L, Yu L, Jiang D-H, Huang H-C, Zhuang W-Y. 2010. Botrytis fabiopsis, a new species causing chocolate spot of broad bean in central China. Mycologia. 102:1114–1126. https://doi.org/10.3852/09-217.