ABSTRACT
Cowpea is an important food and nutritional security grain legume crop in the semi-arid regions of sub-Saharan Africa. Its production is limited by biotic factors including pre-harvest insect pests such as aphids. Aphids cause yield reductions, promotes the growth of sooty moulds, honeydew and as a result reduce photosynthetic processes and rates. Various methods have been deployed to try to control the damage of aphids in cowpea plants. The methods include the use of chemical insecticides, cultural, mechanical, biological controls and integrated pest management. However, these methods have not been very effective as smallholder farmers growing cowpea in marginal areas cannot afford them. Hence, host plant resistance/genetic control is the best and effective method for controlling aphids in cowpea plantations. Breeding for aphid resistance is one of the effective methods that can sustain the production and productivity of the cowpea for longer periods. Furthermore, assessing the presence of genetic diversity can also help in sourcing genes of novelty for addressing this important issue to increase the production and productivity of the crop. Recently, scientists have used various strategies to try to solve the challenge of aphid damage on cowpea. The objective of this review was to document the research progresses on aphid resistance breeding of cowpea to facilitate the breeding and conservation of cowpea germplasm. The review serves as a baseline information to guide future cowpea breeding for resistance to aphids.
Introduction
Nutritional value and the ability of cowpea to withstand drought and heat makes it an important crop for food and nutritional security, particularly in the sub-Saharan Africa and globally at large. It is high in protein content (28%) and minerals, which include magnesium and calcium (Gerrano et al. Citation2019), vitamin A, and vitamin B6. Hence, It is regarded as a poor man’s meat and rich man’s vegetable crop. It plays an important role as a cash-crop in semi-arid areas. It exhibits tolerance to shade, fixes atmospheric nitrogen and fits well in several low input agricultural farming systems (Francis Citation1989; Saiful et al. Citation1991). Cowpea has been considered as a neglected crop species being underutilised in the past (Mabhaudhi et al. Citation2017) and currently, its potential has been recognised and needs to be unleashed.
According to an FAO (Citation2019) report, cowpea production is about 89,03,329 tonnes in a harvested area of 14,447,336 hectares worldwide with the major production coming from the African continent accounting for 95.9%. The major production is reported to come from Western Africa with an average yield of 5, 564 hg/ha. The major producers are Nigeria, Niger, Burkina Faso, Tanzania and Mali. In South Africa, the production is about 4, 801 tonnes in an area of 10, 990 hectares and is mainly by small-scale farmers in marginal areas (FAO Citation2019; DAFF Citation2012).
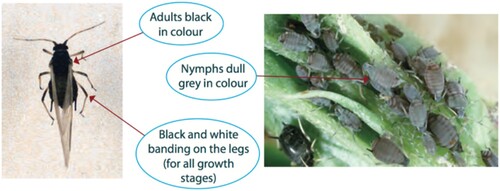
However, among the biotic stresses, aphids hinder cowpea growth, development and production (Souleymane et al. Citation2013). Among the insect pests that affect cowpea production, aphids are a major challenge. Cowpea aphids, Aphis craccivora, are small dark brown insects with white and black legs that feed together in small groups on young shoots of plants (Pettersson et al. Citation1998) including immature pods and leaves. The insect has four stages to complete its life cycle, thus egg to larva, pupa and adult (Obopile and Ositile Citation2010) (). These aphids are more prevalent in hot and dry areas. These aphids primarily infest seedlings, although large populations also infest flower buds, flowers and pods. The damage of aphids has been reported in other crops such as groundnuts, potato, cotton, some ornamental plants and weeds (Kataria and Kumar Citation2013). Losses in grain or foliage attributed to field pests of cowpea ranges from 20% to almost 100% including aphids (Kataria and Kumar Citation2013).
Figure 1. Life cycle of cowpea aphids. Source: https://cesaraustralia.com/pestnotes/aphids/cowpea-aphid/, accessed 23 January 2021.
Various methods have been employed to control infestations of aphids on cowpea. Among the control strategies such as the biological control (use of natural enemies) (Milán Vargas et al. Citation2005; Pervez and Omkar Citation2005), cultural control (mixed cropping systems) (Abdallah et al. Citation2016), mechanical control, chemicals have been used extensively to control heavy infestations of aphids (Hasken and Poehling Citation1995). However, the chemicals used are often not accessible to small-scale cowpea growers (Souleymane et al. Citation2013) due to the residual effects of some of the chemicals which are faced out as well as the cost of the chemicals. Furthermore, the use of chemicals is not the most economically and environmentally sound method for the sustainable use of the environment. The use of resistant cultivars can be a permanent solution for smallholder farmers to increase the quality of grain and yields at the end of the growing season. Hence, screening, identification and breeding for new sources of resistance to cowpea aphids are empirical with the inclusion of technologies that can help to quicken the improvement for sustainable resistance towards enhanced the production and productivity of cowpea. The article aims to review progress made so far on the genetics and breeding for aphid resistance in cowpea.
Biology, ecology and distribution of cowpea aphids
Aphis craccivora is a polyphagous insect, which prefers plants of the Leguminosae family has shown in fifty host plants of nineteen families (Mehrparvar et al. Citation2012). A. craccivora is a shiny black insect with legs and antennae that are white to pale yellow with black tips (). Immatures are slightly dusted with wax, adults without wax with six-segmented antennae, distal part of femur, siphunculi and cauda black and apterae are 1.4-2.2 mm. Alate viviparous A. craccivora females have abdomens with dorsal crossbars. Alatae 1.4–2.1 mm (Blackman and Eastop Citation2000). The aphid is ovoviviparous, with females retaining eggs inside their bodies and giving birth to small larvae. In areas with colder winters, overwintering may be as egg or hibernation. Optimal development of A. craccivora is dependent on fairly specific climatic conditions, such as temperatures 24-28.5 °C and around 65% relative humidity (Réal Citation1955; Mayeux Citation1984). In the field conditions, aphids do not generally survive periods of heavy rain (Réal Citation1955; Mayeux Citation1984).
The cowpea aphid mainly attacks legume crops such as cowpea, groundnuts, faba bean, chickpea, pigeon pea, lentil, mung bean, lucerne but occasionally can infest common beans especially in low altitude areas. This type of aphid has a wide host range in the leguminous family including weeds and some ornamental plants. This aphid is widely distributed globally in all continents, with predominance in the tropics and sub-tropics. It is usually dispersed through winds in winged forms with plant material being the minor distributor. It is also a vector of virus transmission between plants and host crops. Virus-infected planting material offers an easy means of virus transmission.
Effect of aphids on cowpea
Aphid infested cowpea plants are characterised by the presence of honeydew and ants moving up and down on the plant (). Sticky honeydew produced by aphids provides a rich medium to entrap fungal spores and promote growth of moulds. This results in the reduction of effective leaf area for light absorption and thus significantly limits photosynthetic efficiency (Smith and Boyko Citation2007). The aphids suck the plant sap by piercing plant tissues, sometimes therefore transmitting phytopathogen viruses. This might affect the absorption, translocation and the physiological process of mineral elements from the soil to the plant system and affect the genetic potential of cowpea (Shegro et al. Citation2012; Gerrano et al. Citation2017). As a result, fewer nutrients are available for plant growth and development. Hence, the crop becomes stunted, delaying the initiation of flowering and results in the transmission of viruses (Davis et al. Citation1991; Obopile and Ositile Citation2010). A. craccivora transmits over 14 viruses to cowpea and other legumes (Blackman and Eastop Citation1984; Thottapilly et al. Citation1990). The cowpea aphids have been implicated as the main vector of the non-persistent cowpea aphid-borne mosaic virus (Atiri et al. Citation1986). Damage by aphid-transmitted viruses is often additive to direct damage by aphids. Aphid imbibition of plant sap and injection of saliva into plants during feeding by aphids altered photosynthetic potential of the crop by partitioning patterns in shoots, reduced flux of phloem translocate to roots, and induced assimilate sources in plants to become sinks (Hawkins et al. Citation1987). As the susceptible crop grows, the population of the aphids also does (Kataria and Kumar Citation2013).
Figure 2. Presence of aphids and nymphs on the leaves, flower and green pods of a cowpea plant. Source (Navas Citation2014).

Management strategies of cowpea aphid infestations
Biological control
Biological control entails the use of natural enemies to control aphids. This method is non-toxic, non-polluting for the sustainability of the environment, self-perpetuating, long-lasting and can be implemented at low costs for producers and consumers. Natural enemies serve as control agents of aphid infestations including parasitoids such as Aphidiids and Aphelinids (Mackauer and Chow Citation1986). The aphidiidae are strictly specific solitary endophagous parasitoids and are the aphelinidde are hymenopterous insects (Mackauer and Chow Citation1986).
The ten most common genera of aphidiids are Adialytus foster, Aphidius nees, Diaeretiella stary, Ephedrus haliday, Lipolexis foster among others (Ghorpadé Citation1981; Singh and Narasimham Citation1992; Poorani Citation2002; Rakhshani et al. Citation2019). The most common genera of aphelinids are Aphelinus dalman and Mesidia foster. Joshi (Citation2005) reported 14 species of aphidiid and aphelinid parasitoids parasitising 19 aphid species in Karnakata. Predators also parasitise aphids. The most common predatory species include Coccinellidae (Poorani Citation2002), Chrysopida (Singh and Narasimham Citation1992), and Syrphidae (Ghorpadé Citation1981). Pathogens such as fungi also form part of the biological control of some of the aphididae species as they are very effective in the natural and laboratory conditions. The most common species belong to the order of Zygomycetes (Latge and Papierok Citation1998). Several studies have previously reported that several fungal pathogens serve as means of aphid control (Li et al. Citation2005; Lagte et al. Citation1983; Zimmermann Citation1983). Nonetheless, parasitoids are most preferred over predators as they are specific to hosts they parasitise, require lower foods per individual, therefore, maintains a balance between them and the hosts at lower densities and their larvae have sufficient food and do not need to search for food from a far host (Hansen Citation2018).
The use of natural enemies is advantageous over chemicals as they are host specific, killing only one or a few related species of prey/host. Biocontrol programs have no side effects, unlike with chemicals (van Lenteren et al. Citation2006). In India, populations of A. craccivora were reduced by releases of the reduviid predators Rhynocoris marginatus (Sahayaraj and Martin Citation2003), Rhynocoris kumraii (Sahayaraj and Ravi Citation2007) and the chrysopid predator Chrysoperla zastrowi sillemi (Baskaran and Rajavel Citation2013) and by sprays with the fungus Verticillium lecanii (Sahayaraj and Namachivayam Citation2011).
Cultural control
Cultural controls include intercropping, alteration of planting dates, plant density, adjustment of fertiliser applications, mulching, intercropping and weed control. According to Umina and Hangartner (Citation2015), one must remove unwanted plants/weeds in and around the crops of interest, particularly legumes, to reduce the availability of alternate hosts between growing seasons. Plant crops of interest early to enable plants to begin flowering before aphid numbers peak in spring and use a high sowing rate to achieve a dense crop canopy, which will assist in deterring aphid landings. Select cultivars that are less susceptible to aphid feeding damage towards increased yield for food security (Jackai and Singh Citation1988). Another strategy is the use of smaller amounts of fertiliser throughout the growing season that can help to reduce potential aphid outbreaks. Another effective preventative method is the use of reflective silver mulch (a Mylar like film placed over the soil surface), especially in vegetable production. A side benefit of reflective mulch is that it can increase crop yields because of the increased amount of solar energy reflected onto the leaves. Intercropping can serve as another effective method for controlling aphid populations in fields. For instance, intercropping faba bean with coriander (Coriandrum sativum) significantly decreased the population of A. craccivora while simultaneously significantly increased the numbers of associated predators and increased seed yields (Rizk Citation2011).
Mechanical control
A water hose and nozzle with adequate pressure is enough to knock the aphids from the foliage, but not to damage the plant. The jet or shower setting on a dial nozzle is enough to dislodge the aphid insect pests. Once off the plant, aphids cannot climb back up the plant and will often starve to death. Aphids can also be rubbed off the plants with fingers or a wet cloth. This method is effective against small aphid populations and at the very early stages of infestation. Physical removal by rubbing would be ineffective at removing large infestations. Many other approaches are susceptible to reduce plant contamination by aphid or/and aphid multiplication on crops, like plastic mulches and kaolin particle films.
Chemical control
Chemical control involves the use of insecticides that are effective against aphids infestations but have minimal impact on pollinators and natural enemies. Insecticides are safe for the environment, non-toxic and cost-effective and minimise the aphid populations. The most common insecticides used to control aphids include synthetic pyrethroids including cypermethrin, alphacypermethrin, deltamethrin and lambdacyhalothrin are the most commonly used chemical pesticides against sucking insects (Ali Citation2016). The use of insecticide seed treatments is also effective in delaying aphid colonisation and reduces early infestation and aphid feeding (Dedryver et al. Citation2010). A border spray in autumn/early winter, when aphids begin to move into crops, may provide sufficient control without the need to spray the entire field.
In addition, chemical control has inherent disadvantages as pests develop resistance against them. Most synthetic pesticides kill other non-target animals including their natural enemies, hence caution has to be taken into account when spraying to control aphids particularly in spring when these natural enemies can play a very important role in suppressing aphid populations if left untouched (Umina and Hangartner Citation2015). Although insecticides were used to control cowpea aphids, the increasing awareness of environmental and human health hazards has led researchers to develop alternative control measures with integrated pest management (IPM) (Shannag and Ja'far Citation2007).
Integrated pest management
Integrated Pest Management (IPM) is the use of multiple control strategies in a comprehensive and preventative approach to reduce pest populations, maintain plant health and minimise the use and impact of pesticides in the environment. IPM is much better when involving a combination of control strategies such as cultural, physical or mechanical control, host plant resistance, chemical control and biological control strategies than when using a single strategy (Adipala et al. 2000). A strategy that combined early planting, close planting (30 × 20 cm) and three insecticide applications, once each at the budding, flowering and podding stages, resulted in the highest yields of 791 kg/ha with a 51% yield gain over the farmers’ traditional practices (Nabirye et al. Citation2003). The best option is regularly monitoring the fields for aphid occurrence and spread is a basic step in crop protection. Early detection in many cases can help address the pest situation by low-cost spot treatment or removal of pests or infested plant material (Nabirye et al. Citation2003).
Genetic control
Host plant resistance (HPR) is an economical and environmental method of pest control (Adipala et al. 2000). Producers are encouraged to grow cowpea varieties/cultivars that are resistant to cowpea aphids in their fields as this will control numbers of aphid populations. Cultivars such as TVu-6464, TVu-1583, and TVu-15445, TVu-801, TVU 3000, IT81D-1020, and IT82E-1-108 have been developed and released by the International Institute of Tropical Agriculture (IITA) for resistance to aphids (Togola et al. Citation2020; Nkansah-Poku and Hodgson Citation1995). Cowpea variety resistance to aphid is conferred by severely high levels of antibiosis in some cultivars for example, TVu 310 and TVu 810. In addition, the cowpea varieties, TVu 2897, TVNu1158 and IT84S-2246, showed a high level of resistance to aphid colonisation (Afun et al. Citation1991; Omoigui et al. Citation2017). Moreover, a number of high-yielding varieties with aphid and virus resistance have now been developed at IITA and should be available to the small-scale farmers in the near future in the African continent and beyond.
Screening for aphid resistance in cowpea
In order to combat the problem of aphid damage, screening and breeding for aphid resistance damage are necessary. This is because some of the reported resistant lines or cultivars break down under heavy infestations, hence, it is critical to continue to seek for new resistant genotypes. shows some of the resistant cultivars reported to have resistance to cowpea aphids. For instance, Souleymane et al. (Citation2013) screened a hundred and five wild cowpea genotypes for resistance to aphids and found that TVNu 1158 was consistently resistant to aphids and should be a good source of resistance genes for incorporation into cultivated cowpea. According to preliminary studies conducted by Alabi et al. (Citation2012), Erusu, Berret and Modupe cowpea cultivars hold promise as potential sources of resistance genes for developing resistant varieties against cowpea aphid. Kusi et al. (Citation2020) screened four cowpea cultivars and the authors found SARC1-57-2 to have resistance against cowpea aphids in Ghana.
Table 1. Sources of cowpea aphid resistance recorded/identified.
Molecular screening for aphid resistance
According to Omoigui et al. (Citation2017), molecular and phenotypic screening revealed that TVU-2876 has a strong resistance to cowpea aphid and should be a good source of resistance genes that can be used in breeding to develop new aphid resistant cowpea cultivars. Although the results of phenotypic tests and molecular marker detection agreed in most cases, molecular markers detection was found more reliable in identifying genotypes for resistance to cowpea aphid attack (CPA). The results also revealed that among the cowpea genotypes studied, TVu 2897 and TVNu 1158 supported lowest number of aphids and plant damage scores. TVu-2876 showed to have a strong resistance to cowpea aphid and was a good source of resistance gene that can be used in breeding to develop new aphid resistant cowpea cultivars. Although the results of phenotypic tests and molecular marker detection agreed in most cases, molecular markers detection was found more reliable in identifying genotypes for resistance to cowpea aphid. The segregation in F2 and BC1 populations derived from the cross between TVNu 2876 and Keffi local indicated that resistance to cowpea aphids in TVu-2876 is controlled by a single dominant gene. Allelism test revealed that resistance gene in TVNu 2876 is non-allelic with the gene that confers resistance in SARC 1-57-2 and TVNu 1158 in Nigeria.
Genetics of the aphid resistance
Breeding for cowpea aphid resistance is needed to reduce further losses from field outbreaks (Singh et al. Citation1996). Boateng (Citation2015) conducted a genetic study for cowpea aphid resistance, where the results showed most of the direct and reciprocal F1 generations to be resistant to the cowpea aphid and the F2 segregating generations were intermediate between the resistant and susceptible parents but were skewed towards the resistant parent. Results also showed that a single gene controlled the resistance in Hewale and Asomdwee and the F2 generation conformed to the 3 resistant and 1 susceptible after a χ2 test. Pathak (Citation1988) studied the genetic resistance of cowpea aphid and reported that a single dominant gene, designated as Rac1 and Rac2, conferred the cowpea aphid resistance. Ombakho et al. (Citation1987) also studied in F1 and F2 generations of cowpea (TVU 310, ICV10 and ICV 11) and reported that resistant gene in TVU 310 and ICV 10 were designated by Ac1, while the resistant gene in ICV11 was Ac2. However, plant reactions to insect attack may depend on plant genotype, insect biotypes and environmental factors. Bioscience for the future (Citation2010) reported that there was a 3:1 ratio between the crosses of susceptible and resistant genotypes of cowpea. Furthermore, in the same study, it was also reported that one Simple Sequence Repeat (SSR) maker was linked to the aphid resistance gene.
Bao-Lam et al. (Citation2015) mapped recombinant inbred lines (RILS) and the parents for cowpea aphid attack resistance using field-based screenings during two main crop seasons in a ‘hotspot’ location of the Central Valley of California. One minor and one major quantitative trait locus (QTL) were consistently mapped on linkage groups 1 and 7, respectively, both with favourable alleles contributed from genotype IT97K-556-6. The major QTL appeared dominant based on a validation test in a related F2 population. SNP markers flanking each QTL were positioned in physical coatings carrying genes involved in plant defense based on synteny with related legumes. These markers could be used to introgress resistance alleles from IT97K-556-6 into locally adapted and susceptible varieties.
Progress in breeding for aphid resistance in cowpea
Considerable progress has been made in the past years in developing cowpea varieties resistant to aphids. Singh et al. (Citation1996) reported several improved cowpea varieties with combined resistance to aphid, thrips and bruchid. Attempts to improve cowpea aphids resistance through conventional breeding programs have met with limited success because aphid resistance is a genetically monogenic trait. Hence, researchers have resorted to the use and implementation of molecular markers in their breeding programmes. Marker-assisted selection has been used as an approach to trace resistance genes in cowpea. Omoigui et al. (Citation2017) screened cowpea genotypes against cowpea aphid and found that TVNu 1158 and TVNu 2897 were resistant. However, the resistance in TVNu 1158 did not seem strong compared to the genotype TVU 2897 that was confirmed to be resistant to multiple aphid biotypes. The mechanism of resistance in TVU 2897 and TVNu 1158 were expressed as a hypersensitive response at the site of infestation on the leaves. Molecular and phenotypic screening revealed that TVU-2876 has a strong resistance to cowpea aphid and should be a good source of resistance gene that can be used in breeding to develop new aphid resistant cowpea cultivars. Although the results of phenotypic tests and molecular marker detection agreed in most cases, molecular markers detection was found more reliable in identifying genotypes for resistance to aphids.
Using marker-assisted selection (MAS) will expedite cowpea breeding procedures but it is limited by the lack of information on marker associations of the aphid resistance. However, Qin et al. (Citation2017) reported that 338 cowpea varieties were grouped into three clusters according to their genetic structures when screened using 1047 SNP markers identified from DNA sequencing. The association study revealed that two SNP markers, C35011941_894 and Scaffold30061_3363, were strongly associated with aphid resistance by using three different methods of analysis. Thus far, limited technologies have been used with the aim of improving cowpea for aphid resistance. More technologies need to be employed to identify markers that can be used for breeding for resistance to cowpea aphid damage. Bao-Lam et al. (Citation2015) mapped aphid resistance quantitative trait loci using Single Nucleotide Polymorphism (SNPs) and reported the SNP markers flanking each QTL were positioned in physical contigs carrying genes involved in plant defense based on synteny with related legumes. These markers could be used to introgress resistance alleles from IT97K-556-6 into susceptible local blackeye varieties by backcrossing. Kusi et al. (Citation2020) combined SSR and SNPs to map the aphid resistance genes using marker-assisted backcross breeding and reported that the study identified a novel aphid resistance locus and demonstrated the effectiveness of integrating SSR and SNP markers for trait mapping and marker-assisted breeding. On the other hand, Souleymane et al. (Citation2013) confirmed the tolerance to aphids of the improved line IT97K-556-6, in which two resistance loci were mapped (Huynh et al. Citation2013) and also identified a new source of aphid resistance from a cowpea wild relative, TVNu 1158.
Future directions
Although a lot of works have been done on breeding cowpea for aphid resistance. Few markers have been used to mapping aphid resistance genes in breeding populations. Developing markers such as Kompetitive Allele-Specific PCR (KASP), diversity array sequencing (DArTSeq) derived SNPs, genotype by sequencing (GBS), next-generation sequencing (NGS) can also for screening for aphid resistance and QTL linkage maps be of paramount importance. Of late the technology has advanced, the CRISPR/Cas9-mediated genome editing technology has been the most recent technique used by breeders to alter the genome. The CRISP/Cas9 has been used to efficiently disrupt the representative symbiotic nitrogen fixation (SNF) gene in Vigna unguiculata (Jie et al. Citation2019). More research work is required to be conducted on cowpea for major production constraints including aphid resistance. CRISP/Cas9 has been used in soybean (Jean-Michel et al. Citation2015), Arabidopsis (Cermak et al. Citation2011), rice (Li et al. Citation2013), tobacco (Zhang et al. Citation2013) and Brachypodium (Shan et al. Citation2013). Only a few crop species have been researched using CRISPR technology (Jiang et al. Citation2013) hence, a need for more research in cowpea.
In conclusion, Cowpea’s nutritional value and ability to withstand drought makes it an important crop for food and nutritional security in the sub-Saharan Africa and globally. However, its production is limited by cowpea aphids causing yield and biomass reduction. Chemicals, cultural, mechanical, biological and integrated pest management controls were used as control strategies. However, these methods have not been very effective as smallholder farmers growing cowpea in marginal areas cannot afford the cost of them. Hence, host plant resistance is the best and effective method for controlling aphids. Breeding for aphid resistance is one of the effective methods that can sustain the production and productivity of the cowpea for longer periods. Assessing the presence of genetic diversity can also help in sourcing genes of novelty for addressing this issue. Furthermore, more research work is needed using the most recent techniques.
Acknowledgements
The first author would like to thank the Department of Science and Innovation for funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Maletsema Alina Mofokeng
Dr Maletsema Alina Mofokeng is a Researcher in Plant Breeding at the Agricultural Research Council-Grain Crops, Potchefstroom, South Africa.
Abe Shegro Gerrano
Dr Abe Shegro Gerrano is a Senior Research Scientist in Plant Breeding at the Agricultural Research Council-Vegetable, Industrial and Medicinal Plants, Pretoria, South Africa.
References
- Abdallah IS, Abou-Yousef HM, Fouad EA, Kandil MAEH. 2016. The role of detoxifying enzymes in the resistance of the cowpea aphid (Aphis craccivora Koch) to thiamethoxam. J Plant Prot Res. 56:67–72.
- Afun JVK, Jackai LEN, Hodgson CJ. 1991. Calendar and monitored insecticide application for the control of cowpea pests. Crop Prot. 10:363–370.
- Alabi OY, Aziza E, Omoloye AA. 2012. Preliminary evaluation of selected cowpea varieties for resistance to cowpea aphid, Aphis craccivora. Nig J Ecol. 12:45–55.
- Ali OSM. 2016. Effect of salicylic acid and its mixtures with three insecticides on some cotton insect pests [MSc thesis]. Tanta University. Egypt.
- Atiri GI, Enobakhare DA, Thottapilly G. 1986. The importance of colonizing and non-colonizing aphid vectors in the spread of cowpea aphid-borne mosaic virus in cowpea. Crop Prot. 5:406–410.
- Bao-Lam H, Jeffrey D E, Arsenio N, Steve W, Mitchell RL, Timothy JC, Philip AR. 2015. Genetic mapping and legume synteny of aphid resistance in African cowpea (Vigna unguiculata L. Walp.) grown in California. Mol Breeding. 35:36.
- Baskaran RKM, Rajavel DS. 2013. Management of sucking insect pests of groundnut through bio-agents and botanicals. Ann Plant Prot Sci. 21(2):286–290.
- Benchasri S, Nualsri C, Santipracha Q, Ngampongasi A. 2007. Evaluation of aphid (Aphis carccivora Koch.) resistance in 24 accessions of yardlong bean and cowpea. Proceeding of the 1st Joint PSU-UNS International Conference of Bioscience: Food, Agriculture, and the Environmen, Songkhla, Thailand, Aug 17–19, 2006. p. 215–222.
- Bioscience for the future. 2010. The 7th IMT-GT Uninet and the 3rd Joint International PSU-UNS Conference: Proceeding: Oct 7–8, Prince of Songkla University, Hat Yai, Songkla, Thailand.
- Blackman RL, Eastop VF. 1984. Aphids on the world's crops – an identification guide. New York: John Wiley and Sons.
- Blackman RL, Eastop VF. 2000. Aphids on the world’s crops: An identification and information guide. 2nd ed. Chichester: Wiley; p. 414.
- Boateng AB. 2015. Genetic studies of aphids (Aphis craccivora Koch) resistance in cowpea [MPhil thesis]. Kwame Nkrumah University of Science and Technology. Ghana.
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. 2011. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39:e82. doi:10.1093/nar/gkr218.
- DAFF. 2012. Production guidelines for Cowpeas. Department of Agriculture, Forestry and Fisheries. Directorate Plant Production.
- Davis DW, Oelke EA, Oplinger ES, Doll JD, Hanson CV, Putnam DH. 1991. Cowpea: Alternative field crops manual. http://www.hort.purdue.edu/newcrop/ afcm/cowpea.html.
- Dedryver CA, Le Ralec A, Fabre F. 2010. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C R Biologies. 333:539–553.
- FAO. 2019. FAOSTAT. Accessed 20 April 2021.
- Francis CA. 1989. Biological efficiencies in multiple-cropping systems. Adv Agron. 42:1–42.
- Gerrano AS, van Rensburg WSJ, Adebola PO. 2017. Nutritional composition of immature pods in selected cowpea [Vigna unguiculata (L.) Walp.] genotypes in South Africa. Aust J Crop Sci. 11(02):134–141.
- Gerrano AS, van Rensburg WSJ, Venter SL, Shargie NG, Amelework BA, Shimelis HA, Labuschagne MT. 2019. Selection of cowpea genotypes based on grain mineral and total protein content. Acta Agric Scand B Soil Plant Sci. 69(2):155–166.
- Ghorpadé KD. 1981. Insect prey of Syrphidae (Diptera) from India and neighbouring countries: a review and bibliography. Tropical Pest Manag. 27:62–82.
- Hansen T. 2018. Identifying mechanisms of host plant specialization in Aphis craccivora and its bacterial symbionts [MSc thesis]. University of Kentucky. Kentucky.
- Hasken K-H, Poehling H. 1995. Effects of different intensities of fertilisers and pesticides on aphids and aphid predators in winter wheat. Agric Eco Environ. 52(1):45–50.
- Hawkins CDB, Whitecross MI, Aston MJ. 1987. The effect of short-term aphid feeding on partitioning of carbon14 dioxide photo assimilate in three legume species. Canad J Bot. 65(4):666–672.
- Huynh B-L, Close TJ, Roberts PA, Hu Z, Wanamaker S, Lucas MR, et al. 2013. Gene pools and the genetic architecture of domesticated cowpea. Plant Genom. 6:1–8.
- Jackai LEN, Singh SR. 1988. Screening techniques for host plant resistance to insect pests of cowpea. Trop Grain Legum Bull. 35:2–18.
- Jean-Michel M, Xiaobo W, Junqi L, Shaun JC, Thomas JYK, Robert MS. 2015. CRISPR/Cas mutagenesis of soybean and Medicago truncatula using a new web-tool and a modified Cas9 enzyme. GM Crops Food. 6(4):243–252.
- Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP. 2013. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 41:e188. doi: 10.1093/nar/gkt780.
- Jie J, Chunyang Z, Zhongfeng S, Longlong W, Deqiang D, Qiuling F. 2019. Genome editing in cowpea Vigna unguiculata using CRISPR-Cas9. Int J Mol Sci. 20:2471. doi:10.3390/ijms20102471.
- Joshi S. 2005. Faunistic studies on Aphididae 9Hemiptera) of Karnataka and bioecology of the aphid parasitioid, Diateretiella rapae (M’Intosh) (Hymenoptera: Braconidae) [PhD thesis]. University of Agricultural Sciences, Bangalore.
- Kataria R, Kumar D. 2013. On the Aphid-ant association and its relationship with various host plants in the Agroecosystems of Vadodara, Gujarat, India. Halteres. 4:25–32.
- Kusi F, Nboyine JA, Attamah P, Awuku JF, Sugri I, Zakaria M, Lamini S, Mensah G, Larweh V, Owusu RK, et al. 2020. Stability of sources of resistance to cowpea aphid (Aphis craccivora Koch, Hemiptera: Aphididae) across major cowpea production zones in Ghana. Int J Agron. 2020:1–8. doi:10.1155/2020/8869334.
- Latge JP, Papierok B. 1998. Aphid pathogen. In: Minks AK, Hourenijin P, editor. Aphdis: their biology, natural enemies and control. Vol 2B. Itally: Portici; p. 323–335.
- Lagte JP, Silvie P, Papierok B, Remaudiere G, Dedryver CA, Rabasse JM. 1983. Advantages and disadvantages of Conidiobolus obscurus and of Erynia neoaphidis in the biological control of aphids. In: Cavallora R, editor. Aphid antagonists. Rotterdam: AA Balkema; p. 20–32.
- Li J, Norville JE, Aach J, McCormack M, Zhang D, Bush J, et al. 2013. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 31:688–691. doi:10.1038/nbt.2654.
- Li W, Xuan W, Wang H, Sheng C, Miao C. 2005. Review of entomogenous fungi infecting aphids in China. Entomol Knowledge. 42:3–35.
- Mabhaudhi T, Vimbayi GP, Chimonyo MA. 2017. Status of underutilised crops in South Africa: opportunities for developing research capacity. Sustainability. 9:1569.
- Mackauer M, Chow FJ. 1986. Parasites and parasite impact on aphid population. In: McLean GD, Garret RG, Ruesink WG, editors. Plant virus epidemics – Monitoring, modelling and predicting outbreaks. Sydney: Academic Press; p. 95–118.
- Mayeux A. 1984. The groundnut aphid. Biology and control. Oleagineux. 39:425–434.
- Mehrparvar M, Madjdzadeh SM, Mahdavi Arab N, Esmaeilbeygi M, Ebrahimpour E. 2012. Morphometric discrimination of Black Legume Aphid, Aphis craccivora Koch (Hemiptera: Aphididae), populations associated with different host plants. North-West J Zool. 8(1):172–180.
- Milán Vargas O, Cueto Zaldívar N, Hernández Pérez N, Ramos Torres T, Pineda Duvergel M, Granda Sánchez R. 2008. Prospección de los coccinélidos benéficos asociados a plagas y cultivos en Cuba. Fitosanidad. 12:71–78.
- Nabirye J, Nampalaa P, Ogenga-Latigoa M, Kyamanywaa S, Wilsonb H, Odekec V, et al. 2003. Farmer-participatory evaluation of cowpea integrated pest management (IPM) technologies in Eastern Uganda. Crop Prot. 22:31–38.
- Navas M. 2014. Basis for agroecological management of aphids (Aphis craccivora Koch) on cowpea (Vigna unguiculata L.) in Cuban agroecosystems. Biol. 2014. pp. 1–86.
- Nkansah-Poku J, Hodgson CJ. 1995. Interaction between aphid resistant cowpea cultivars, three clones of cowpea aphid, and the effect of two light intensity regimes in this interaction. Int J Pest Manag. 41:161–165.
- Nwosu DJ, Falusi AO, Gana AS, Olayemi IK. 2019. Sourcing for cowpea aphid (Aphis craccivora) Resistance Gene among cowpea wild relatives. Int J Adv Sci Eng Inf Technol. 6(7):10060–10069.
- Obopile M, Ositile B. 2010. Life table and population parameters of cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae) on five cowpea Vigna unguiculata (L. Walp.) varieties. J Pest Sci. 83:9–14.
- Ombakho GA, Tyagi AP, Pathak RS. 1987. Inheritance of resistance to the cowpea aphid in cowpea. Theor Appl Genet. 74(6):817–819.
- Omoigui LO, Ekeuro GC, Kamara AY, Bello LL, Timko MP, Ogunwolu GO. 2017. New sources of aphids [Aphis craccivora (Koch)] resistance in cowpea germplasm using phenotypic and molecular marker approaches. Euphytica. 213(8):178.
- Ouédraogo PA, Benoit JB, Fousseni T, Jean-Baptiste T, BaoLam H, Philip AR, Timothy C, Jeremy TO. 2018. Screening of cowpea (Vigna unguiculata (L.) Walp.) lines for resistance to three Aphids (Aphis craccivora Koch) strains in Burkina Faso. Afr J Agric Res. 13(29):1487–1495.
- Pathak R. 1988. Genetics of resistance to aphid in cowpea. Crop Sci. 28:474–476.
- Pervez A and Omkar O. 2005. ‘Functional responses of Coccinellid predators: An illustration of a logistic approach. J Insect Sci. 5:1–6.
- Pettersson J, Karunaratne S, Ahmed E, Kumar V. 1998. The cowpea aphid, Aphis craccivora, host plant odours and pheromones. Entomol Exp Appl. 88:177–184.
- Poorani J. 2002. An annotated checklist of the Coccinellidae (Coleoptera) (excluding Epilachninae) of the Indian subregion. Oriental Insect. 36:307–383.
- Qin J, Shi A, Mou B, Bhattarai G, Yang W, Weng Y, Motes D. 2017. Association mapping of aphid resistance in USDA cowpea (Vigna unguiculataL. Walp.) core collection using SNPs. Euphytica. 213:36. doi:10.1007/s10681-016-1830-z.
- Rakhshani E, Barahoei H, Ahmad Z, Starý P, Ghafouri-Moghaddam M, Mehrparvar M, Kavallieratos NG, Čkrkić J, Tomanović Z. 2019. Review of Aphidiinae parasitoids (Hymenoptera: Braconidae) of the Middle East and North Africa: key to species and host associations. Eur J Taxon. 552:1–132.
- Réal P. 1955. Le cycle annuel du puceron de l’arachide (Aphis leguminosae Theob.) en Afrique noire française et son déterminisme. Rev Pathol Vég d’entomol agricole de France. 34(1-2):1–122.
- Rizk AM. 2011. Effect of strip-management on the population of the aphid, Aphis craccivora Koch and its associated predators by intercropping faba bean, Vicia faba L. with coriander, Coriandrum sativum L. Egyptian J Biol Pest Cont. 21(1):81–87.
- Saiful H, MacKown CT, Poneleit CG, Hildebrand DF. 1991. Growth and N accumulation in maize and winged bean as affected by N level and intercropping. Ann Bot. 68:17–22.
- Sahayaraj K, Martin P. 2003. Assessment of Rhynocoris marginatus (Fab.) (Hemiptera: Reduviidae) as augmented control in groundnut pests. J Cent Eur Agric. 4(2):103–110.
- Sahayaraj K, Namachivayam SKR. 2011. Field evaluation of three entomopathogenic fungi on groundnut pest. Tropicultura. 29(3):143–147.
- Sahayaraj K, Ravi C. 2007. Small-scale mass production strategy for a reduviid predator Rhynocoris longifrons Stal (Heteroptera: Reduviidae). In: Guptha VK, Verma AK, editors. Perspective in animal ecology and reproduction. Vol. 4. New Delhi: Daya Publishing House; p. 53–81; ISBN 9788170354598.
- Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, et al. 2013. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 31:686–688. doi:10.1038/nbt.2650.
- Shannag H, Ja'far A. 2007. Biometry and responses of faba bean varieties to blackbean aphid, Aphis fabae Scopoli. Am-Eurasia J Agric Environ Sci. 2:328–334.
- Shegro A, Shargie NG, van Biljon A, Maryke TL. 2012. Diversity in starch, protein and mineral composition of sorghum landrace accessions from Ethiopia. J Crop Sci Biotechnol. 15:275–280.
- Singh BB, Chambalis OL, Sharma B. 1996. Recent advance in cowpea breeding. Advance in cowpea research. Ibadan: IITA. p. 182–189.
- Singh SP, Narasimham AU. 1992. Indian chrysopidae. Bangalore: Biological Control Centre. p. 34.
- Smith C, Boyko E. 2007. The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl. 122:1–16.
- Souleymane A, Aken’Ova ME, Fatokun CA, Alabi OY. 2013. Screening for resistance to cowpea aphid (Aphis craccivora Koch) in wild and cultivated cowpea (Vigna unguiculata L. Walp.) accessions. Int J Environ Sci Technol. 2(4):611–621.
- Togola A, Boukar O, Servent A, Chamarthi S, Tamò M, Fatokun C. 2020. Identification of sources of resistance in cowpea mini core accessions to Aphis craccivora Koch (Homoptera: Aphididae) and their biochemical characterization. Euphytica. 216:88.
- Thottapilly G, Rossel HW, Reddy DVR, Morales FJ, Green SK, Makkouh KM. 1990. Vectors of virus and mycoplasm diseases: an overview. In: Singh SR, editor. Insect pests of tropical food legumes. Chichester: John Wiley and Sons Ltd; p. 323–342.
- Umina P, Hangartner S. 2015. ‘Cabbage aphid’, Cesar Australia. [accessed 2021 Feb]. http://cesaraustralia.com/sustainable-agriculture/pestnotes/insect/Cabbage-aphid.
- van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJM. 2006. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol. 51:609–634.
- Zhang Y, Zhang F, Li X, Baller JA, Qi Y, Starker CG, et al. 2013. Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol. 161:20–27. doi: 10.1104/pp.112.205179.
- Zimmermann G. 1983. Biological control of aphids by entomopathogenic fungi: present state and prospects. In: Cavallo R., editor. Aphid antagonists. Proceedings of a meeting of the EC Experts’ group. Rotterdam: Balkema; p. 33–40.
