 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Root-knot nematodes pose a severe threat to worldwide agricultural development. Due to the high toxicity of chemical nematicides, eco-friendly control strategies against root-knot nematodes need to be established. A pot and in vitro experiment were performed to estimate nematicidal potential of Pochonia chlamydosporia. P. chlamydosporia was used alone or in combination with two botanicals for controlling Meloidogyne incognita in chickpea. The laboratory assessment was performed with four prepared concentrations (S, S/2, S/10, S/25) of fungal inoculum of P. chlamydosporia against egg hatching and second-stage juvenile's mortality of M. incognita. All four concentrations reduced egg hatching and increased mortality of J2s. In pot experiment, P. chlamydosporia was used with chopped leaves of two botanicals viz., Ageratum conyzoides and Eichhornia crassipes against M. incognita in chickpea. All the treatments found significantly suppressed root infestation caused by M. incognita and improved growth and physiological attributes of chickpea. The combined application of P. chlamydosporia + A. conyzoides was found highly effective, and E. crassipes alone was least. Therefore, using P. chlamydosporia with botanicals is a promising sustainable strategy in agriculture against M. incognita infected chickpea.
Introduction
Pulses seem to be the essential foods for vegetarians in the entire world. Pulses are continuously gaining prominence as an affordable protein source and a vital component for sustainable agriculture. Chickpea (Cicer arietinum L., Family-Fabaceae) is the world's second-largest legume crop grown globally across an area of 14.6 M hac (FAOSTAT Citation2017). Numerous factors significantly reduce chickpea production, including insect pests, fungi, bacteria, and nematodes (Zwart et al. Citation2019). Meloidogyne incognita is a root-knot nematode (RKN) and a sedentary endo-parasite (SEP) that affects thousands of plant species, including pulses and vegetables, and is among the most dangerous agricultural ailments. In India, Meloidogyne species such as M. incognita, M. javanica have been estimated to show losses of 19–40% and 24–61% to chickpea production, respectively (Ali and Naimuddin Citation2010). RKNs reduce the growth of host crops and intervene with nodulation and nitrogen fixation (Rehman et al. Citation2012). Indeed, considering the high economic effect of RKNs, several management techniques in agricultural production have been introduced, viz., use of chemical nematicides, biocontrol agents from botanicals, and resistant varieties. The most successful way of handling RKNs, primarily through the use of chemical nematicides, but, on the other hand, most of them are forbidden due to their toxic impact on flora and fauna, which has also disrupted soil structure and the climate. Applying biocontrol agents is suggested as a much better option and highly feasible for the RKN management programme. Fungal biocontrol agents may suppress the RKNs by various modes, producing toxins, antibiotics, and enzymes or interference with the detection of the nematode-plant host. Colonisation of root by Pochonia chlamydosporia provided good efficacy and advantages for host plant about growth parameters, defense mechanism against many pathogens viz., nematodes, bacteria, and fungi (Siddiqi and Akhtar Citation2008). Several biocontrol agents viz. P. chlamydosporia, Paecilomyces lilacinus, and Trichoderma harzianum have extremely antagonistic impact on RKNs in glasshouse as well as in field conditions (Huang et al. Citation2016; Naz et al. Citation2021). Abd-Elgawad (Citation2016) and Khan et al. (Citation2020) reported in their study that some selected bacteria and fungi strains were successfully control the root infection in plants caused by plant-parasitic nematodes (PPNs). Various plant parts have also been reported for their nematicidal activities and effectively killed RKNs (El-Deen et al. Citation2013; Yahia et al. Citation2020). Due to the increasing environmental pollution caused by chemical nematicides, the use of botanical extracts to control Meloidogyne spp. is gaining attention due to their availability, cost-effectiveness, pollution-free and environmentally safe. Azadirachtin and limuloids (meliantriol, salannin, nimbin, and nimbidin) present in neem (Azadirachta indica A. Juss.) extracts have nematostatic and nematicidal properties and also minimise the hatching of eggs (Babu et al. Citation2019). Therefore, in this study, we conducted egg hatching, nematode mortality and glasshouse experiment separately to test the nematicidal efficacy of P. chlamydosporia with two combinations of botanicals against M. incognita. In one experimental set-up, P. chlamydosporia was used with chopped leaves of Ageratum conyzoides and Eichhornia crassipes separately. Another set-up, P. chlamydosporia was planned to use a leaves mixture of plants Ageratum conyzoides and Eichhornia crassipes. The root-knot disease and plant-growth parameters were evaluated in the chickpea plant.
Materials and methodology
Plant, fungi, and root-knot nematode
Chickpea cv. Avrodhi was used as a host crop. Seeds were surface sterilised in a 1.0% NaOCl for 15 min, washed 3–4 times in Double Distilled Water (DDW) for 10 s each. The pure culture of a fungal biocontrol agent, P. chlamydosporia (ITCC No. 6898), was purchased from the Indian Agricultural Research Institute (IARI), New Delhi, India. Fungal culture was preserved on the medium of Potato Dextrose Agar (PDA). The identification of pure fungal culture was based on microscopic (conidial, colourful, conidium, vesicle shaped) and macroscopic features (diameter, colour, and colony texture). For the mass production of P. chlamydosporia, Richards Medium was utilised. The RKN, M. incognita, was used as a pathogen.
Maintenance of the inoculum of root-knot nematode, M. incognita
The inoculum of M. incognita was maintained in a glasshouse on eggplant. Identification of M. incognita was performed based on perineal pattern (Sasser and Carter Citation1982). Eggs of M. incognita separated from infected eggplant roots by dipping roots in 0.05% NaOCl for 3-4 min (Hussey and Barker Citation1973). Then eggs were collected and washed with DDW on 25 μm pore size sieves. The egg masses were left for hatching in a BOD incubator, and J2s that hatched from the eggs on a sieve were collected after five days. After that, fresh egg masses and J2s were used for the hatching, mortality, and pots bioassay.
Preparation of cultural filtrate of P. chlamydosporia
To obtain adequate inoculum, P. chlamydosporia inoculated by a sterile inoculation needle in Richard's liquid medium (Riker and Riker Citation1936). The fungal mycelia mat on filter paper was washed in DDW, washed away excess water and nutrients with blotting paper. The fungal inoculum was prepared by mixing 10 g of mycelium mat in 100 ml of double distilled water and blending it (10,000 rpm) in a waring blender for 30 s. Ten millilitres of the fungal inoculum of P. chlamydosporia were used for inoculation in pots. The fungal inoculum obtained was labelled as ‘S’ (Standard) and being used as a stock suspension. Successive concentrations viz., S/2, S/10, S/25 were prepared by adding the required amount of DDW for hatching and mortality experiments (Mukhtar et al. Citation2013).
Effect of different concentrations of fungal inoculum of P. chlamydosporia on the mortality of j2s of M. incognita
Four concentrations (S, S/2, S/10, S/25) of fungal inoculum of P. chlamydosporia were prepared to determine the effect on mortality of J2s. For the mortality test, 1 ml of DDW containing 150 freshly hatched J2s poured into petri dishes having 9 ml of different concentrations of fungal inoculum mentioned above. Petri dishes having only DDW were used as control. Each concentration had five replicates. The petri dishes were sealed with a lid and wrapped in parafilm. Petri dishes were placed at 28°C, and the number of live and dead J2s counted after 6, 12, 24, and 48 hrs of incubation using a stereoscopic microscope. The mortality of J2s was recorded according to the mean percentage of dead J2s. The J2s displayed flexibility or looked as winding shapes declared alive (El-Rokiek and El-Nagdi Citation2011), and if J2s did not show any motion and their body outline looks straight, they were found dead. Living and dead J2s were calculated under the binocular microscope, and the percentage of mortality was determined using the formula (Sun et al. Citation2006).
Effect of different concentrations of fungal inoculum of P. chlamydosporia on egg hatching of M. incognita
The egg inhibitory activity of various concentrations (S, S/2, S/10, S/25) of fungal inoculum of P. chlamydosporia was tested by egg mass dipping method. Six healthy egg masses of M. incognita were collected from the infected root of eggplant using forceps and poured into petri dishes with 10 ml of the different concentrations of fungal inoculum referred to above. Petri dishes were coated with parafilm to avoid evaporation and then placed at 28°C. Egg masses were dipped in DDW served as control. Each concentration had been repeated five times, excluding control. The hatching value was recorded by counting the number of hatched J2s in each replicate after 3, 5, and 7 days of incubation using a binocular microscope and calculating the per cent inhibition in egg hatching of each replicate using the following formula (Khan et al. Citation2019).
Where,
C0 = Number of J2s that emerged from egg masses in control (DDW)
Tα = Number of J2s emerged from egg masses in each concentration of fungal inoculum of P. chlamydosporia
In planta bioassay (pot study)
A pot experiment was laid out in Plant Pathology and Plant Nematology glasshouse, Department of Botany, Aligarh Muslim University, Aligarh. Seeds of susceptible chickpea cv. Avrodhi was purchase from the local market of Aligarh. The earthen pots (15 cm wide) loaded with 1 kg of autoclaved soil in proportion to 3:1 (sandy loam: farmyard manure). Pots were amended with 15 g of freshly chopped leaves of A. conyzoides and E. crassipes. The pots were arranged according to the treatment set-up and put in a completely randomised glasshouse (Temperature-18-25°C; Relative humidity-95-100%). Pots were regularly watered for the complete decomposition of chopped leaves. After 10 days of adjustment, 4-5 sterilised chickpea seeds sown 1-2 in. deep in each pot. When the seedlings grew two sets of leaves, the plants were thinned. Each of the healthy and stable seedlings was selected per pot, and the remaining ones removed from the ground level, including control. Aqueous suspension with 2500 freshly hatched J2 of M. incognita was inoculated evenly around each seedling root. Two days after the inoculation, a sterile pipette added an S concentration level (10 mL) of P. chlamydosporia around chickpea roots. In control, pots were inoculated with 10 ml of DDW in place of fungal inoculum of P. chlamydosporia. Plants have been irrigated sufficiently throughout the experiment and carefully to eliminate errors during the examination. After 60 days, the plants were uprooted and washed with flowing tap water to remove adhered soil particles, and assessments were performed. The growth, physiological and pathological parameters of chickpea plants are presented in tables and figures.
Photosynthetic pigments analysis
Chlorophyll content estimation bioassay
The chlorophyll content of fresh leaves of each treatment was estimated using the Mackinney (Citation1941) process. Take 1 g of leaves from each treatment, ground in a blender with 20 ml of 80% acetone, and centrifuge at 5000 rpm for 5 min. The supernatant was collected, and absorption determined at 645 and 663 nm against (blank) 80% acetone on the spectrophotometer (UV 1700 Shimadzu, Japan). The chlorophyll content of each treatment (mg g-1 tissue) was evaluated using the following formula.
A = Absorbance of leaf sample read at 645 and 663 nm
V = Final volume,
W = Fresh weight of leaves,
D = Length of the path of light
Carotenoid content estimation bioassay
The carotenoid content in leaves samples was observed using the method given by MacLachlan and Zalik (Citation1963). To determine carotenoid content, the protocol for preparation of extract of leaves of each treated pot is the same as of chlorophyll bioassay. The absorbance of the extracts was observed at wavelength 480 and 510 nm against blank (80%) acetone on the spectrophotometer. The carotenoid value was calculated using the following formula.
O.D. = Optical density of extract of leaf sample read at 480 and 510 nm
V = Final volume
W = Fresh weight
D = Length of the path of light
Pathological analysis
Nematode population in soil
At termination, soil samples were taken from each treated pot using a garden trowel then mixed thoroughly. Weigh 250 g of well-mixed soil from each treatment to estimate the population of J2s. Estimation of the nematode population (J2s) was done by Cobb's sieving and decanting technique (Cobb Citation1918), followed by modified Baermann's funnel method (Southey Citation1986). Counting of J2s was done under the stereoscope microscope (Motic SMZ 168 series).
Statistical analysis
Statistical analysis was performed using the Duncan Multiple Range Test (DMRT) of complete randomised block design using R (version 2.14.1 software). The standard error of the mean (±SE) and Least Significant Difference (LSD) was calculated at a 5% significance level. The Principal Component Analysis (PCA) was calculated using Origin software [version 2019b (9.65)]. Microsoft Excel assessed the coefficient of correlation.
Results
Nematicidal effect of P. chlamydosporia on egg hatching of M. incognita in vitro
All four selected concentrations (S, S/2, S/10, S/25) of fungal inoculum were significantly inhibited egg hatching of M. incognita at (p < 0.05). There was an increased inhibition in egg hatching when the concentration of P. chlamydosporia was increased from S/25 level to S level. Maximum inhibition in egg hatching was observed at S concentration, while minimum inhibition was reported at S/25. S/10 and S/25 concentrations of P. chlamydosporia also showed a decrease in efficacy as compared to S and S/2. The individual inhibitory effect of the used concentration of P. chlamydosporia is given in .
Table 1. Effect of different concentrations of P. chlamydosporia on egg hatching of M. incognita at 3, 5 and 7 days of incubation period.
Nematicidal effect of P. chlamydosporia on second-stage juvenile's mortality of M. incognita in vitro
The findings showed an apparent effect of four concentrations (S, S/2, S/10, S/25) of P. chlamydosporia on the mortality of J2s of M. incognita after 6, 12, 24, and 48 hrs of the incubation period (p < 0.05). The P. chlamydosporia were toxic to J2s of M. incognita compared to distilled water (control). The standard concentration (S) showed maximum mortality compared to other concentrations (S/2, S/10, S/25). All concentrations showed the highest mortality of J2s at 48 hrs of incubation period compared to 6, 12, 24 hrs. The S concentration was found highly toxic to J2s at 48 hrs of incubation, and this toxicity was found significant compared to the other concentrations. Similarly, the incubation period also significantly influenced the mortality of J2s and reached the maximum after 48 hrs of incubation compared to 6, 12, and 24 hrs. As the concentration of fungal inoculum increase from S/25 to S, there is an increase in the mortality of J2s. Individual toxic effect of four concentrations of P. chlamydosporia on J2s of M. incognita shown in .
Table 2. Effect of different concentrations of P. chlamydosporia on mortality of J2s of M. incognita at 6, 12, 24 and 48 hrs of incubation period.
Pot experiment
The nematicidal potential of P. chlamydosporia alone or in combination with chopped leaves of botanicals on the growth attributes of chickpea
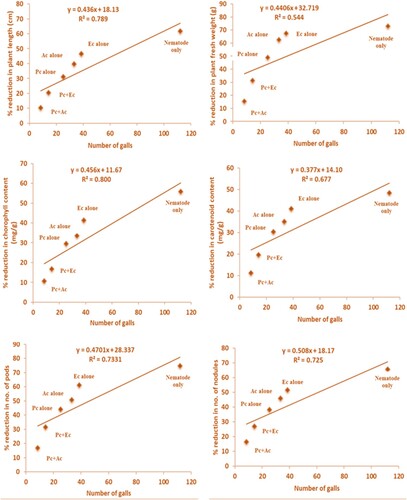
The incoulum of P. chlamydosporia (10 mL) alone or combined with chopped leaves (15 g) of A. conyzoides and E. crassipes showed improvement in infected chickpea's growth attributes. All treatments substantially (p<0,05) enhanced the chickpea growth attributes (). Application of combined treatment of P. chlamydosporia + A. conyzoides gave the highest improvement in plant length (56 ± 2.17 cm), plant fresh weight (137.4 ± 4.12 g), number of pods and nodules (34.80 ± 1.74 and 34.60 ± 1.83), and followed by P. chlamydosporia + E. crassipes (plant length −49.6 ± 1.33 cm, plant fresh weight −111.20 ± 3.64 g, number of pods −28.60 ± 2.60, number of nodules −30.20 ± 1.74), P. chlamydosporia alone (plant length −43 ± 1.76 cm, plant fresh weight −83 ± 2.65 g, number of pods −23.40 ± 2.02, number of nodules −25.60 ± 2.82), A. conyzoides alone (plant length −37.6 ± 1.01 cm, plant fresh weight −60.80 ± 2.45 g, number of pods −20.60 ± 1.30, number of nodules −22.40 ± 1.77), and least was observed in E. crassipes alone (plant length −33.4 ± 1.53 cm, plant fresh weight −52.80 ± 1.64 g, number of pods −16.20 ± 1.74, number of nodules −20 ± 1.15) as compared to those plant treated with nematode only (plant length −23.8 ± 1.24 cm, plant fresh weight −44 ± 2.16 g, number of pods −10.60 ± 0.87, number of nodules −14.20 ± 1.47) (). The findings revealed that there exists a strong linear relationship between galls/plant and plant length (R2 = 0.78); plant fresh weight (R2 = 0.54); number of pods (R2 = 0.73); the number of nodules (R2 = 0.72); chlorophyll content (R2 = 0.80); carotenoid content (R2 = 0.67). As the relation is positive, the increase in the number of galls increased the per cent reduction of growth attributes of chickpea ().
Figure 1. Nematicidal effect of P. chlamydosporia alone or in combination with chopped leaves of A. conyzoides and E. crassipes on the growth attributes of chickpea infested with M. incognita (Pc + Ac = P. chlamydosporia + A. conyzoides; Pc + Ec = P. chlamydosporia + E. crassipes; Pc alone = P. chlamydosporia alone; Ac alone = A. conyzoides alone; Ec alone = E. crassipes alone).
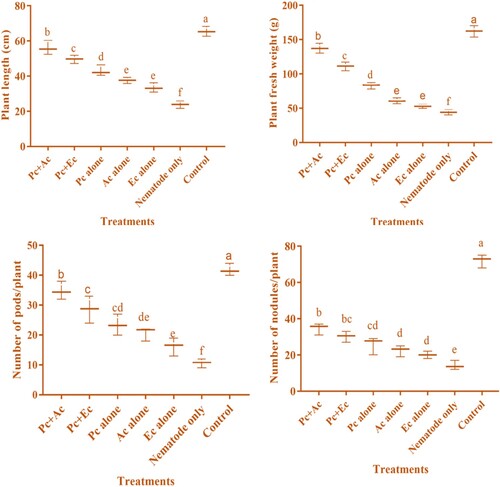
Figure 2. Relationship between the number of galls and per cent reduction in various growth attributes of chickpea (Pc + Ac = P. chlamydosporia + A. conyzoides; Pc + Ec = P. chlamydosporia + E. crassipes; Pc alone = P. chlamydosporia alone; Ac alone = A. conyzoides alone; Ec alone = E. crassipes alone).
Nematicidal potential of P. chlamydosporia alone or in combination with chopped leaves of botanicals on the physiological attributes of chickpea
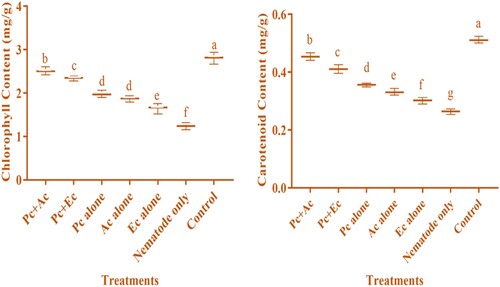
The application of P. chlamydosporia (10 ml) alone or in combination with chopped leaves (15 g) of A. conyzoides and E. crassipes enhanced the physiological attributes (chlorophyll and carotenoid content) of chickpea. Significantly, maximum chlorophyll content (2.51 ± 0.05 mg/g) and carotenoid content (0.454 ± 0.007 mg/g) were achieved when combined treatment P. chlamydosporia + A. conyzoides was applied. Other treatments also enhanced physiological attributes (chlorophyll and carotenoid content) viz., P. chlamydosporia + E. crassipes (2.34 ± 0.03 and 0.411 ± 0.13 mg/g), P. chlamydosporia alone (1.98 ± 0.04 and 0.356 ± 0.11 mg/g), A. conyzoides alone (1.87 ± 0.04 and 0.332 ± 0.11 mg/g), and E. crassipes alone (1.65 ± 0.07 and 0.302 ± 0.10 mg/g) as compared to those plant treated with nematode only (1.24 ± 0.04 and 0.264 ± 0.08 mg/g) ().
Figure 3. Nematicidal effect of P. chlamydosporia alone or in combination with chopped leaves of A. conyzoides and E. crassipes on the physiological attributes of chickpea infested with M. incognita (Pc + Ac = P. chlamydosporia + A. conyzoides; Pc + Ec = P. chlamydosporia + E. crassipes; Pc alone = P. chlamydosporia alone; Ac alone = A. conyzoides alone; Ec alone = E. crassipes alone).
The nematicidal potential of P. chlamydosporia alone or in combination with chopped leaves of botanicals on the nematode population and galls/plant
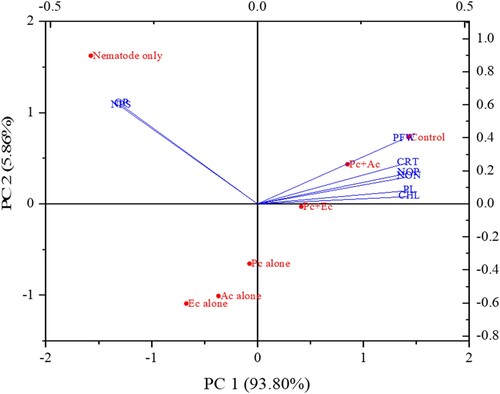
The treatments significantly (p < 0.05) reduced the pathological parameters such as nematode population, and galls/plant (). Treatment with combined application of P. chlamydosporia + A. conyzoides achieved the highest reduction in galls (8.40 ± 0.41) followed P. chlamydosporia + E. crassipes (14.20 ± 1.41), P. chlamydosporia alone (25.20 ± 1.64), A. conyzoides alone (33.30 ± 1.81), E. crassipes alone (38.60 ± 2.05) compared to the pot treated with nematode only (112 ± 7). When counting the nematode population in 250 g soil, a significant reduction was seen compared with the control. In the same manner, P. chlamydosporia + A. conyzoides was most prominent in suppressing the nematode population (455 ± 16.07). The least suppressive effect was found in E. crassipes alone (1002 ± 24.84) as compared to pot treated with an only nematode (3024 ± 28.21) (). The outcome of the principal component analysis showed that the population of M. incognita in soil and galls per plant was strongly correlated with other parameters of chickpea. Scatter biplot showed that P. chlamydosporia combined with chopped leaves of A. conyzoides was found highly effective, reduced the infestation caused by M. incognita considerably, and improved chickpea's growth attributes ().
Figure 4. Nematicidal effect of P. chlamydosporia alone or in combination with chopped leaves of A. conyzoides and E. crassipes on the pathological attributes of chickpea (Pc + Ac = P. chlamydosporia + A. conyzoides; Pc + Ec = P. chlamydosporia + E. crassipes; Pc alone = P. chlamydosporia alone; Ac alone = A. conyzoides alone; Ec alone = E. crassipes alone).
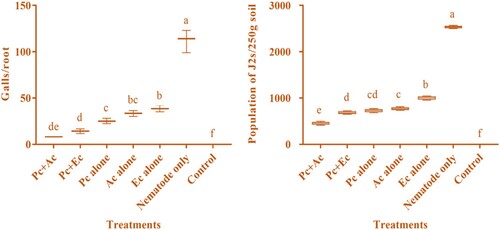
Figure 5. The biplots of principal component analysis, comparing the effects of P. chlamydosporia alone or in combination with chopped leaves of A. conyzoides and E. crassipes on various studied parameters of chickpea infected with M. incognita (PFW = Plant fresh weight; PL = Plant length; NOP = Number of pods; NON = Number of nodules; CHL = Chlorophyll content; CRT = Carotenoid content; NPS = Nematode population in 250 g soil; GPP = Galls per plant; Pc + Ac = P. chlamydosporia + A. conyzoides; Pc + Ec = P. chlamydosporia + E. crassipes; Pc alone = P. chlamydosporia alone; Ac alone = A. conyzoides alone; Ec alone = E. crassipes alone).
Discussion
In in vitro experiment, the tested concentrations viz., S, S/2, S/10, S/25 of fungal inoculum were effectively inhibited egg hatching of egg masses. We also found J2s mortality of M. incognita in the same fungal concentration ( and ). Standard concentration ‘S’ was found highly effective in reducing egg hatching of M. incognita, followed by S/2, S/10, S/25 (). However, contrary findings were also reported by Uddin et al. (Citation2019) and Singh and Mathur (Citation2010). They found in their studies that treatment with P. chlamydosporia gave no significant result in egg hatching inhibition. The maximum mortality was 85% observed at 48 hrs of incubation in standard ‘S’ concentration of P. chlamydosporia followed by S/2, S/10, S/25 (). In contrast, chlamydosporia showed 11.3-76.3% mortality of J2s of M. incognita after 72 hrs of exposure, according to a published report by Uddin et al. (Citation2019). The nematicidal effect of fungal inoculum was increased when exposure time extended. Meyer et al. (Citation2004) and Elbadri et al. (Citation2008) reported in their study that the impact of fungal inoculum varied from concentration to concentration, thus confirming these findings. In our study, the concentration and incubation period were important factors. The nematicidal action of P. chlamydosporia may be due to direct parasitism of fungus mycelium on nematode, or perhaps secondary metabolites released in surroundings, which caused mortality of J2s after treatment. Fungal activity as the nematicidal impact on adult nematodes, suppression of egg hatching and juvenile growth have also been tested (Meyer et al. Citation2004). Benedict and Brady (Citation1972) found that antagonistic compounds were released, responsible for reducing the J2s population.
Similarly, in pot study, P. chlamydosporia alone or in combination with chopped leaves of A. conyzoides and E. crassipes significantly enhanced growth attributes of chickpea and decreased disease severity caused by M. incognita to varying degrees. The combined application of P. chlamydospore with chopped leaves of A. conyzoides caused the highest reduction in root galling and nematode population (). It improved the growth and physiological attributes of chickpea ( and ). However, contrary findings were also reported by Podestá et al. (Citation2013), who found that fungus P. chlamydosporia had little effect on destroying the nematode eggs, reducing the number of galls by only 2.68%. Analysis of correlation coefficient exhibited that number of galls had a positive correlation with plant length, plant fresh weight, chlorophyll and carotenoid content and number of pods and nodules (). Scattered points which are existing in graphs show whether two variables have a relationship or not. Maximum scattered points with minimum correlation were found between the number of galls and plant fresh weight (R2 = 0.544) with positive correlation and maximum correlation with highly condensed points observed number of galls index and chlorophyll content (R2 = 0.800) (). Our finding confirmed with Rich et al. (Citation1984), reported that significant positive correlations were observed between nematode numbers and plant yield of tobacco.
As we observed in our pot study that P. chlamydosporia and botanicals have a significant impact against nematodes. Our finding confirmed with Naz et al. (Citation2021), reported that applying biocontrol agents in combination may be an attractive measure because the combined use of different organisms may produce synergistic effects that facilitate the management of RKNs. That might be due to the release of bioactive compounds after decomposing plant leaves, altering the current nematode pattern and enhancing chickpea's growth. Adding botanicals to soil improves soil quality, acts as a nutrient reservoir and provides the perfect habitat for plant growth. Our findings are similar to Aktar and Malik (Citation2000), they reported that the addition of organic matter increases the nutrient content and improves the soil texture, promoting the development of antagonistic microorganisms, while its breakdown produces toxic compounds against RKNs. Biocontrol agents colonised roots better in organic amendments soil, resulting in better protection of plants from various pathogens and thus improved plant growth. Once organic matter is applied in soil may release compounds with nematicidal effect and support the increase in native antagonist population in the soil or serve as the substrate for the development and the establishment of antagonists in soil (Cannayane and Rajendran Citation2001). Egg parasitic fungi, P. chlamydosporia and P. lilacinus are attractive biocontrol agents for RKNs management programmes in a wide variety of crops (Anastasiadis et al. Citation2008; Ebrahim et al. Citation2008). The fungus P. chlamydosporia colonises and infects RKNs eggs and exposed females, reducing the number of infective second stage juveniles (Manzanilla-López et al. Citation2013). After decomposition of organic additives/matters, release some nutrients responsible for the improvement in plant nutrition (Reiner Citation2015) and the development of plant resistance against nematode infection (Reiner Citation2015). A synergistic impact observed on the management of M. incognita on chickpea was due to P. chlamydosporia with botanicals. A significant decrease in nematode infection could be due to release of secondary metabolites by Pochonia spp. and botanicals that have nematicidal behaviour. Several metabolites have been isolated from various plants with a wide variety of structures and their nematicidal activities are assessed against multiple species of nematodes (Faria et al. Citation2016; Khan et al. Citation2017). The assumption derived from the analysis is that biocontrol agents and botanicals enhanced growth attributes and yield of chickpea and reduced the infestation caused by M. incognita. Applying these commodities, which are readily available domestically, would be far more beneficial from the economic perspective of farmers if adequately used, especially for small-scale farmers who could not buy expensive chemical nematicides. Therefore, our approach to applying P. chlamydosporia alone or in combination with chopped leaves of selected botanicals in agricultural practices could benefit the soil's physicochemical attributes and provide an environmentally friendly option for the sustainable management of root-knot nematode. Our findings supported the benefits of biocontrol agents and botanicals by keeping down the root-knot disease in chickpea plants. Therefore, using fungal inoculum (P. chlamydosporia) with selected botanicals may be a suitable environmentally friendly option to keep the nematode population below the threshold level.
Declaration of interest statement
The authors declare that there is no conflict of interest.
Acknowledgement
The authors are grateful to the University Grants Commission (UGC-BSR Research Start-up-Grant: F30-409/2018) and the Deanship of Scientific Research, King Khalid University (Grant Number R.G.P. 2/11/42) for financial assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Amir Khan
Amir Khan, completed his PhD in Botany with specialization in Plant Pathology and Nematology from the Department of Botany, Aligarh Muslim University, Aligarh. He has done his B.Sc. and M.Sc. from the AMU, Aligarh in 2012 and 2014 respectively. Besides, thrust area of Dr. Amir and his group to promote organic farming by utilizing organic matter (plant parts, oil cakes, agricultural waste, etc.), bio-fertilizer and biocontrol agents for the sustainable management of root-knot nematodes as well as enrich the soil with nutrients necessary for plant growth and development. He is a Life Member of Indian Phytopathological Society, New Delhi (India), and an Editor of Journal of Ecology & Natural Resources.
Manar Fawzi Bani Mfarrej
Manar Fawzi Bani Mfarrej, received her PhD in Sustainability and Environmental Studies in 2010 from the University of Jordan. She is currently working as Assistant Professor of Environmental Science in the College of Natural and Health Sciences at Zayed University, United Arab Emirates. Dr. Manar's research interests include air quality, environmental sustainability, plant protection, pesticide residues, environmental pollution, and waste management.
Moh Tariq
Moh Tariq, working as an Assistant Professor & Head of Department in Botany at Lords University, Alwar (Rajasthan). The thrust area of Moh Tariq and his group is to promote organic farming by utilizing organic matter, biofertilizer, and biocontrol agents for the sustainable management of plant-parasitic nematodes. Nowadays, organic farming has been an important sector of sustainable agriculture without disturbing the microfauna and microflora. He is an associate editor in the ‘Saudi Journal of Pathology and Microbiology’ (SJPM) ISSN 2518-3370. He has published more than 30 research papers in reputed national and international journals.
Mohd Asif
Mohd Asif, completed his PhD in Botany from the section of Plant Pathology and Nematology, Department of Botany, Aligarh Muslim University, Aligarh. He is actively engaged in the characterization and identification of novel and natural bio-pesticides of origin for nematode disease management and the promotion of organic farming. He has received the best and second-best presentation awards for their outstanding contribution to boosting organic farming. Currently, he is engaged in the Medico- Ethno Botanical Survey and cultivation of Medicinal plants and is also the Co- PI of the two IMR, projects funded by CCRAS. He has published more than 30 research articles in national and international journals of repute.
Hera Nadeem
Hera Nadeem, is a research scholar in the Department of Botany, Aligarh Muslim University, Aligarh. Presently, she is working on the management of root-knot nematode disease in vegetables through microbial-based compound.
Mansoor Ahmad Siddiqui
Mansoor Ahmad Siddiqui, is a professor at the Department of Botany, Aligarh Muslim University. His research area is the studies on the effect of organic soil amendments and biocontrol agents on plant-parasitic nematodes. He attended about 32 National/International conferences including XVIII IPPC in July 2015 in Berlin, Germany. He received Young Scientist Award (1994) by BRS, Allahabad, for outstanding contribution in the field of Plant Nematology.
Mohamed Hashem
Mohamed Hashem, is a professor of microbiology at King Khalid University, Saudi Arabia, and Assiut University, Egypt. He was awarded as a distinguished professor at KKU in 2015. His research interest includes mycology, plant pathology, biological control, microbial biotechnology, bioenergy, and bionanotechnology. He supervised fifteen Ph.D. and master students to completion of their studies. He published more than 130 scientific papers in international journals and implemented 20 projects in collaboration with international scientists.
Saad Alamri
Saad Alamri, is a professor of microbiology at King Khalid University, Saudi Arabia. He occupied many administrative positions, and his last position was a vice-president of KKU. His research interest includes bacteriology, environmental toxicology, microbial biotechnology, waste management, and bionanotechnology. He supervised ten MSc thesis in the field of interest. He published more than 100 scientific papers in international journals and implemented many funded local and international projects.
Faheem Ahmad
Faheem Ahmad, is an Assistant Professor at the Department of Botany, Aligarh Muslim University. He has done his PhD from the AMU, Aligarh in 2009. Earlier, he has worked as a postdoctoral fellow at the Ehime University (Japan), North-West University (South Africa), and National Sun Yat-Sen University (Taiwan). His research interests include plant-associated microbe identification, biological control of plant-parasitic nematodes, and plant disease studies of economic crops. He published more than 30 research articles in peer-reviewed journals and supervised master students. He was the Principal Investigator of UGC-BSR Start-up Grant from 2018 to 2020.
References
- Abd-Elgawad MMM. 2016. Biological control agents of plant-parasitic nematodes. Egypt J Biol Pest Cont. 26:423–429.
- Aktar M, Malik A. 2000. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Biores Technol. 74:35–47.
- Ali SS, Naimuddin, Ali M. 2010. Nematode infestation in pulse crops. In: M.R. Khan, M.S. Jairajpuri, editors. Nematode Infestations, Part I: Field Crops. Allahabad. India: The National Academy of Sciences; pp. 288–325.
- Anastasiadis IA, Giannakou IO, Prophetou ADA, Gowen SR. 2008. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus with various practices for the control of root-knot nematodes. Crop Prot. 27:352–361.
- Babu GS, Chand R, Verma VK, Kumar M, Veer R. 2019. Effect of neem plant parts and its products on egg hatching and larval penetration of Meloidogyne incognita on tomato. Ann Plant Protect Sci. 27:122–125.
- Benedict RG, Brady LR. 1972. Antimicrobial activities of mushroom metabolites. J Pharm Sci. 61:1820–1822.
- Cannayane I, Rajendran G. 2001. Application of biocontrol agents and oil cakes for the management of Meloidogyne incognita in brinjal (solanum melongena L.). Curr Nemat. 12:51–55.
- Cobb NA. 1918. Estimating the nema population of soil, with special reference to the sugar-beet and root-gall nemas, Heterodera schachtii Schmidt and Heterodera radicicola (Greef) Müller. Agric Tech Circ Bur Pl Ind US Dep Agric. 1:48.
- Ebrahim A, Seddighe F, Hassan RE. 2008. Potential for biocontrol of heterodera schachtii by Pochonia chlamydosporia var. chalmydosporia on sugar beet. Biocon Sci Technol. 18:157–167.
- Elbadri GA, Lee DW, Park JC, Yu HB, Choo HY. 2008. Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita. J Asia-Pac Entomol. 11:99–102.
- El-Deen NAH, Abdel-Kafie OM, El-Ghareb NM. 2013. Evaluation of seaweed extract and various plant products against Meloidogyne incognita on basil. Georgi Agricul. 16(1):29–34.
- El-Rokiek KG, El-Nagdi WM. 2011. Dual effects of leaf extracts of eucalyptus citriodora on controlling purslane and root-knot nematode in sunflower. J Plant Protec Res. 51(2):121–129.
- FAOSTAT. 2017. Available at: http://faostat.fao.org/faostat/.
- Faria JMS, Sena I, Ribeiro B, Rodrigues AM, Maleita CMN, Abrantes I, Bennett R, Mota M, Figueiredo ACS. 2016. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J Pest Sci. 89(1):207–217.
- Huang WK, Cui JK, Liu S, Kong LA, Wu QS, Peng S, He WT, Sun JH, Peng D. 2016. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of syncephalastrum racemosum and Paecilomyces lilacinus as being most effective. Biol Cont. 92:31–37.
- Hussey RS, Barker K. 1973. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Repor. 57:1025–1028.
- Khan A, Tariq M, Asif M, Siddiqui MA. 2017. Evaluation of botanicals toxicants against root-knot nematode, Meloidogyne incognita in vitro. Asian J Biol. 4(3):1–7.
- Khan F, Asif M, Khan A, Tariq M, Ansari T, Shariq M, Siddiqui MA. 2019. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In vitro and greenhouse study. Curr Plant Biol. 20:100115. https://doi.org/10.1016/j.cpb.2019.100115
- Khan RAA, Najeeb S, Xie B, Li Y. 2020. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 8:817. https://doi.org/10.3390/microorganisms8060817
- Mackinney G. 1941. Absorption of light by chlorophyll solutions. J Biol Chem. 140:315–322.
- MacLachlan S, Zalik S. 1963. Plastid structure chlorophyll concentration and free amino acid composition of a chlorophyll mutant of barley. Cana J Bot. 41:1053–1062.
- Manzanilla-López RH, Esteves I, Finetti-Sialer MM, Hirsch PR, Ward E, Devonshire J, Hidalgo-Díaz L. 2013. Pochonia chlamydosporia: advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J Nematol. 45:1–7.
- Meyer SLF, Huettel RN, Liu XZ, Humber RA, Juba J, Nitao JK. 2004. Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile motility. J Nematol. 36:23–32.
- Mukhtar T, Kayani MZ, Hussain MA. 2013. Nematicidal activities of Cannabis sativa L. and Zanthoxylum alatum Roxb. against Meloidogyne incognita. Indust Crops Prod. 42:447–453.
- Naz I, Khan RAA, Masood T, Baig A, Siddique I, Haq S. 2021. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol Cont. 152:104429. https://doi.org/10.1016/j.biocontrol.2020.104429
- Podestá GS, Freitas LG, Dallemole-Giaretta R, Zooca RJF, Caixeta LB, Ferraz S. 2013. Meloidogyne javanica control by Pochonia chlamydosporia, Gracilibacillus dipsosauri and soil conditioner in tomato. Summa Phytopathol. 39:122–125.
- Rehman B, Ganai MA, Parihar K, Siddiqui MA, Usman A. 2012. Management of root -knot nematode, Meloidogyne incognita affecting chickpea, Cicer arietinum for sustainable production. Bios Intern. 1:1–5.
- Reiner DA. 2015. Subproduto da indústria vinícola no controle de Meloidogyne javanica. Dissertação apresentada à Universidade Tecnológica Federal do Paraná no Programa de Pós-Graduação em Agronomia, pp. 77.
- Rich JR, Hodge C, Johnson JT. 1984. Population development and pathogenicity of Meloidogyne javanica on flue-cured tobacco as influenced by ethoprop and DD. J Nemat. 16:240–245.
- Riker AJ, Riker RS. 1936. Introduction to research on plant diseases. St. Louis, Chicago, New York & Indianapolis: John's Swift Co., pp. 117.
- Sasser JN, Carter CC. 1982. Root-knot nematodes (Meloidogyne spp.): identification, morphological and physiological variation, host-range, ecology, and control. In: Riggs R.D., editor. Nematology in the Southern region of the United States. Southern cooperative series bulletin 276. Fayetteville, Arkansas: Arkansas Agricultural Experiment Station; p. 21–32.
- Siddiqi ZA, Akhtar MS. 2008. Synergistic effects of antagonistic fungi and a plant growth promoting rhizobacterium, an arbuscular mycorrhizal fungus, or composted cow manure on populations of Meloydogyne incognita and growth of tomato. Biocon Sci Technol. 18:207–290.
- Singh S, Mathur N. 2010. In vitro studies of antagonistic fungi against the root-knot nematode, Meloidogyne incognita. Biocon Sci Technol. 20:275–282.
- Southey JF. 1986. Laboratory methods for work with plant and soil nematodes. London: Ministry of Agriculture, Fisheries and Food, Reference Book 402, pp. 202.
- Sun MH, Gao L, Shi YX, Li BJ, Liu XZ. 2006. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J Invert Pathol. 93:22–28.
- Uddin MN, Saifullah AM, Khan W, Khan BM. 2019. Evaluation of Pochonia chlamydosporia (goddard) isolates for suppression of Meloidogyne incognita, root-knot nematode of tomato. J Agricul Sci. 11:70–81.
- Yahia Y, Benabderrahim MA, Tlili N, Bagues M, Nagaz K. 2020. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus mill. species. PLoS ONE. 15:e0232599. https://doi.org/10.1371/journal.pone.0232599
- Zwart RS, Thudi M, Channale S, Manchikatla PK, Varshney RK, Thompson JP. 2019. Resistance to plant-parasitic nematodes in chickpea: current status and future perspectives. Fron Plant Sci. 10:966. https://doi.org/10.3389/fpls.2019.00966
