ABSTRACT
Integrating a combination of organic matter management (OMM) practices can increase soil fertility, biomass, and nutrient recycling, but evidence of this potential is limited. This study tested the impact of integrating a combination of OMM practices on soil fertility, biomass, and nutrient recycling on smallholder farms. Following a randomised complete block design, a four-season experiment was conducted in 2018-2019 on 10 farms. The treatments (T) included T1: cowpea-maize-bean-maize rotation; T2: cowpea-maize-bean-maize rotation + farmyard manure; T3: Faidherbia albida alleys + cowpea-maize-bean-maize rotation; T4: F. albida alleys + cowpea-maize-bean-maize rotation + farmyard manure; and T5 (control): maize monocrop with diammonium phosphate application at 50 kg/ha application rate. T1-T4 are the OMM practices. The maize in T2-T4 was undersown with Mucuna pruriens. Soil fertility parameters (i.e. pH, water holding capacity, nitrogen, phosphorus, and potassium), biomass, and nutrients in the biomass were determined. There were no differences in soil fertility parameters among all treatments (P > 0.05). From the second to the fourth season, biomass was consistently higher under T3 and T4 than in other treatments. Moreover, the nutrients in biomass were higher in T3 and T4 than in other treatments, an indicator that OMM practices with alley crops can increase nutrient recycling.
Introduction
To achieve the United Nations’ sustainable development goals of ‘no poverty, zero hunger, and good health and wellbeing’, soil fertility improvement in nutrient-depleted smallholder farms is paramount. Moreover, soil nutrient depletion has continued to affect farming systems in most Sub-Saharan African countries (Tully et al. Citation2015; Zingore et al. Citation2015). As a result, negative nutrient balances have been observed in 21 countries of Sub-Saharan Africa, including Uganda, and have been estimated to be higher than 60 kg NPK/ha/year by 2002-2004 (Henao and Baanante Citation2006). These negative nutrient balances are contributed by several factors, including soil erosion, especially in sloping areas, limited nutrient input, monocropping, and poor crop residue (stover) management within the field (Haileslassie et al. Citation2005; Gebresamuel et al. Citation2020). As a result, they are more significant in conventional and organic farming systems with low nutrient inputs than in systems with high inputs (Adamtey et al. Citation2016).
An effective way to minimise the negative nutrient balances in the organic fertiliser management systems could be to apply a combination of organic matter management (OMM) practices such as alley cropping, farmyard manure (FYM) application, crop residue incorporation into the soil, and cover crop integration in a well-planned rotation system. Alley cropping increases organic matter (OM), mainly in the topsoil (Beuschel et al. Citation2019). This OM is formed from the accumulated tree leaf and root litter, and it then mineralises to increase soil nutrients in the upper soil layer that support crop growth (Beuschel et al. Citation2019; Birhane et al. Citation2018; Yengwe et al. Citation2018). In this context, deep rooting alley shrubs and trees (> 60 cm soil depth) transfer nutrients from below ground. With that, the trees increase nutrient availability for annual crops, and if they are legumes, they can increase the net profit via nitrogen fixation. The increased carbon offer leads to increased soil microbial biomass and thus nutrient availability. Studies have shown that FYM application increases soil organic carbon and the availability of nutrients supporting crop growth and increases crop biomass, providing a significant residual advantage to crops in the subsequent seasons (Meena et al. Citation2019; Basha et al. Citation2017). However, the quality of the FYM in terms of nutrient content depends on the type of fodder given to the livestock (Kato et al. Citation2013; Snijders et al. Citation2009). For instance, feeding livestock with different supplements, including legumes, can improve the nutrients in the FYM (Snijders et al. Citation2009). When cover crops are integrated into a farming system, a substantial improvement of OM content with or without the addition of mineral fertilisers can be realised (Beltrán et al. Citation2018). These cover crops can be integrated as intercrops in combination with the main crop or between the main crops in a rotation system. Cover crops offer several services in a cropping system, which include soil structure improvement when the roots bind the soil particles together, soil aeration improvement, protection of soil against rain splash, recycling of unused nutrients, providing animal feed, and controlling competing weeds (Baraibar et al. Citation2018; Pérez-Álvarez et al. Citation2015; Gómez et al. Citation2018; Uzoh et al. Citation2019). An additional benefit of biological nitrogen fixation can be obtained by integrating leguminous forage cover crops such as Mucuna pruriens into a rotation system (Muoni et al. Citation2019). Integration of grain legumes as intercrops or in a rotation can also improve soil chemical and physical conditions for the subsequent crops and improve the yield of the following crops (Nassary et al. Citation2020). When biomass from legumes and crop residues is incorporated into the soil, further improvement in soil structure, water holding capacity (WHC), nitrogen, phosphorus and potassium content is achieved (Bu et al. Citation2020; Malobane et al. Citation2020). The integration of legumes and incorporation of legume residues can improve soil properties, nutrient balances, and recycling (Lele and Tunya Citation2016; de de Tombeur et al. Citation2021). Moreover, the integration of OMM practices into a cropping system has been reported for improving biomass in terms of dry matter (DM) grain yield and DM residue yield (Basha et al. Citation2017; Meena et al. Citation2019; Sanginga Citation2003). The grains can be eaten by the family or sold in the market, while the crop residues can be sold or fed to livestock or incorporated into the field for soil nutrient recycling.
The authors hypothesise that integrating a combination of OMM practices in a crop rotation system can double or triple biomass and soil nutrients even better than the application of inorganic fertilisers as a sole strategy or in combination with a single OMM practice. It is further hypothesised that OMM practices, including recycling nutrients from animal husbandry and alley cropping, increase soil nutrient content and availability. The performance of such a cropping system with a combination of OMM practices may be high in humus production, nitrogen fixation, weed control, soil erosion management, and further nutrient recycling as the accumulated biomass decomposes.
Evidence of nutrient recycling and biomass contribution to a farming system via the integration of a combination of different OMM practices is limited. This study aimed at modifying the annual cropping systems by applying a combination of forage legumes, grain legumes, alley crops, crop residue incorporation, and FYM to understand their impact on soil nutrient availability, biomass contribution, and nutrient recycling on smallholder farms. The specific objectives of this study were to (1) understand the impact of a combination of OMM practices on the soil physical–chemical properties of pH, OM, WHC, and the plant available nutrients of N, P, and K; (2) determine the nutrient content of grains that are harvested from the different OMM practices; (3) determine the biomass contribution of the different OMM practices and (4) ascertain the contribution of OMM practices on nutrient recycling.
Materials and methods
Description of the study area
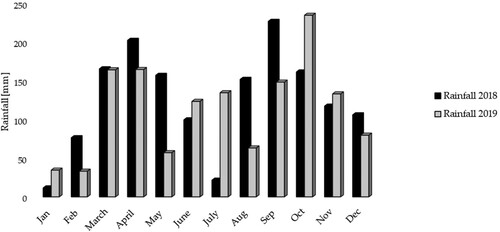
This study was conducted on 10 smallholder farms in the Nyabbani sub-county of Kamwenge district that is located in the Rwenzori region in Western Uganda (). The elevation of this area is between 1140 and 1184 m above sea level. The area has a temperature distribution of 20–25°C and bimodal rainfall distribution, where the first season is the short rainy season (SR) that stretches from March to May while the second is the long rainy season (LR), which stretches from August to December (Kamwenge Citation2016). From 2018 to 2019, the study area received 384–525 mm of rain during the SR and 570–660 mm during the LR (). The area is dominated by sandy clay loam – Acrisols, which are acidic with a pH (H2O) of approx. 5.1 and have organic carbon of 0.8% (FAO Citation2012). Such soils require the integration of OMM strategies for pH adjustment and further organic carbon improvement.
Experimental design
In January 2018, a multi-locational field experiment was set up following a randomised complete block design on 10 smallholder farms. These farms had undergone a fallow period of at least six months before they were subjected to experimental conditions. Each of the 10 farms was considered a block. Five treatments that involved crop rotations for four consecutive crop-growing seasons (two years) were randomly allocated to each block. Each treatment was allocated to a plot of 10 × 10 m dimensions. The five treatments (T1-T5) were:
T1: Vigna unguiculata (cowpea)- Zea mays (maize)-Phaseolus vulgaris (bean)-maize rotation;
T2: cowpea-maize-bean-maize rotation + Farmyard Manure (FYM);
T3: cowpea-maize-bean-maize rotation + Faidherbia albida alleys;
T4: cowpea-maize-bean-maize rotation + FYM + F. albida alleys; and
T5 (control): maize monocrop with diammonium phosphate (DAP) fertiliser (18-46-0) application at a rate of 50 kg/ha (N: 9 kg/ha; P: 10 kg/ha). This rate is affordable to most maize subsistence farmers in the Rwenzori region.
T1-T4 are treatments with OMM strategies, while T5 is the treatment with an inorganic fertiliser application strategy, and it served as a control. The maize in T2-T4 was undersown with M. pruriens. For T2 and T4, FYM at a rate of 2.5 tons/ha (N: 13 kg/ha; P: 6 kg/ha; K: 18 kg/ha) was incorporated into the soil. The FYM required for this application rate can be obtained from 4–6 tropical cows per season. In all treatments, crop residues from the previous harvest were chopped into pieces of about 3 cm in length and then incorporated into the soil of the same plots where they were obtained. This was done to further improve soil organic matter and nutrient recycling. gives details of the four-season arrangement of the treatments.
Table 1. Arrangement of treatments for the entire experiment in 2018 and 2019.
Maize was the main crop selected for this experiment because of its ability to tolerate the drought conditions of the study area (FAO Citation2017). Longe 5 maize variety was planted in the long rainy season of 2018 (LR2018) and the long rainy season of 2019 (LR2019), and was selected because it matures very fast in only 115 days. The legume cowpea (Vigna unguiculata) was planted in the short rainy season of 2018 (SR2018) as a pre-crop for maize due to its capacity to produce high biomass and nitrogen in a short time (Omae et al. Citation2014). SECOW 5 T cowpea variety was planted, as it is the one that was recommended for the study site conditions by the National Agricultural Research Organisation of Uganda. M. pruriens var. utilis is a forage legume that was undersown into the 30 days old maize crop during LR2018 and LR2019. The aboveground biomass from M. pruriens was later incorporated into the soil as green manure in the same field where it had grown. F. albida is a leguminous tree with reversal phenology since it develops leaves during the dry season and sheds them off during the rainy season. This reversal phenology enables the tree to grow well with minimum water competition with other crops (Roupsard et al. Citation1999). F. albida of Moroto provenance was planted as it was the only seed provenance available at the Uganda National Tree seed centre. The F. albida seedlings were introduced into the plots with treatments T3 and T4 when they were only three months old at a height of about 30 cm tall. By the fourth season, F. albida had grown to a height of about 4 m tall. At the end of every season, the leaves from the prunings of this tree were incorporated into the soil of the same plots where the trees were growing for further organic matter improvement. The grain legume beans (Phaseolus vulgaris) were planted during the short rainy season of 2019 (SR2019) as a pre-crop for the maize planted in LR2019. The bean crop was selected since it is a common source of proteins in both urban and rural areas (Aseete et al. Citation2018). Nabe 4 bean variety was planted because of its fast maturity (matures after 80–85 days) and has high resistance against anthracnose and the bean common mosaic virus, which are prevalent in the Rwenzori region (Kankwatsa Citation2018). The seed/seedling planting rates and spacing of all crops are shown in .
Table 2. The seed planting rates and spacing of crops during the field experiment.
Soil sampling and testing
A composite soil sample was collected from each of the 50 study plots at 0–15 cm soil depth in January 2018 (before experimenting) and in April and September 2018 and 2019 (one month after sowing). Soil physical–chemical properties (pH, OM, WHC, and macronutrient content of N, P, and K in plant-available forms) were tested. Soil pH was determined through an electrometric method using a pH meter (Eutech pH 700 meter). In contrast, OM was determined through the ignition of the samples at high temperatures (up to 550 °C) (Okalebo et al. Citation2002). WHC was obtained by calculating the difference between the air-dried soil and the soil saturated with water (Pawar et al. Citation2009). The plant-available nitrogen was determined through the use of H2O2 and KCl following a procedure by Sahrawat (Citation1982) and Tie et al. (Citation2013). In this soil test procedure, the soil was sorted out of plant roots and stones, air-dried, and sieved through a 2 mm sieve. 5 g of the sieved soil was placed into a 300 ml conical flask where 50 ml of 25% H2O2 was added. The conical flask with its content was then placed into a ventilated oven and heated at a temperature of 60°C for 6 h. The suspension was cooled and 1 M KCl was added. The flask with its content was then rotated for 30 min and then filtered. N-NH4+ from the filtrate was determined through distillation (Ibid).
Plant available phosphorus was determined through Bray 1 extraction method in the procedure by Kovar and Pierzynski (Citation2009). In this procedure, 20 ml of Bray1 extraction solution (0.025M HCl in 0.03 M NH4F) were added into a 50 ml conical flask containing 2 g of soil. The contents of the flask were shaken at 200 revolutions per minute at room temperature. The extract was filtered using the Whitman No. 42 filter paper and the plant-available P was measured by use of a spectrophotometer (VWR- UV- 6300PC) at a wavelength of 880 nm (Kovar and Pierzynski Citation2009). The results obtained were converted from mg P/kg soil to Kg/ha.
Plant available potassium was determined following a flame photometry procedure using chemical atomic absorption (Pawar et al. Citation2009; Okalebo et al. Citation2002). Under this procedure, 100 ml of Ammonium acetate (NH4OAc) was added to a conical flask containing 5 g of air-dry soil sample. The flask and its content were shaken at 200 oscillations per minute for 30 min. The solution was left to stand for 30 min and the supernatant was filtrated through the Whatman No. 42 filter paper. The extracted solution was diluted 10 times and 5 ml of the solution was pipetted into a 50 ml volumetric flask. 1 ml of lanthanum chloride solution was added to the volumetric flask with its contents. The contents were then diluted with NH4OAc extraction solution to the mark. The solution was sprayed onto the flame of the flame photometer (PFP7 model), starting with standards, the sample, and blank solutions to determine the K content. K concentration was calculated by reference to the calibration graph (Pawar et al. Citation2009; Okalebo et al. Citation2002). The ratings of the nutrient content and pH results in all the samples were based on Pawar et al. (Citation2009).
Plant sampling and testing
Depending on the crop type grown in a particular season, we obtained aboveground biomass from each plot by randomly cutting ten different maize, beans, and cowpea plants from the root collar. All plant samples were oven-dried at 70 °C until each sample attained a constant weight. The total dry weight of all crops and trees per plot, including grains, crop residues, and leaves was measured and then expressed in kg/ha. Biomass from each plot that can be incorporated into the soil for organic matter enhancement was calculated by adding the total dry weight of crop residues, and leaves of F. albida and M. pruriens.
The grains, plant residues, and leaves of M. pruriens and F. albida were analyzed for N, P, and K content. Nitrogen was tested using the Kjeldahl nitrogen procedure with selenium catalyst and it was determined through distillation and titration (Muñoz-Huerta et al. Citation2013; Okalebo et al. Citation2002). Phosphorus was tested by the total phosphorus without pH adjustment, using the ascorbic acid procedure. The P content in the plant parts was then measured with a spectrophotometer at a wavelength of 880 nm. A flame photometer was used to determine potassium concentration in the digested plant samples (Okalebo et al. Citation2002). The nutrients in the grains of maize, cowpea, and beans were considered as nutrients taken out of the field, while the nutrients in biomass were considered as nutrients that can be recycled into the field for organic matter and nutrient improvement.
Data analysis
One-way multivariate analysis of variance (one-way MANOVA) test in IBM SPSS version 26 was applied to determine the differences in the soil physical–chemical properties of pH, WHC, OM, and the plant available forms of N, P, and K. The pre-requisite for the ANOVA test was the normal distribution of the residuals as determined by the Kolmogorov–Smirnov and Shapiro–Wilk test, and the homogeneity of variance of the data as determined by the Levene Statistics. The data about soil physical–chemical properties was further subjected to Tukey’s HSD post hoc test for pairwise comparison between the means of each studied property.
The differences in biomass and nutrients in both grains and biomass among the treatments in each season were determined through the one-way MANOVA test. MANOVA was conducted after the data had undergone a Log10 transformation. Furthermore, a Tukey’s HSD post hoc test was used for pairwise comparisons of the Log10 transformed total biomass and nutrients in grains and biomass among the treatments in each season. Both the untransformed and the log10-transformed data were presented for easy comparison.
Results
Soil physical–chemical properties
The baseline study indicates that the soils had a pH value of 5.8 with 2.5% organic matter and 62.01% water holding capacity. Further tests revealed that the mean plant-available N, P, and K were 256.2, 21.1, and 198.5 kg/ha respectively. According to the classification by Pawar et al. (Citation2009), the baseline soils can be categorised as acidic with high organic carbon (mean content is more than 1%). These soils can further be categorised as relatively low in plant-available N (mean content is within the 140–280 kg/ha rating), moderate in plant-available P (mean content is within 13–22 kg/ha), and moderate in plant-available K (mean content is within the 181–240 kg/ha rating). In a comparison of soil physical–chemical properties between seasons, it can be seen that soil pH values were however generally lower in the first season (SR2018) than in the baseline soil. In the subsequent seasons, the soil pH values increased irrespective of the treatments. According to Pawar et al. (Citation2009), the soils, irrespective of the treatments, can be described as moderately acidic (pH value of 5.3–6.0) before subjecting the fields to the different treatments. The moderately acidic soils became strongly acidic (pH value of 4.6–5.2) during the first season and then moderately acidic during the fourth crop-growing season (LR2019). However, there were no major differences in the pH values observed among treatments of the same season (P > 0.05). Similarly, there were no major differences in WHC observed among treatments of the same season (P > 0.05), but the amounts increased in the subsequent seasons. For OM content, minor differences were observed among all treatments (P > 0.05), and the amount was not affected by the change in seasons. The soil OM content remained high in organic carbon for all treatments during the four study seasons (Pawar et al. Citation2009).
There were minor differences in soil N content among treatments (P > 0.05), but the amounts increased in the subsequent crop-growing seasons. Based on Pawar et al. (Citation2009), soil N content was relatively low before the study, increased to medium amounts (281–420 kg/ha) right during the first season and to moderately high amounts (421–560 kg/ha) at the end of the fourth season. Like soil N, there were minor differences in soil K content among all the treatments (P > 0.05). K content, however, increased in the subsequent seasons. The amounts were moderate before the study, and they became moderately high (241–300 kg/ha) during the third season (Pawar et al. Citation2009). Like N and K, there were minor differences in P content among all treatments (P > 0.05), but the change in the content did not follow any specified trend. Notwithstanding, the P content in the soil remained within the medium range for all treatments in the four seasons (Pawar et al. Citation2009).
Nutrient content of grains
The total N content in grains for the entire four-season rotation was lower in T1-T4 with OMM practices than in T5 (P < 0.05). In SR2018, the N content in grains was lower in the treatments with OMM practices than in T5 (). For SR2019, the N content in grains was highest under T5 (P < 0.05). However, minor differences in N content were observed between T5 and the treatments T1, T2 and T4 with the OMM practices (P > 0.05) (). In both LR2018 and LR2019, there were no major differences observed among all treatments-T1-T5 (P > 0.05) (). P and K content in grains followed a similar trend as observed in N ().
Table 3. Comparison of N, P, and K content in the grains between treatments T1-T5 over four seasons during 2018 and 2019.
Biomass contribution of the OMM practices
The total biomass for the entire four-season rotation system was lower under T5 than in T1-T4 and was highest under T3 and T4 in comparison with other treatments (P < 0.05). In the first season, the biomass was higher under T5 than the treatments with the OMM practices (P < 0.05). However, this tendency changed in the subsequent seasons and the biomass became higher under treatments T3 and T4 with F. albida alleys than in other treatments (). From the second season onwards, the biomass in T3 and T4 was consistently higher than in other treatments (). Apart from T5, which did not show any specified trend, biomass for other treatments increased in the subsequent seasons except in LR2019 ().
Table 4. Comparison of total biomass between treatments T1-T5 over four seasons during 2018 and 2019.
Nutrient composition of biomass
For the entire four-season rotation system, the total N content in biomass was lower in T5 than in T1-T4 (P < 0.05). The total N content in biomass was however higher in T3 and T4 than in other treatments (P < 0.05). In a season-per-season nutrient comparison, it can be seen that N content was higher in T5 than in T1-T4 during the first season (P < 0.05) (). In the subsequent seasons, the N content in biomass was however higher in T3 and T5 than in other treatments (). P and K content in the biomass followed a similar trend as the N content.
Table 5. Comparison of N, P, and K content in biomass between treatments T1-T5 over four seasons during 2018 and 2019.
Discussion
Soil physical–chemical properties
It was found that OM had high organic carbon while P and K were moderate in the baseline soil samples. The high organic carbon and moderate P and K content in the baseline soil samples could be attributed to the six-month fallow period the farmers had subjected to the fields before the experiment was set. The soil pH values throughout the experimental period were lower than what was observed under the baseline conditions possibly because of the difference in rainfall intensities between January 2018, when the baseline samples were collected, and the months of April and September during the experimental period. Besides, January is within the dry season, while April and September are months of high rainfall amounts in the Rwenzori region (). The rains received one month after we had sowed could have leached some of the alkaline elements making the soil more acidic than in the dry season when the baseline samples were collected. Moreover, high rainfall intensities have been documented for accelerating soil nutrient leaching rates (Hougni et al. Citation2021; Lehmann and Schroth Citation2003). In the first season, cowpea was expected to produce more biomass that would decompose with an eventual increase in pH than the sole maize cropping system. However, there were minor differences in pH results between the cowpea and maize fields, as no treatment had enough time to accumulate OM responsible for nutrients and pH change. These results did not agree with other studies (Jan et al. Citation2016; Latati et al. Citation2014; Wang et al. Citation2015; Butterly et al. Citation2013) where lower pH values were obtained from the cowpea-maize intercrop than in the monocropping system. The lower pH values obtained in the cowpea-maize intercrop system than in the sole maize or sole cowpea cropping system in the previous studies could be explained by the higher cation uptake in the fields with an intercropping system than in the monocrop system (Latati et al. Citation2014). Soil pH in our study increased from season to season and this trend differed from Rezig et al. (Citation2012), where the values under crop residue incorporation further increased soil acidity. The soil pH increase that we observed could be attributed to the accumulation of alkaline elements into the soil as the organic material decomposes.
During the experimental period, soil organic carbon remained high despite the cropping systems. These high amounts of organic carbon could be attributed to the decomposition of the crop residues incorporated into the soil during land preparation. Moreover, retaining crop residues within the field has proved to improve soil OM in other studies (Raffa et al. Citation2015). Apart from crop residue incorporation, a maize-legume rotation system, maize/ M. pruriens, and F. albida alleys in this study could have increased biomass that decomposed to keep the organic carbon high. This is expected since the maize-legume rotation system, maize/ M. pruriens and F. albida integration have been documented for improving soil organic carbon and OM (Pikuła and Rutkowska Citation2020; Birhane et al. Citation2018).
Like pH, there were no major differences in WHC among all treatments, but it increased in the subsequent seasons. From the baseline conditions to the fourth crop growing season, the WHC increased by 19.6% under T4 with a combination of OMM practices and 16.5% under T5 with the inorganic fertiliser application practice. The higher WHC in T4 than in T5 could be explained by the higher biomass in T4, which could have decomposed, contributing to higher WHC than in T5. Since crop residues were the main constituent of biomass under this investigation, they could have contributed to the higher WHC in the soil. Besides, other studies have indicated an improvement in WHC through crop residue incorporation into the field (Rezig et al. Citation2012). Furthermore, the OM accumulated by the decomposed FYM, and M. pruriens and F. albida leaves could further improve the soil WHC if such soil amendment strategies are continued for more than the four study seasons.
In this study, there were no major differences in N content between the OMM practices and the inorganic fertiliser application strategy. This could imply that the OMM practices increased soil N content to amounts similar to those under the inorganic fertiliser application strategy. In SR2018, which was the first crop-growing season, the results did not agree with what was observed in other studies, for example, Jan et al. (Citation2016) where higher N content under cowpea than in the sole maize cropping system was reported. The difference in our results and those of Jan et al. (Citation2016) could have resulted from the type and rates of fertilisers applied in the field. Moreover, the field studies by Jan et al. (Citation2016) involved inorganic N, P, and K fertiliser application in both maize and cowpea fields, while the current study did not involve any inorganic fertiliser application during the SR2018. In our subsequent seasons, the increase in N and P content under the inorganic fertiliser application strategy could be explained by the DAP applied to the field. The increase in N, P and K content under the OMM practices could be attributed to the mineralisation of OM from the biomass accumulated within the fields in the subsequent seasons. Besides, FYM application, alley cropping, integration of the forage legume- M. pruriens and grain legume- beans into the cropping system are recognised for improving N, P and K content in the soil (Akpalu et al. Citation2020; Umar et al. Citation2013; Birhane et al. Citation2018; Gatsios et al. Citation2021; Uzoh et al. Citation2019; Yengwe et al. Citation2018). Crop residue incorporation could have also contributed to the observed increase in soil N, P, and K during the subsequent seasons as the residues decomposed, mineralising into soil nutrients as indicated in other studies (Bu et al. Citation2020; Thapa et al. Citation2018; Malobane et al. Citation2020). Our results suggest that the OMM practices can improve soil N, P and K to amounts observed when the inorganic fertiliser- DAP, at 50 kg/ha is applied. The continuation of the OMM practices is expected to lead to optimised nutrient recycling in the future.
Nutrient content of grains
The nutrients in the grains provide an insight into the amount of nutrients that are transported out of the field for consumption and or sale, which need to be replaced through external fertiliser application. It was found that N, P, and K content in grains during SR2018 were lower under T1-T4 than in T5 (), which could be explained by the variation of crops planted in that season, i.e. maize in T5 and cowpeas in T1-T4. Obtaining a similar effect in N, P, and K content in grains among all treatments (T1-T5) in LR2018 and LR2019, where maize was a common crop among all the five treatments could imply that both the OMM practices and inorganic fertiliser application can result in similar nutrient concentrations in the grains. However, nutrient depletion is expected if nutrients are continuously carried away from the field via the grains without any effort to recycle the biomass and integrate legumes or add other external fertilisers.
Biomass contribution of the OMM practices
The results show that total biomass in SR2018 was lower under T1-T4 than in T5 (), which could be explained by the lower weight of cowpea residues under T1-T4 than the maize residues in T5. The higher biomass we observed under T3 and T4 from SR2019 to LR2019 than in other treatments () could be an indicator that alley cropping greatly contributes to the improvement of biomass. As the biomass from the F. albida leaves decomposes releasing nutrients, other crops take up these nutrients hence improving their growth and producing more biomass. A combination of FYM and F. albida alleys in a cropping system was expected to increase biomass more than the other treatments. The contrary results in the current study could be explained by the ability of the microorganisms to take up some of the nutrients during the tree leaves and FYM decomposition process, temporarily fixing the nutrients that would be available for crop growth and biomass accumulation. Moreover, FYM is one of the amendments reported to increase microbial population growth (Basha et al. Citation2017). In addition, T5 had less biomass possibly due to the lower N (9 kg/ha) and P (10 kg/ha) content with no potassium in DAP than T3 and T4 where the integration of a combination of F. albida and M. pruriens resulted into high biomass and the nutrients therein. Biomass of F. albida had N, P and K content of 2512, 165 and 1194 kg/ha in T3 and 1193, 81 and 580 kg/ha in T4 respectively while the biomass of M. pruriens, had N, P and K content of 60, 5 and 39 kg/ha in T3 and 61, 5 and 31 kg/ha in T4 respectively. Since F. albida and M. Pruriens were integrated into the cropping system, their nutrients could have contributed to the increased biomass observed in T3 and T4. T3 and T4 could be recommended for integration into the nutrient-depleted smallholder farms as they exhibited the highest biomass compared to other OMM practices and the inorganic fertiliser application strategy.
Nutrient composition of biomass
The higher N, P and K content in biomass for the treatments with OMM practices (T1-T4) than in T5 during LR2018, SR2019, and LR2019 () could be explained by the OM generated from the biomass that was accumulated by the legume-maize rotation system, intercrop of maize with M. pruriens and the crop residues incorporated into the field. Moreover, biomass from crop residues has been reported for releasing nutrients upon decomposition (da da Silva et al. Citation2021). The higher amount of nutrients in biomass that we observed in the treatments with OMM than in T5 could have also resulted from the FYM incorporation in T2 and T4, nitrogen fixation, and/or the uptake of leached nutrients from deeper layers by F. albida for T3 and T4.
The high nutrient content in the grains and biomass (crop residues, as well as leaves of leguminous trees and forage legumes) observed in this study might increase the risk of nutrient depletion if farmers do not increase the input of biomass into their cropping systems. The higher biomass and the associated available macronutrients of N, P and K in OMM treatments than in T5 with DAP application can indicate that the integration of OMM practices can maintain the soil fertile.
We conclude that the incorporation of biomass into the soil increases the availability of soil nutrients and leads to more efficient use of farm own nutrients, which in turn reduces the external inputs.
As the dominating farming practices are low in OMM investment and are critical in terms of soil erosion, climate change, and yield development, the findings of this study are of relevance to motivating farmers to change their practices. Our findings can also inform extension workers on the use of a combination of OMM strategies for improved soil nutrients, biomass and nutrient recycling within their fields.
This study was conducted in four crop-growing seasons with a rotation of two food crop families (Fabaceae and Poaceae). If a rotation based on only two crop families continues for more than two seasons, soil-borne diseases are likely to emerge. Future studies should be conducted for more than four seasons and the rotation system should involve more than two crop families. This can enable us to know if the OMM practices will further increase the soil physical–chemical and biological properties, biomass and the nutrients in biomass.
Author contributions
Conceptualisation, DME, TTK and BF; methodology, DME, TTK, JF and BF; investigation, DME, BA; writing – original draft preparation, DME; writing – review and editing, DME, TTK, PD, BA, JK, JF, AM and BF; supervision, JK, JF, AM and BF. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors acknowledge the farmers of Nyabbani Sub County for availing their land for the experiment and for fully participating in the field experiment. Bosco Bwambale (PhD) is acknowledged for reviewing this paper and Collins Muhangi for his assistance in soil and plant testing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Deous Mary Ekyaligonza
Deous Mary Ekyaligonza she is a Lecturer at Mountains of the Moon University (MMU) and a PhD candidate at the University of Natural Resources and Life Sciences (BOKU), Vienna. Her research interest is in natural resources management and sustainable agricultural systems. She has a Master of Science in Natural Resources Management Degree from Mountains of the Moon University in Uganda and a Bachelor of Science in Forestry degree from Makerere University in Uganda.
Thaddeo Tibasiima Kahigwa
Thaddeo Tibasiima Kahigwahe is a PhD candidate at the University of Natural Resources and Life Sciences, Vienna. His main research focus is on the sustainability of smallholder farming systems in tropical areas. He has vast experience in working with agroecological farming systems and teaching sustainable farming systems both at the farm and university levels.
Phillipp Dietrich
Phillipp Dietrichhe is born in Linz/OÖ; his educational background is in agricultural sciences (BOKU, Vienna) and development studies (University of Vienna). Since 2009, he has been involved at BOKU´s Division of Organic Farming in project activities (ERASMUS+, APPEAR & ELLS), teaching, and coordination of the international MSc EUR-Organic.
Bendicto Akoraebirungi
Bendicto Akoraebirungiis a horticulturist at Mountains of the Moon University. He has a Master of Science in Natural Resources Management with a specialty in physical resources from Mountains of the Moon University (MMU) in Uganda and a Bachelor of Science in Horticulture and Entrepreneurship Management from MMU.
John Patrick Kagorora
John Patrick Kagorora he is an Associate Professor in the Faculty of Agriculture and Environmental Sciences at Mountains of the Moon University in Uganda. He has vast experience in agricultural research as he worked with the Uganda National Agricultural Research Organisation (NARO), World Agroforestry Centre (ICRAF) in Uganda for five years and the university for 15 years. He has supervised several students at PhD, masters and bachelors levels.
Jürgen Kurt Friedel
Dr. Jürgen Kurt Friedelis an Associate Professor in the Division of Organic Farming at BOKU. His research area is soil science; agronomy; agricultural-chemical research; agricultural ecology; organic farming; and soil science. He has supervised several PhD and master's students and has been a reviewer of several scientific journals.
Andreas Melcher
Dr. Andreas Melcheris a Deputy Head at the Institute for Development Research and Cluster (CDR) for Development Research at BOKU. He has a PhD in Environmental Engineering and Water Management, Applied Aquatic Ecology from BOKU. Andreas has over 25 years of experience in Biodiversity research; Biostatistics; bioclimatology; water pollution control; hydrobiology; limnology; agricultural ecology; pisciculture; ichthyology; and environmental protection. Has supervised many Masters and PhD students and has been a reviewer for several journals.
Bernhard Freyer
Dr. Bernhard Freyerserves as the chair of the Division of Organic Farming at BOKU, leads the working group- Transdisciplinary System Research, and is a senior fellow of agricultural systems, MISA, and CFANS at the University of Minnesota in the USA. His main research focuses on combining natural and social sciences, (organic) agriculture, and food systems embedded in society, recognizing that society is in the process of ongoing differentiation, with a focus on systems in East Africa. He has organized and headed several national and international research projects and supervised PhD and master theses for more than 25 years. He offers lectures in a broad field of topics within organic agriculture, methods and theories in social sciences. Furthermore, he is a reviewer in several journals in the field of social sciences, ethics, and ecology.
References
- Adamtey N, Musyoka MW, Zundel C, Cobo JG, Karanja E, Fiaboe KKM, Muriuki A, Mucheru-Muna M, Vanlauwe B, Berset E. 2016. Productivity, profitability and partial nutrient balance in maize-based conventional and organic farming systems in Kenya. Agric Ecosyst Environ. 235:61–79.
- Akpalu SE, Dawoe EK, Abunyewa AA. 2020. Effects of Faidherbia albida on some important soil fertility indicators on agroforestry parklands in the semi-arid zone of Ghana. Afr J Agri Res. 15:256–268.
- Aseete P, Katungi E, Bonabana-Wabbi J, Birachi E, Ugen MA. 2018. Consumer demand heterogeneity and valuation of value-added pulse products: a case of precooked beans in Uganda. Agric Food Secur. 7:51.
- Baraibar B, Mortensen DA, Hunter MC, Barbercheck ME, Kaye JP, Finney DM, Curran WS, Bunchek J, White CM. 2018. Growing degree days and cover crop type explain weed biomass in winter cover crops. Agron Sustain Dev. 38:65.
- Basha SJ, Basavarajappa R, Shimalli G, Babalad HB. 2017. Soil microbial dynamics and enzyme activities as influenced by organic and inorganic nutrient management in vertisols under aerobic rice cultivation. J Environ Biol. 38:131.
- Beltrán MJ, Sainz-Rozas H, Galantini JA, Romaniuk RI, Barbieri P. 2018. Cover crops in the Southeastern region of Buenos Aires, Argentina: effects on organic matter physical fractions and nutrient availability. Environ Earth Sci. 77:1–11.
- Beuschel R, Piepho H, Joergensen RG, Wachendorf C. 2019. Similar spatial patterns of soil quality indicators in three poplar-based silvo-arable alley cropping systems in Germany. Biol Fertil Soils. 55:1–14.
- Birhane E, Gebremeskel K, Taddesse T, Hailemariam M, Hadgu KM, Norgrove L, Negussie A. 2018. Integrating Faidherbia albida trees into a sorghum field reduces striga infestation and improves mycorrhiza spore density and colonization. Agrofor Syst. 92:643–653. doi:10.1007/s10457-016-0027-8.
- Bu R, Ren T, Lei M, Liu B, Li X, Cong R, Zhang Y, Lu J. 2020. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under rice-rice-rapeseed rotation system. Agric Ecosyst Environ. 287:106681.
- Butterly CR, Baldock JA, Tang C. 2013. The contribution of crop residues to changes in soil pH under field conditions. Plant Soil. 366:185–198.
- da Silva JJP, Teixeira R, da Silva IR, Soares EMB, Lima AMN. 2021. Decomposition and nutrient release from legume and non-legume residues in a tropical soil. Eur J Soil Sci. 73:e13151.
- de Tombeur F, Roux P, Cornelis J. 2021. Silicon dynamics through the lens of soil-plant-animal interactions: perspectives for agricultural practices. Plant Soil. 1–28.
- FAO. 2012. Harmonised World Soil Database Viewer (Version 1.2). FAO, IIASA, ISRIC and ISS-CAS, JRC FAO. Rome, Italy and IIASA, Laxenburg, Austria. http://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/harmonized-world-soil-database-v12/en/.
- FAO. 2017. Drought-tolerant maize varieties in Uganda. http://www.fao.org/3/CA2545EN/ca2545en.pdf.
- Gatsios A, Ntatsi G, Celi L, Said-Pullicino D, Tampakaki A, Savvas D. 2021. Impact of legumes as a pre-crop on nitrogen nutrition and yield in organic greenhouse tomato. Plants. 10:468.
- Gebresamuel G, Opazo-Salazar D, Corral-Núnez G, van Beek C, Elias E, Okolo CC. 2020. Nutrient balance of farming systems in Tigray, Northern Ethiopia. J Soil Sci Plant Nutr. 1–14.
- Gómez JA, Campos M, Guzmán G, Castillo-Llanque F, Vanwalleghem T, Lora A, Giráldez JV. 2018. Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ Sci Pollut Res. 25:977–989.
- Haileslassie A, Priess J, Veldkamp E, Teketay D, Lesschen JP. 2005. Assessment of soil nutrient depletion and its spatial variability on smallholders’ mixed farming systems in Ethiopia using partial versus full nutrient balances. Agric Ecosyst Environ. 108:1–16.
- Henao J, Baanante C. 2006. Agricultural production and soil nutrient mining in Africa: Implications for resource conservation and policy development. IFDC: Muscle Shoals, Alabama, U.S.A. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Henao%2C+Julio%2C+and+Carlos+Baanante.+2006.+Agricultural+production+and+soil+nutrient+mining+in+Africa.
- Hougni DGJM, Schut AGT, Woittiez LS, Vanlauwe B, Giller KE. 2021. How nutrient rich are decaying cocoa pod husks? The kinetics of nutrient leaching. Plant Soil. 1–16.
- Jan R, Saxena A, Jan R, Khanday MA, Jan R. 2016. Impact of intercropping patterns (Maize+ Black cowpea) on yield attributes and soil physico-chemical properties. Ecol Environ Conserv. S13–S16.
- Kamwenge DLG. 2016. Kamwenge District Local Government: Five- Year Development Plan 2015/2016–2019/2020 http://npa.ug/wp-content/uploads/2017/05/KAMWENGE-DDP-2015-2020-FINAL.pdf.
- Kankwatsa P. 2018. Efficacy and cost-benefit analysis of indigenous technical knowledge versus recommended integrated pest and disease management technologies on common beans in South Western Uganda. Open Access Libr J. 5:1.
- Kato H, Bareeba FB, Sabiiti EN. 2013. Productivity of soil fertilised with faecal manure of cattle fed Calliandra, Gliricidia and Luecaena browse/maize silages. Afr J Agri Res. 8:634–638.
- Kovar JL, Pierzynski GM. 2009. Methods of phosphorus analysis for soils, sediments, residuals, and waters second edition. Southern cooperative series bulletin 408.
- Latati M, Blavet D, Alkama N, Laoufi H, Drevon J, Gerard F, Pansu M, Ounane SM. 2014. The intercropping cowpea-maize improves soil phosphorus availability and maize yields in an alkaline soil. Plant Soil. 385:181–191.
- Lehmann J, Schroth G. 2003. Nutrient leaching. In: Trees, crops and soil fertility. Wallingford: CABI Publishing; p. 151–166.
- Lele JJ, Tunya BA. 2016. Contribution of legumes and phosphorus fertilizer to nutrient balances in a sorghum based cropping system in Njoro Kenya. J Exp Agri Int. 1–12.
- Malobane ME, Nciizah AD, Mudau FN, Wakindiki IIC. 2020. Tillage, crop rotation and crop residue management effects on nutrient availability in a sweet sorghum-based cropping system in marginal soils of South Africa. Agronomy. 10:776.
- Meena BP, Kumar A, Lal B, Meena RL, Shirale AO, Dotaniya ML, Kumar K, Sinha NK, Meena SN, Ram A. 2019. Sustainability of popcorn-potato cropping system improves due to organic manure application and its effect on soil health. Potato Res. 62:253–279.
- Muñoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV. 2013. A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances. Sensors. 13:10823–10843.
- Muoni T, Öborn I, Mhlanga B, Okeyo I, Mutemi M, Duncan A. 2019. The role of Mucuna pruriens in smallholder farming systems of Eastern and Southern Africa: a review. Agronomic Crops. 485–498.
- Nassary EK, Baijukya F, Ndakidemi PA. 2020. Intensification of common bean and maize production through rotations to improve food security for smallholder farmers. J Agri Food Res. 2:100040.
- Okalebo JR, Gathua KW, Woomer PL. 2002. Laboratory methods of soil and plant analysis: a working manual second edition. Nairobi, Kenya.
- Omae H, Saidou AM, Tobita S. 2014. Improving millet-cowpea productivity and soil fertility with crop rotation, row arrangement and cowpea density in the Sahel, West Africa. J Agric Environ Sci. 14:110–115.
- Pawar DR, Randhe DB, Shaikh M, Korde AK, Shinde KA, Yadav MP, Chavan AE, Sapkale VM. 2009. Laboratory testing procedure for soil & water sample analysis. Water Resources Department Directorate of Irrigation Research & Development, Pune. https://docplayer.net/26211159-Laboratory-testing-procedure-for-soil-water-sample-a …
- Pérez-Álvarez EP, Garde-Cerdán T, Santamaría P, García-Escudero E, Peregrina F. 2015. Influence of two different cover crops on soil N availability, N nutritional status, and grape yeast-assimilable N (YAN) in a cv. Tempranillo vineyard. Plant Soil. 390:143–156.
- Pikuła D, Rutkowska A. 2020. Selected chemical properties of sandy soil after 36 years of differential fertilization with mineral nitrogen and manure without liming in two crop rotation. Soil Sci Annu. 71:246–251.
- Raffa DW, Bogdanski A, Tittonell P. 2015. How does crop residue removal affect soil organic carbon and yield? A hierarchical analysis of management and environmental factors. Biomass Bioenergy. 81:345–355.
- Rezig AMR, Elhadi EA, Mubarak AR. 2012. Effect of incorporation of some wastes on a wheat-guar rotation system on soil physical and chemical properties. Int J Recycl Org Waste Agric. 1:1–15.
- Roupsard O, Ferhi A, Granier A, Pallo F, Depommier D, Mallet B, Joly HI, Dreyer E. 1999. Reverse phenology and dry-season water uptake by Faidherbia albida (Del.) A. Chev. in an agroforestry parkland of Sudanese west Africa. Funct Ecol. 13:460–472.
- Sahrawat KL. 1982. Evaluation of some chemical indexes for predicting mineralizable nitrogen in tropical rice soils. Commun Soil Sci Plant Anal. 13:363–377.
- Sanginga N. 2003. Role of biological nitrogen fixation in legume based cropping systems; a case study of West Africa farming systems. Plant Soil. 252:25–39.
- Snijders PJM, Davies O, Wouters AP, Gachimbi L, Zake J, Ergano K, Abduke M, Keulen HV. 2009. Cattle manure management in East Africa: review of manure quality and nutrient losses and scenarios for cattle and manure management. Wageningen UR Livest Res. 258.
- Thapa B, Pande KR, Khanal B, Marahatta S. 2018. Effect of tillage, residue management and cropping system on the properties of soil. Int J Appl Sci Biotech. 6:164–168.
- Tie TB, Diby LN, Seyo E, Assa A. 2013. Estimating soil available nitrogen with a hot H2O2/KCl extraction. Sci Res Essays. 5:1455–1462.
- Tully K, Sullivan C, Weil R, Sanchez P. 2015. The state of soil degradation in Sub-Saharan Africa: Baselines, trajectories, and solutions. Sustainability. 7:6523–6552.
- Umar BB, Aune JB, Lungu OI. 2013. Effects of Faidherbia albida on the fertility of soil in smallholder conservation agriculture systems in Eastern and Southern Zambia. Afr J Agri Res. 8:173–183.
- Uzoh IM, Igwe CA, Okebalama CB, Babalola OO. 2019. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci Rep. 9:1–9.
- Wang Z, Bao X, Li X, Jin X, Zhao J, Sun J, Christie P, Li L. 2015. Intercropping maintains soil fertility in terms of chemical properties and enzyme activities on a timescale of one decade. Plant Soil. 391:265–282.
- Yengwe J, Amalia O, Lungu OS, De Neve S. 2018. Quantifying nutrient deposition and yield levels of maize (Zea mays) under Faidherbia albida agroforestry system in Zambia. Eur J Agron. 99:148–155.
- Zingore S, Mutegi J, Agesa B, Tamene L, Kihara J. 2015. Soil degradation in sub-Saharan Africa and crop production options for soil rehabilitation. Better Crops. 99:24–26.