ABSTRACT
Onions are produced and consumed in large amounts all over the world. Even though the dry onion bulb is well suited to storage, significant losses occur due to sprouting and diseases during the storage period. The objective of this study was to find methods to support decision-making in storage and prevent some of these losses. Three onion cultivars were tested during two storage seasons, and quality indicators such as firmness, sugar content, dry matter content and mineral content were measured. All but one of the samples were treated with maleic hydrazide for sprouting inhibition. Contents of fructose and glucose were found to be connected to the extent of sprouting, with the highest contents coinciding with the onset of sprouting in spring. Firmness and dry matter losses differed between samples from different growing conditions with firmness losses up to 35.2% and dry matter losses between 4.9% and 11.1% found. Dry matter content was significantly connected to the fraction of sprouted bulbs in a sample. While firmness had a decreasing trend for all but one sample, the firmness measurements carried out with a handheld penetrometer were not consistent enough to be a reliable indicator of sprouting.
Introduction
As stated both by the Sustainable Development Goals of the United Nations, specifically goal 12, target 12.3 (UN General Assembly Citation2015) and the Recommendations for Action in Food Waste Prevention produced by the European Union (EU Platform on Food Losses and Food Waste Citation2019), reducing losses and waste of food is an important step towards sustainability. The bulb onion, Allium cepa L., is often stored for several months after harvest and during storage it suffers losses. These losses are caused by a variety of factors including storage diseases, caused by a number of different fungal and bacterial pathogens (Schwartz and Mohan Citation2007), sprouting and related weight loss due to transpiration and respiration consuming stored carbohydrates (Jaime et al. Citation2001). As stated in the UNECE standard for onions, later stages of sprouting, where visible leaf growth has occurred, render the bulbs unsellable (UNECE Citation2019). The amount of postharvest losses on a national level vary depending on cultivation and storage circumstances, and examples of losses as low as 13% (Franke et al. Citation2013) and as high as 50% (Olanipekun Citation2018) have been reported. With a worldwide production of over 100 million tons each year, onions are one of the most produced and consumed vegetable crops, second only to tomatoes (FAOSTAT Citation2023). Sprouting can be inhibited using chemical control; one common method is application of maleic hydrazide before harvest, which inhibits cell division in the bulb meristem (Greulach and Atchison Citation1950). Exposing the bulbs to ethylene or 1-methylcyclopropene is another method that has been shown to inhibit sprouting (Downes et al. Citation2010). Ionising irradiation is a third method that is used in many places (Sharma et al. Citation2020). The methods available to a grower depend on regional laws and regulations, as not all countries allow the use of maleic hydrazide (Põldma et al. Citation2011) or irradiation (Stefanova et al. Citation2010). Preventing development of storage diseases is more challenging. Ozone application has been found to reduce growth of fungal and bacterial causers of storage diseases (Shelake et al. Citation2022). The effect of air temperature and humidity during storage has been studied by many; it has been found that temperatures of 0–5°C inhibit both sprouting and disease development, while temperatures of 25–30°C inhibit sprouting but may promote disease development (Abdalla and Mann Citation1963; Islam et al. Citation2015; Petropoulos et al. Citation2017) A RH of 55–70% is beneficial as it helps limit disease and root development while also preventing excessive fresh weight loss (Gubb and MacTavish Citation2002). However, no method can guarantee complete long-term prevention of sprouting or disease. Therefore, there is a need for tools that help predict quality decline and support decision making regarding which batches are suitable for long-term storage.
Factors influencing the storage potential of a bulb are affected to varying degrees both by the genetics of the particular cultivar and by cultivation and handling. Approximately 80–90% of the onion bulb’s dry matter content consists of non-structural carbohydrates such as fructans, sucrose, fructose and glucose (Darbyshire and Henry Citation1979). Contents of nonstructural carbohydrates change during the storage season and these changes are connected to the sprouting process (Downes et al. Citation2010). The content of fructans and the rate of fructan hydrolysis has been shown to be connected to onion bulb storability (Jaime et al. Citation2001). Onions containing high amounts of fructans and with low rates of fructan hydrolysis can be stored for longer periods of time before sprouting. The changing ratio between monosaccharides and disaccharides over the course of a storage period has also been pointed out as a potential predictor of sprouting (Chope et al. Citation2012). Additionally, bulbs with high firmness typically also have higher dry matter content, making firmness another indicator of storability (Coolong et al. Citation2008). A third factor influencing onion quality and storability is the content of mineral nutrients in the bulb, which is affected by fertiliser application. It has been shown that reduced doses of nitrogen fertiliser application can result in better storability (Golubkina et al. Citation2022). Increased doses of sulphur fertilisation can improve bulb growth, firmness and sulfur content, but no effect on total soluble solids or storability has been found (Lancaster et al. Citation2001; Forney et al. Citation2010).
Numerous studies have been performed over the years in an attempt to elucidate the effect of cultivar choice, cultivation methods and storage technology on onion quality and losses, and many have delved into the finer details of the bulbs’ carbohydrate metabolism and enzymatic activity, and how this connects to the sprouting process (e.g. Pak et al. Citation1995; Carter et al. Citation1999; Ko et al. Citation2002; Benkeblia et al. Citation2005; Yasin and Bufler Citation2007; Chope et al. Citation2012; Sharma et al. Citation2015; Golubkina et al. Citation2022). In this grower initiated study we aim to look into how some of these well studied aspects of onion physiology could be of use for storage planning in commercial onion production under Nordic conditions. In order to do so, we worked in collaboration with a large scale commercial onion producer and took monthly samples of onions of three cultivars in their storage over the course of two whole storage seasons. The cultivars chosen were ‘Hystore’, ‘Hytech’ and ‘Saskia’, three commonly grown very long day cultivars with similar traits, all well adapted to Nordic conditions and suited to long term storage.
The objective of this study was to compare measurements of onion quality indicators with sprouting, with the goal of identifying methods that could be easily applied in storage facilities. In this study, we used a combination of easily applicable methods and well-developed scientific methods. The quality indicators measured were firmness, sugar composition, dry matter and mineral content. We hypothesise that these indicators are connected to sprouting status and can be used to predict storability of onions.
Materials and methods
Plant material
The onions were produced by commercial growers in the region of Skåne, Sweden, in 2019 and 2020. Harvested onion bulbs were kept in a storage facility operated by Almhaga Grönsaker AB (Höllviken, Sweden). The three cultivars, Hystore (HYS), Hytech (HYT) and Saskia (SAS), were harvested in September and sampled once a month during storage, from October until June. To obtain four replicates, bulbs were collected from squares placed at least 40 m apart in each field. For each sampling occasion, samples consisting of five random average sized (diameter 65–80 mm) onion bulbs without visible defects were taken from each replicate batch. In 2019, each cultivar was grown in two different fields for a total of six sample IDs, and in 2020, HYS and SAS were grown in two fields each while HYT was grown in three fields, for a total of seven sample IDs (). Before harvest, all batches except HYT20k were treated with full dose maleic hydrazide (Fazor, Arysta LifeScience Great Britain Ltd, United Kingdom) at 80% tops down. Bulbs were lifted in August or September () and allowed to dry in field until September 25th in 2019 or until October 5th in 2020. After field drying, the bulbs were dried for an additional four weeks in trays, protected from rain by a roof but otherwise exposed to ambient conditions, before being placed in refrigerated storage. The refrigerated storage was kept between 0 and 1°C with a RH of 80 to 85%. Onions intended for the experiment were kept separate from the bulbs meant for commercial sale, but in identical storage conditions. In 2019, grower reported losses, defined as the fraction of stored bulbs not suitable for retail sale, were 19% (HYS19a), 17% (HYS19b), 7% (HYT19c), 22% (HYT19d), 6.5% (SAS19e) and 7% (SAS19f). In 2020 the losses were 7% (HYS20 g), 9% (HYS20 h), 7% (HYT20i), 100% (HYT20j), 8% (HYT20k), 9% (SAS20 l) and 100% (SAS20 m). The 100% losses recorded for HYT20j and SAS20 m were due to the whole batches being sold to industrial processing at the end of storage as a large percentage of the bulbs had sprouted.
Table 1. A list of the onion cultivars used and the ID each batch was given.
Table 2. The sowing dates for the sampled onions and the pH, content of clay, sand and organic matter in soil samples from the fields where the onions were grown.
Field data
Soil samples were taken from the fields during the respective growing seasons (18 September 2019 and 15 August 2020), fractions of clay, sand and organic matter were measured and ammonium lactate (AL) analyses for mineral contents were performed (LMI, Helsingborg, Sweden). Results are shown in .
Sample preparations and firmness measurements
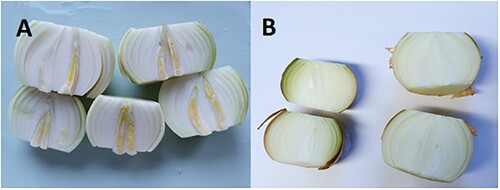
The bulbs were rinsed with tap water, and the top and basal plate and all fully or partially dry scales were removed. Bulb firmness was measured at three points along each bulb’s equator using a handheld penetrometer (6 mm pin, Digital Fruit Hardness Tester 41050, Step Systems GmbH, Germany). For better uniformity in measurements, the penetrometer was laid flat on a table with the measuring pin sticking out and held still while the bulb was pushed onto the pin. After this, the bulbs were cut into quarters and sprouting status was noted. Bulbs where internal sprout elongation had started and the sprouts had visibly changed colour were deemed to be sprouting (). The quartered bulbs were turned into a fine mash using a Waring blender (Model 91-358, USA). For sugar content analysis, onion mash was placed in a 50 mL polypropylene tube (Sarstedt, Germany), weighed and frozen at −80 °C. The samples were then lyophilised at −90°C using a Hetosicc CD 13-3 (Heto InterMed, Denmark) for five days, and weighed again.
Sugar analysis
To analyse the contents of sucrose, glucose and fructose using HPLC, sugars were extracted from the samples by adding 5 mL MilliQ filtered water to 50 mg of finely ground lyophilised onion sample and shaking overnight at 120 rpm and 4°C. Samples were then centrifuged for five minutes at 18000 G and 4 °C, and the supernatant was collected for analysis. For the samples collected in October 2020 and June 2021, a Phenomenex LUNA omega column and a mobile phase consisting of 75% acetonitrile was used. For all other samples, a Sarasep Car-H column was used with a mobile phase consisting of water and 3 mM HCl. Standards containing D-(+)-saccharose (VWR, USA), D-(+)-glucose (Merck, Germany) and D-(−)-fructose (Sigma-Aldrich, USA) at known concentrations were run using the same methods to ensure consistent measurements.
Mineral and dry matter content
After samples for flavonoid and sugar analysis had been collected, samples to be sent for mineral content analysis were prepared from the remaining onion mash. Each replicate was poured into an aluminium tray and weighed before and after being fully dried in an oven at 60 °C for seven days. The dry matter content was recorded. In order to study the mineral content dynamics, the dried samples from the storage season starting in 2019, months October, February and June, were analysed regarding a plant nutrient status panel of 17 minerals (Eurofins Agro Testing Sweden AB, Kristianstad, Sweden).
Statistics
Statistical analysis was carried out using Minitab version 19.2020.1 (Minitab, LLC) and R version 4.1.2. The correlation between mineral and dry matter content was calculated using Pearson pairwise correlation in Minitab. The connections between sprouting, mineral content and sugar content were calculated using the Anova function in R. A general mineral model was applied to the sprouting, sugar and mineral data in Minitab. Tukey pairwise comparisons were made for average sprouting and mineral content, and a main effects plot of the sugar content and sprouting was created using Minitab.
Ethics statement
This study did not involve human or animal subjects and therefore ethical approval was not necessary.
Results
Firmness
Firmness, as measured with the penetrometer, decreased significantly over time (p<0.001). There was a large variability between individual measurements, and no significant correlation with sprouting could be found. The total firmness decline varied somewhat, but it did not significantly differ between most sample IDs ().
Table 3. The average firmness of onion bulbs of the cultivars ‘Hystore’ (HYS), ‘Hytech’ (HYT) and ‘Saskia’ (SAS) harvested in 2019 (19) or 2020 (20) from 13 fields (a-m) in Skåne, Sweden.
Sprouting
The first signs of sprouting were observed in January of both years, with an average of 26% of the bulbs showing signs of sprouting from January to February. By March, 72% of the bulbs showed signs of sprouting, increasing to 92-99% in the period April-June. Slight differences between sample IDs could be seen, but the only significant difference found when IDs were compared using the Tukey method was that the group HYT20k, HYT20j and HYS19a had a higher percentage of sprouted bulbs than HYT19d (). The fact that HYT20k was not treated with maleic hydrazide did not have a significant effect on the sprouting status of the tested bulbs.
Table 4. Sprouting in onion bulbs of the cultivars ‘Hystore’ (HYS), ‘Hytech’ (HYT) and ‘Saskia’ (SAS) harvested in 2019 (19) or 2020 (20) from 13 fields (a-m) in Skåne, Sweden.
Sugars and dry matter content
The HPLC analysis of sugar concentration throughout the storage seasons showed that concentrations of the sugars fructose, glucose and sucrose were higher around mid-storage season, coinciding with the start of the internally visible sprouting process (). This trend was particularly clear with fructose and glucose ((A, B)), which showed a marked increase coinciding with many of the bulbs starting their internal sprout growth. The monosaccharide content tended to peak at the time when 60–80% of the bulbs had sprouted, after which content decreased. The sucrose content did not peak in the same way. As sprouting progressed further (), the fructose levels dropped off to near the levels found at the start of each storage season. Fructose was found to be significantly connected with sprouting status, both as a standalone factor (p<0.05) and when taking sampling month and sample ID into account (p<0.01). A significant connection was found between glucose content ((B)), month, and sprouting (p<0.01). Sucrose content ((C)) was significantly connected with sprouting on its own (p<0.01) and in connection to month (p<0.05), but not when sample ID was taken into account. No differences in average peak area could be found between samples run with the two different HPLC columns and solvents, and data have therefore been treated as part of the same set. Dry matter content varied between sample IDs at the beginning of the season and decreased at similar rates after that point (). Dry matter had a significant correlation of −0.390 with sprouting (p<0.001, 95% confidence interval −0.497; −0.270). On average, dry matter contents were higher in 2020 compared to 2019.
Figure 2. The relation between the percentage of sprouted onion (Allium cepa L.) bulbs and the contents of fructose, glucose and sucrose (expressed as HPLC peak area). Bulbs were harvested in 2019 and 2020, and sugar content was measured once a month from October until June using HPLC.
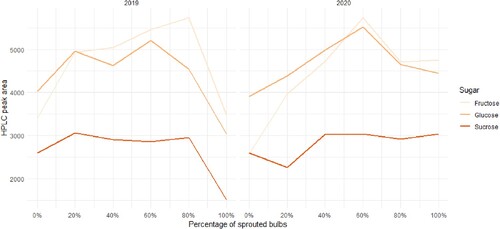
Figure 3. The amounts of fructose (A), glucose (B) and sucrose (C) found when sugars were extracted from homogenised and lyophilised onion bulbs collected from storage facilities from October (1) until June (9). Data shown as HPLC chromatogram peak area per mg extracted dry onion. Error bars represent the average value (n = 4) ± the standard deviation for the sample.
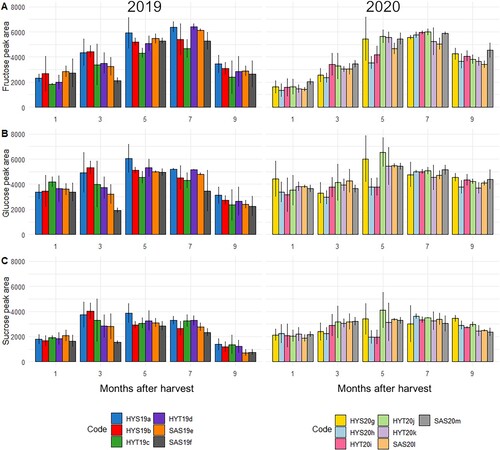
Table 5. The measured dry matter content of onion bulbs of the cultivars ‘Hystore’ (HYS), ‘Hytech’ (HYT) and ‘Saskia’ (SAS) harvested in 2019 (19) or 2020 (20) from 13 fields (a-m) in Skåne, Sweden.
Mineral content
The concentration of certain minerals in the bulb dry weight was found to be significantly connected to the sprouting status. Concentrations of nitrogen (p < 0.001), sulphur (p < 0.01), calcium (p < 0.001) and potassium (p < 0.01) were significantly connected to the extent of sprouting when taking the sampling month into account. Additionally, sulphur content was significantly connected with sprouting when taking both the sampling month and sample ID into account (p < 0.05). Contents of calcium (p < 0.05) and chlorine (p < 0.01) also showed a significant connection with sprouting without consideration of sampling month or sample ID. No significance was found for the other tested minerals: magnesium, copper, zinc, iron, sodium, manganese, boron and selenium. Nitrate and cobalt levels were below the detection threshold (0.3 g kg−1 and 43 μg kg−1, respectively) and molybdenum content was either exactly at or below the detection threshold (0.2 mg kg−1) in all samples. The overall dry weight percentages of the major minerals N, P, K, Ca and Mg were 1.28%, 0.34%, 1.76%, 0.26% and 0.09%, respectively. Appendix 1 lists all measured mineral contents. All minerals except magnesium, sodium and iron showed negative correlations with the dry matter content of the bulbs ().
Table 6. Pairwise Pearson correlations for the dry matter and mineral content in onion bulbs during storage.
Discussion
The purpose of this project was to measure a variety of onion quality indicators and identify those that showed promise as storability indicators, in order to help reduce storage losses. The hypothesis was that the measured quality indicators could be used to predict the storability of onions. We chose to focus on how the measured quality indicators changed over time and in relation to internal sprout development, as sprouting is a common cause of storage losses (Anbukkarasi et al. Citation2013; Sharma et al. Citation2016). We found that contents of sugars, dry matter and certain minerals were connected to the fraction of sprouting bulbs over time, while firmness showed no significant connection, but also tended to decrease over time. It can be assumed that the increased content of minerals per gram of dry matter with time in storage () are merely artefacts of the decreasing dry matter content, and as such are only indirect predictors of sprouting. This is in line with findings by Grevsen and Sorensen (Citation2004), who found that the content of ash per fresh weight increased towards the end of the storage season, as the bulbs lost both carbohydrates and water. The contents of the minerals N, P, K, Ca and Mg found in the samples in this study were similar to values reported in the literature (Furlan and Bernier-Cardou Citation1989). In conclusion, the content of minerals at harvest had no effect on other quality indicators in this study.
As dry matter content seemed to decrease at similar rates for all samples (), it seems onion bulbs with a high initial dry matter content will retain a relatively high content later in the season, when compared to bulbs with a lower initial content. Jaime et al. (Citation2001) found that onions with an initial dry matter content of at least 16% were better suited for storage, as fewer had sprouted after a six-month storage period. In our samples, we had no measured dry matter content above 13%, and most samples started the storage seasons with values below 11% in 2019 and below 12% in 2020. These values seem typical of onions produced in Scandinavia, as other studies have found that dry matter content of 11–13% is common at the start of storage and that this decreases to 9–11% towards the end of storage (Hansen Citation1999; Mogren et al. Citation2007). It has been shown that a relatively early harvest, at 20–50% so called ‘tops down’ rather than 80%, can result in a higher dry weight percentage in the harvested bulb and somewhat slower sprouting (Grevsen and Sorensen Citation2004). However, the earlier harvest also resulted in a lower total weight of marketable bulbs, and the long-term sprouting reduction was not as effective as for bulbs treated with maleic hydrazide (ibid.). We found that SAS20 m, which was sown later than any other ID in 2020, had the lowest dry matter content, but did not differ from the other IDs in terms of sprouting (). We also found a significant connection between the onset of sprouting and the increase in content of the monosaccharides glucose and fructose (). The changing ratio between mono- and disaccharides has previously been reported to be a good indicator of sprouting status by Chope et al. (Citation2012). The subsequent decrease in fructose, glucose and sucrose in our study is also as expected, as mono- and disaccharides are consumed in the sprouting process (Sheikh et al. Citation2022). The peak of mono- and disaccharide content coinciding with the start of sprouting can be attributed to an increased activity of fructan-degrading enzymes, a process that has been pointed out as a possible strong indicator of the start of the sprouting stage (Benkeblia et al. Citation2005). In our study, we did not examine enzyme activity, but it seems the increase in monosaccharides can serve as an easily measurable, if a bit later, indicator of the same process. HPLC analysis of sugar content showed changes over time (). Similar measurements could also be made with simple and relatively cheap methods, e.g. Merck reflectoquant system (Kleman et al. Citation2023). Another process that can be indicative of sprouting initiation is the redistribution of fructans in the bulb (Ohanenye et al. Citation2019). However, we homogenised all non-dry scales of the bulbs and could therefore not detect movement of sugars. Further studies regarding the redistribution of sugars in bulbs and whether this could be useful in storage decision making may be relevant.
The varying rates of decrease in firmness is another interesting quality indicator. We found significant differences in loss of firmness for different samples of the cultivar ‘Hytech’ (). It could be assumed that this was influenced by the cultivation and handling of the onions, and could be predictive of storability. Firmness is connected to dry matter content and, therefore, storability (Coolong et al. Citation2008), and is a relatively easy parameter to measure. Regular penetrometer measurements could easily be applied in storage, and batches of onions with faster rates of decline in firmness could be sold sooner than batches that retain their firmness well. A mounted penetrometer should be used, as we found that the values obtained from the handheld penetrometer were rather variable, even if the overall trend remained clear. The high variability in the measurements obtained from the handheld penetrometer may be part of the reason we did not see any significant connection between sprouting and firmness. However, it has been shown that a handheld penetrometer can give relatively reliable and consistent measurements in the case of sugar beets, but it requires a defined operating procedure and training of the person performing the measurements (English et al. Citation2022). Dry matter content is another quality indicator that is easy, albeit slower, to measure and only requires access to an oven or lyophiliser and scales.
Conclusion
In summary, measurements of dry weight, firmness and sugar content are useful ways of following the quality of a batch of onion bulbs over the course of a storage season. The content of monosaccharides is a good indication of sprouting status and could aid in deciding which stored batches to sell and thus help reduce losses in storage.
CRediT authorship contribution statement
Isabella Kleman: PhD student, performed majority of experiments, data curation, prepared the manuscript. Anna Karin Rosberg: Supervision, edited and reviewed the manuscript. Lars Mogren: Performed early experiments, method supervision, edited and reviewed the manuscript. All authors have reviewed the final version of the manuscript and agree to the submission and to be accountable for all aspects of the work.
Acknowledgements
The authors wish to thank Oskar Hansson and Almhaga Grönsaker for providing us with the onions and Karl-Erik Gustavsson for the help with running the HPLC sugar samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, IK, upon reasonable request.
Additional information
Funding
Notes on contributors
Isabella Kleman
Isabella Kleman is a PhD student at the Department of Biosystems and Technology, at the Swedish University of Agricultural Sciences in Alnarp, Sweden. Her thesis focuses on the prediction and detection of quality problems during onion storage.
Anna Karin Rosberg
Anna Karin Rosberg is a Senior Lecturer in horticulture with emphasis on systems biology in horticultural value networks at the Department of Biosystems and Technology at the Swedish University of Agricultural Sciences, Alnarp, Sweden.
Lars Mogren
Lars Mogren is an Associate Professor in Horticulture with emphasis on produce quality in vegetable production at the Department of Biosystems and Technology at the Swedish University of Agricultural Sciences, Alnarp, Sweden.
References
- Abdalla AA, Mann LK. 1963. Bulb development in the onion (Allium cepa L.) and the effect of storage temperature on bulb rest. Hilgardia. 35(5):85–112. doi:10.3733/hilg.v35n05p085.
- Anbukkarasi V, Paramaguru P, Pugalendhi L, Ragupathi N, Jeyakumar P. 2013. Studies on pre and post-harvest treatments for extending shelf life in onion – a review. Agricultural Reviews. 34(4):256. doi:10.5958/j.0976-0741.34.4.011.
- Benkeblia N, Ueno K, Onodera S, Shiomi N. 2005. Variation of fructooligosaccharides and their metabolizing enzymes in onion bulb (Allium cepa L. cv. Tenshin) during long-term storage. Journal of Food Science. 70(3):S208–S214. doi:10.1111/j.1365-2621.2005.tb07159.x.
- Carter CE, Partis MD, Thomas B. 1999. The expression of histone 2A in onion (Allium cepa) during the onset of dormancy, storage and emergence from dormancy. New Phytologist. 143(3):461–470. doi:10.1046/j.1469-8137.1999.00484.x.
- Chope GA, Cools K, Hammond JP, Thompson AJ, Terry LA. 2012. Physiological, biochemical and transcriptional analysis of onion bulbs during storage. Annals of Botany. 109(4):819–831. doi:10.1093/aob/mcr318.
- Coolong TW, Randle WM, Wicker L. 2008. Structural and chemical differences in the cell wall regions in relation to scale firmness of three onion (Allium cepa L.) selections at harvest and during storage. Journal of the Science of Food and Agriculture. 88(7):1277–1286. doi:10.1002/jsfa.3219.
- Darbyshire B, Henry RJ. 1979. The association of fructans with high percentage dry weight in onion cultivars suitable for dehydrating. Journal of the Science of Food and Agriculture. 30(11):1035–1038. doi:10.1002/jsfa.2740301103.
- Downes K, Chope GA, Terry LA. 2010. Postharvest application of ethylene and 1-methylcyclopropene either before or after curing affects onion (Allium cepa L.) bulb quality during long term cold storage. Postharvest Biology and Technology. 55(1):36–44. doi:10.1016/j.postharvbio.2009.08.003.
- English W, Ekelöf J, Vancutsem F, Leijdekkers M, Kleuker G, Hoffmann CM. 2022. Method for in-field texture analysis of sugar beet roots using a handheld penetrometer. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science. 72(1):623–634. doi:10.1080/09064710.2022.2042589.
- EU Platform on Food Losses and Food Waste. 2019. Recommendations for action in food waste prevention. EU Platform on Food Losses and Food Waste Brussels.
- FAOSTAT. 2023. FAOSTAT. FAOSTAT Statistical Database. http://www.fao.org/faostat/en/#data/QCL.
- Forney CF, Jordan MA, Campbell-Palmer L, Fillmore S, McRae K, Best K. 2010. Sulfur fertilization affects onion quality and flavor chemistry during storage. Acta Horticulturae. 877:163–168. doi:10.17660/ActaHortic.2010.877.14.
- Franke U, Ministerråd N, Råd N. 2013. Kartläggning av matsvinnet i primärproduktionen. Nordisk Ministerråd : Nordisk Råd : [Eksp.] www.norden.org/order.
- Furlan V, Bernier-Cardou M. 1989. Effects of N, P, and K on formation of vesicular-arbuscular mycorrhizae, growth and mineral content of onion. Plant and Soil. 113(2):167–174. doi:10.1007/BF02280177.
- Golubkina N, Amalfitano C, Sekara A, Tallarita A, Pokluda R, Stoleru V, Cuciniello A, Agafonov AF, Kalisz A, Hamburdă SB, Caruso G. 2022. Yield and bulb quality of storage onion cultivars as affected by farming system and nitrogen dose. Scientia Horticulturae. 293:110751. doi:10.1016/j.scienta.2021.110751.
- Greulach VA, Atchison E. 1950. Inhibition of growth and cell division in onion roots by maleic hydrazide. Bulletin of the Torrey Botanical Club. 77(4):262–267. doi:10.2307/2481897.
- Grevsen K, Sorensen J. 2004. Sprouting and yield in bulb onions (Allium cepa L.) as influenced by cultivar, plant establishment methods, maturity at harvest and storage conditions. Journal of Horticultural Science and Biotechnology. 79:877–884. doi:10.1080/14620316.2004.11511860.
- Gubb IR, MacTavish HS. 2002. 10 onion pre-and postharvest considerations. Allium Crop Science: Recent Advances. 233.
- Hansen SL. 1999. Content and composition of dry matter in onion (Allium cepa L.) as influenced by developmental stage at time of harvest and long-term storage. Acta Agriculturae Scandinavica, Section B-Plant Soil Science. 49(2):103–109.
- Islam MN, Wang A, Skov Pedersen J, Edelenbos M. 2015. Microclimate tools to monitor quality changes in stored onions. In: V International symposium on applications of modelling as an innovative technology in the horticultural supply chain-model-IT 1154. p. 229–234.
- Jaime L, Martín-Cabrejas MA, Mollá E, López-Andréu FJ, Esteban RM. 2001. Effect of storage on fructan and fructooligosaccharide of onion (Allium cepa L.). Journal of Agricultural and Food Chemistry. 49(2):982–988. doi:10.1021/jf000921t.
- Kleman I, Hellström M, Rosberg AK, Becher PG, Mogren L. 2023. Simple quality prediction measurements for stored onions. Acta Horticulturae. 1364:183–186. doi:10.17660/ActaHortic.2023.1364.24.
- Ko S-S, Chang W-N, Wang J-F, Cherng S-J, Shanmugasundaram S. 2002. Storage variability among short-day onion cultivars under high temperature and high relative humidity, and its relationship with disease incidence and bulb characteristics. Journal of the American Society for Horticultural Science Jashs. 127(5):848–854. doi:10.21273/JASHS.127.5.848.
- Lancaster JE, Farrant J, Shaw ML. 2001. Sulfur nutrition affects cellular sulfur, dry weight distribution, and bulb quality in onion. Journal of the American Society for Horticultural Science Jashs. 126(2):164–168. doi:10.21273/JASHS.126.2.164.
- Mogren LM, Olsson ME, Gertsson UE. 2007. Quercetin content in stored onions (Allium cepa L.): effects of storage conditions, cultivar, lifting time and nitrogen fertiliser level. Journal of the Science of Food and Agriculture. 87(8):1595–1602.
- Ohanenye IC, Alamar MC, Thompson AJ, Terry LA. 2019. Fructans redistribution prior to sprouting in stored onion bulbs is a potential marker for dormancy break. Postharvest Biology and Technology. 149:221–234. doi:10.1016/j.postharvbio.2018.12.002.
- Olanipekun CI. 2018, June 15. Increasing production and reducing postharvest losses of onion in Nigeria. World Vegetable Center. https://avrdc.org/increasing-production-and-reducing-postharvest-losses-of-onion-in-nigeria/.
- Pak C, van der Plas LHW, de Boer AD. 1995. Importance of dormancy and sink strength in sprouting of onions (Allium cepa) during storage. Physiologia Plantarum. 94(2):277–283. doi:10.1111/j.1399-3054.1995.tb05312.x.
- Petropoulos SA, Ntatsi G, Ferreira ICFR. 2017. Long-term storage of onion and the factors that affect its quality: a critical review. Food Reviews International. 33(1):62–83. doi:10.1080/87559129.2015.1137312.
- Põldma P, Moor U, Merivee A, Tõnutare T. 2011. Effect of controlled atmosphere storage on storage life of onion and garlic cultivars. In: IV International conference postharvest unlimited 2011 945. p. 63–69.
- Schwartz HF, Mohan SK. 2007. Compendium of onion and garlic diseases and pests, Second Edition. City: The American Phytopathological Society.
- Sharma K, Asnin L, Ko EY, Lee ET, Park SW. 2015. Phytochemical composition of onion during long-term storage. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science. 65(2):150–160. doi:10.1080/09064710.2014.983151.
- Sharma K, Rok Lee Y, Park SW, Nile SH. 2016. Importance of growth hormones and temperature for physiological regulation of dormancy and sprouting in onions. Food Reviews International. 32(3):233–255. doi:10.1080/87559129.2015.1058820.
- Sharma P, Sharma SR, Dhall RK, Mittal TC. 2020. Effect of γ-radiation on post-harvest storage life and quality of onion bulb under ambient condition. Journal of Food Science and Technology. 57(7):2534–2544. doi:10.1007/s13197-020-04290-z.
- Sheikh FR, Jose-Santhi J, Kalia D, Singh K, Singh RK. 2022. Sugars as the regulators of dormancy and sprouting in geophytes. Industrial Crops and Products. 189:115817. doi:10.1016/j.indcrop.2022.115817.
- Shelake PS, Mohapatra D, Tripathi MK, Giri SK, Kate A, Kumar M. 2022. Inactivation of Aspergillus niger and Erwinia carotovora in onion (Allium cepa L.) bulbs subjected to pulsed ozone treatment. Postharvest Biology and Technology. 192:111998. doi:10.1016/j.postharvbio.2022.111998.
- Stefanova R, Vasilev NV, Spassov SL. 2010. Irradiation of food, current legislation framework, and detection of irradiated foods. Food Analytical Methods. 3:225–252.
- UN General Assembly. 2015. Transforming our world: The 2030 Agenda for Sustainable Development (A/RES/70/1). https://www.refworld.org/docid/57b6e3e44.html.
- UNECE. 2019. UNECE Standard FFV-25 concerning the marketing and commercial quality control of onions. UNITED NATIONS New York and Geneva. https://unece.org/fileadmin/DAM/trade/agr/standard/fresh/FFV-Std/English/25_Onions.pdf.
- Yasin HJ, Bufler G. 2007. Dormancy and sprouting in onion (Allium cepa L.) bulbs. I. changes in carbohydrate metabolism. The Journal of Horticultural Science and Biotechnology. 82(1):89–96. doi:10.1080/14620316.2007.11512203.
Appendix
The contents of 14 minerals in onion bulbs of the cultivars ‘Hystore’ (HYS), ‘Hytech’ (HYT) and ‘Saskia’ (SAS) harvested in 2019 from six fields (a-f) in Skåne, Sweden. Four replicates consisting of five random bulbs of each ID were sampled from storage in October, February and June, and the resulting 12 values were pooled to calculate the average contents of each mineral. Mineral contents are given per kg dry matter. 1 n = 7, as five of the samples had iron content below the detection threshold of 22 mg kg−1. Capital letters indicate grouping, generated using Tukey pairwise comparisons and 95% confidence.