Abstract
Field experiments were conducted on sunflower (Helianthus annuus L.) with high- and low-osmotic-adjustment genotypes to ascertain the role of osmotic adjustment (OA) in sustaining achene yield (AY) and its stability under water-deficit conditions. The results indicated that high-osmotic-adjustment (HOA) genotypes were superior in terms of achene yield, root length, dry root weight, root-to-shoot ratio, harvest index (HI), and number of achenes per head under drought stress. The superiority of HOA for AY genotypes was due to efficient translocation of photosynthates to the roots, as indicated by the significant relationship of all root traits with OA and better mobilization of reserves to the developing head by the OA as suggested by a very strong relationship of OA with HI. Among different yield components, OA showed the highest relationship with the number of achenes per head, suggesting that reserves mobilized by OA were utilized to produce a large number of achenes per head. Genotype AMES-10103 belonging to the HOA group showed its superiority over other genotypes under drought stress. Among all traits, HI has shown its promise for selection of high yielding genotypes under drought stress.
Introduction
High yield of crop plants under drought condition is strongly dependent on the efficient partitioning of dry matter. An optimal partitioning of dry matter between root and shoot, and the further separation of aboveground dry matter between the vegetative and reproductive organs, therefore is of key importance for crop yield under drought conditions (Kage et al., Citation2004).
Osmotic adjustment has been identified as a key physiological process affecting dry matter partitioning under drought stress. It has been shown that osmotic adjustment regulates dry matter partitioning by allocation of a larger part of solutes and photosynthates to the roots (Meyer & Boyer, Citation1981; Ogawa et al., Citation2005). As a result of solute and photosynthates accumulation in roots, osmotic potential is lowered, helping the extraction of more water and minerals from the soil, thus maintaining root growth and function under drought. This enhanced root growth eventually reduces the deleterious effects of drought on plants (Kage & Ehlers, Citation1996). Osmotic adjustment has also been reported to facilitate translocation of photosythates to developing grains (Subbarao et al., 1995).
Overall, a positive effect of osmotic adjustment has been shown on grain yield by different researchers working with different species (Angadi & Entz, Citation2002; Chimenti et al., Citation2002; Ludlow et al., Citation1990; Merah, Citation2001). Similarly, there are reports on the superiority, in terms of grain yield and plant growth, of high-osmotic-adjustment (HOA) genotypes over low-osmotic-adjustment (LOA) ones in (Ludlow et al., Citation1990; Chimenti et al., Citation2002; Moinuddin & Chopra, Citation2004). However, there are conflicting reports indicating no differences for grain yield and dry-matter partitioning process between HOA and LOA groups (Thomas & Evans, Citation1989; Tangpremsri et al., Citation1995; Kamoshita et al., Citation2004). Therefore, this aspect requires further investigation and inclusion of data related to biomass partitioning under field conditions by inducing drought stress (Kamoshita et al., Citation2004).
Against this background, the present work was carried out to study the effect of osmotic adjustment on root length and partitioning of dry matter under contrasting water regimes, using field experiments in sunflower genotypes differing in osmotic adjustment. Data obtained would help to develop a better understanding of the osmoregulatory effect on dry-matter accumulation and genotypic responses to the drought.
Materials and methods
Field experiments were conducted during February 2005–2006 at the sunflower experiment field of the Department of Plant Breeding & Genetics, University of Agriculture, Faisalabad, under irrigated and drought conditions. The soil was a sandy-loam soil with low water retention capacity. The laboratory-measured field capacity (FC) −25 kPa and wilting point (NP) −1800 kPa of the soil averaged 35% and 18% by volume, respectively, pH 7.5, organic matter 0.91%, available phosphorus 28.6 ppm, and potassium 140 ppm. The plots were fertilized with 150 kg N ha−1 and 50 kg P ha−1; no K was applied. A split-plot restriction on randomization within a randomized block design (r=3) was used, where levels of water availability were assigned to main plots and genotypes to subplots. Each subplot was 4.8 m wide and 6 m long, consisting of eight rows of a single genotype. The inter-row spacing was 60 cm and interplant spacing was 30 cm.
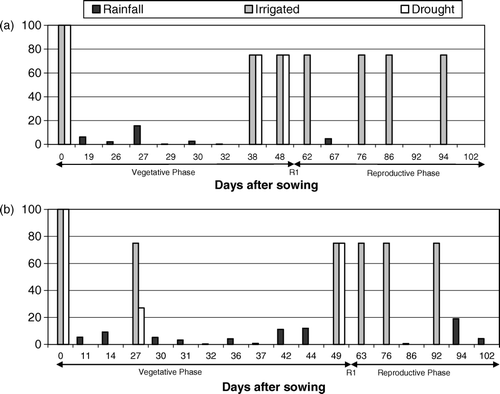
The non-stress regime, henceforth called irrigated, was irrigated during the entire growth cycle to maintain the soil water content close to field capacity. In the stress regime, henceforth called drought, the plots were irrigated at the same time and with the same amount of supplemental water as in the non-stress regime during the vegetative phase; supplemental irrigation was completely withheld just before beginning with the button stage (R1) to achieve low soil moisture content during anthesis. The soil moisture content was measured every 8–10 days up to the range of 0–1.5 m deep (). The total rainfall and supplemental irrigation during crop growth cycle for both years are given in . Rainfall for both years, i.e. 2005 and 2006, was only 31.8 and 75.6 mm, respectively, of which 27 and 51.6 mm, respectively, fell during the vegetative phase while 4.8 and 20 mm fell during the reproductive phase (). Leaf diseases were not present, and weeds were controlled manually.
Figure 1. Volumetric water content for sunflower genotypes irrigated for either the entire growing season (Irrigated) or only during vegetative development (Drought) plus average air temperature during the season (a) 2005 and (b) 2006. As a reference, field capacity (FC 35% v/v) and permanent wilting point (WP 18% v/v) are given as horizontal lines.
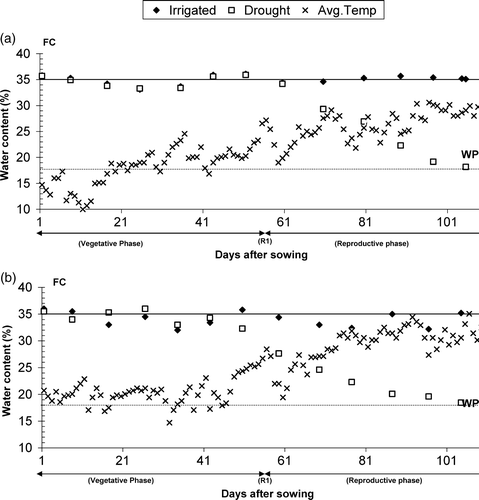
Figure 2. Natural rainfall and water applied through supplemental irrigation for sunflower genotypes irrigated for either the entire growing season (Irrigated) or only during vegetative development (Drought) during year (a) 2005 and (b) 2006.
Selection of genotypes
Thirty-six genotypes collected from North Dakota Research Station, USA and Oil Seeds Research, Institute, Faisalabad-Pakistan were evaluated for their osmotic adjustment by the method narrated by Moinuddin and Chopra, Citation2004. OA was based on the difference between osmotic potential at full turgor (100ψs) of leaves under non stress (W1) and stressed regimes (W2),ΔOA = 100ψs w 1−100ψs w 2. The osmotic potential at full turgor (ψs100) was calculated according to the equation given by Moinuddin and Chopra, Citation2004: ψs100=(corrected ψs×relative water contents)/100, where corrected ψs was ψs+0.1 ψs, for the dilution of symplastic sap by apoplastic water, assuming 10% apoplastic water (Kramer, Citation1983).
From this experiment six HOA and LOA genotypes were selected. Genotypes were classified according to their mean OA values in two classes (), high OA (≥0.4 MPa) and low OA (≤0.1 MPa).
Table I. Genotypes, their origin and osmotic adjustment (OA) values.
Root length and root/shoot ratio measurements
The root length of sunflower cultivars was studied as described by Bohm, Citation1979. For the purpose of measurement of total root length, trenches of 50 cm diameter, were dug and roots were traced up to a depth of 2.5 m using a backhoe. Plants were dug out along with a huge mass of soil. Soil masses were washed with tap water under constant pressure (Entz et al., Citation1992). Care was taken to wash all root systems in the same way (i.e., using the same spatial washing pattern and total washing time). The root system was placed on a clear plastic sheet and root length was determined as described by Bohm, Citation1979. Root-to-shoot ratio was calculated on a dry-weight basis.
Biomass measurements
Total fresh biomass was measured by collecting adjacent plants per plots (1.05 m2). The samples were partitioned into root, stover (stem + leaves + receptacle) and achenes and measured separately, afterward samples i.e. roots and stovers were dried in oven at 70 °C to constant weight. Masses are presented on an oven-dry basis. The harvest index (HI) was calculated as the ratio of achene yield per plot to the total aboveground dry matter per plot. Leaf area was measured in each sample using a planimeter (LI-COR-3100 Nebraska, USA).
Measurement of yield and yield related parameters
At maturity, observations were recorded for achene yield per head, head diameter, 100-achene weight, and number of achenes per head. Heads were harvested separately from each tagged plant per genotype per replication from the field. Head diameter was measured by using a measuring scale. The achenes were harvested manually from dried receptacles and weighed. 100-achene weight was measured by weighing 100 achenes taken randomly from each sample. Number of achenes per head was calculated as follows:
Statistical analysis
The data were analysed in factorial arrangement, assuming years and water regime to have a fixed effect and genotypes to have random effect. The genotypes×years (G×Y) interaction was either nonsignificant (P>0.05) or had a much smaller influence than the main effects. Therefore data were pooled over the two years for each water regime. However, owing to the presence of substantial interaction between genotypes×water regimes (G×W) genotypic mean performance for all traits was shown under both regimes (Figures ). Bars indicated in Figures show standard error of their mean performance. All data were analysed using computer-based program MSTAT-C (MSTAT-C Development Team, Citation1989).
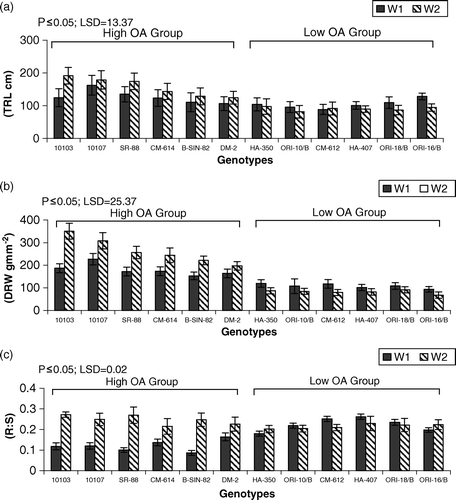
Figure 3. Mean performance of genotypes for (a) total root length (TRL cm) (b) dry root weight (DRW gm m−2) (c) root:shoot ratio (R:S) under irrigated (W1) and drought (W2) regimes. LSD = least significant difference.
Results
Analysis of variance detected significant variation for all traits with respect to year, genotypes, and field water regime. The (G×W) was significant (P<0.01) and (G×Y) was nonsignificant (P>0.05) for all traits. This suggests that the relative ranking of various genotypes for all traits was not consistent across water regimes.
Final root length, root weight, and root-to-shoot ratio of HOA and LOA groups are presented in . The general trend shows that the HOA group produced higher values of root length, root biomass, and root-to-shoot ratio under drought stress in comparison with the LOA group. The LOA group generally showed higher root to shoot ratio in comparison with the HOA group under the nonstress regime. Genotype AMES-10103, belonging to the HOA group, produced the longest root and highest root biomass and root:shoot ratio in drought, while AMES-10107 produced the highest root length and root biomass and HA-407 showed the highest root:shoot ratio under the nonstress regime.
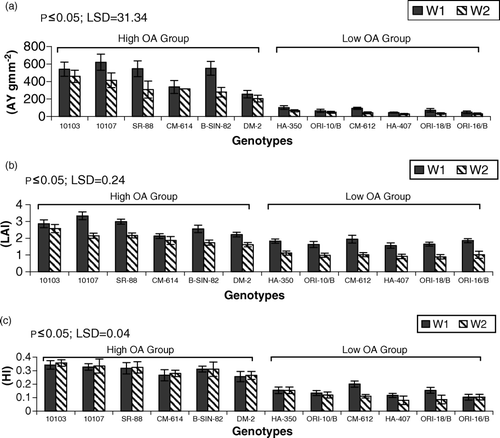
Achene yield (AY) and leaf area index (LAI) were higher in HOA genotypes than in LOA genotypes. All genotypes showed lower AY and LAI under drought stress as compared with nonstress conditions (). Genotype AMES-10103 showed the highest LAI and AY under drought stress while genotype AMES-10107 showed the highest LAI and AY under the nonstress regime (). As far as harvest index (HI) was concerned, the HOA group again showed its superiority over the LOA group. All genotypes belonging to HOA group showed a higher HI under drought stress while a repressing effect of drought was evident on HI in LOA genotypes except for HA-350 and ORI-16/B in which differences were nonsignificant (). Genotype AMES-10103 showed the highest HI under both regimes.
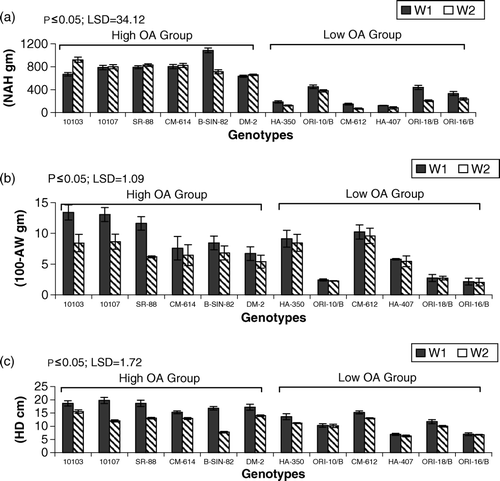
Achene yield components (AYC) are presented in . Both groups showed lower head diameter (HD) and 100-achene weight (100-AW) under drought stress when compared with the nonstress regime. Genotype AMES-10107 showed the highest HD under the nonstress regime while AMES-10103 showed its superiority under drought. Genotype AMES-10103 showed the highest 100-SW under nonstress conditions while genotype CM-612, belonging to the LOA group, showed the highest 100-AW under drought stress ().
HOA group genotypes showed a higher number of achenes per head (NAH) under drought stress when compared with the nonstress regime, except for genotype B-SIN-82 which showed lower NAH under drought conditions. On the other hand, all genotypes belonging to the LOA group showed lower NAH under drought stress. B-SIN-82 showed the highest NAH under the nonstress regime while AMES-10103 showed the highest NAH under drought.
Relationships between osmotic adjustment and different below- and aboveground traits were established and only significant (P ≤ 0.05) relationships are shown in Figures . Among different root traits, OA showed a highly significant positive relationship with root length, root weight, and root-to-shoot ratio (). It showed the highest relationship with root weight. Similarly, OA showed a highly significant positive relationship with aboveground traits such as AY and HI. Among these two traits HI showed the highest relationship with OA ().
Figure 6. The response of (a) root length (cm) (b) root weight (gm m−2) (c) root: shoot ratio to the variation in osmotic adjustment across all genotypes under drought regime.
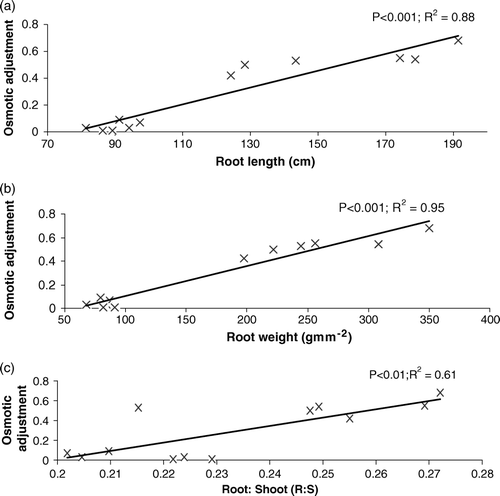
Figure 7. The response of (a) achene yield (b) HI (c) number of achenes per head to the variation in osmotic adjustment across all genotypes under drought regime.
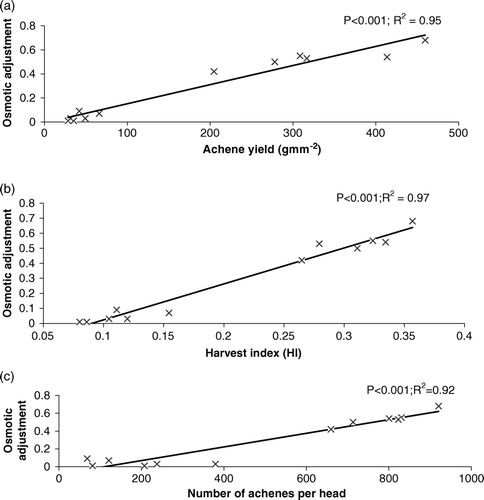
Figure 8. The response of harvest index (HI) to the variation in achene yield (gm m−2) across all genotypes.
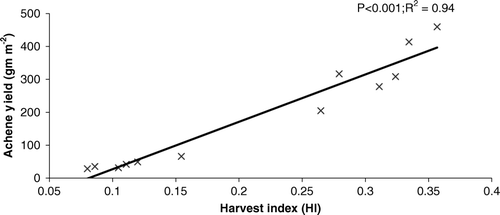
Relationships were also established between OA and achene yield component. Among different yield components, NAH showed a highly significant relationship with OA (). The relationship between HI and AY was also strong and positive ().
Discussion
All root traits showed a significant relationship with OA. However, dry root weight {DRW} showed the highest positive relationship with OA (r 2=0.95) followed by root length (r 2=0.88). Increased root biomass may be attributed to the OA. It may be due to a higher allocation of photosynthates such as sucrose and glucose to the roots (Serraj & Sinclair, Citation2002; Ogawa et al., Citation2005) by HOA genotypes which reduces osmotic potential, helping in extraction of more water and minerals from soil, thus maintaining root growth and function under drought stress.
OA also showed a highly significant relationship with root-to-shoot ratio (r 2=0.61) and a very strong relationship with HI (r 2=0.97), showing that HOA genotypes were more efficient in translocation of photosynthates to the roots and mobilization of their reserves to the developing head. Meyer and Boyer (Citation1981) also found that osmotic adjustment stopped dry matter from accumulating in the hypocotyls, which it bypassed to accumulate in the roots, which grew faster than the control roots and eventually resulted in the increased root-to-shoot ratio. The solutes involved were mostly free amino acids, glucose, fructose, and sucrose, and these accounted for most of the increased dry weight. Efficient translocation of pre-anthesis carbohydrate reserves to the developing grains by HOA genotypes has also been reported before in different species, i.e. sorghum (Ludlow et al., Citation1990), grain legumes (Subbarao et al., Citation2000) and chick pea (Moinuddin & Chopra, Citation2004).
The relationship between AY and OA was strong and positive (r 2=0.95). This positive impact of OA on AY may also be due to a better mobilization of reserves by the OA. Among different traits, OA showed the highest relationship with HI and, furthermore, HI showed the highest relationship with achene yield (r 2=0.95). Among different yield components, OA showed the highest relationship with NAH (r 2=0.92), suggesting that reserves mobilized by OA were utilized to produce a large number of achenes per head. However, in spite of increases in the number of achenes per head, HOA genotypes failed to produce heavier achenes and larger head diameter and experienced a greater decrease in 100-achene weight and head diameter than did LOA genotypes. On the other hand, LOA genotypes, where the number of achenes significantly decreased due to lower osmotic adjustment, maintained their 100-seed weight and head diameter. This may be due to differential response of yield components to OA (Chimenti et al., Citation2006). Similarly, Moinuddin and Chopra, (Citation2004) also showed an increase in the number of seeds in HOA chick pea genotypes at the expense of 500-seed weight under drought.
HOA adjustment genotypes showed higher values for AY, RL, DRW, R:S, HI, and NAH in contrast to LOA genotypes. Genotype AMES-10103 belonging to the HOA group showed its superiority over other genotypes for these traits and thus finally achene yield under drought stress. Among all traits, HI has shown the highest relationship with OA and achene yield. Therefore this trait may be utilized for selection of high-yielding genotypes under drought stress.
Acknowledgements
This study was a part of PhD thesis (Physio-genetics of drought tolerance in sunflower Helianthus annuus L.) and all necessary funds were provided by the higher education commission of Pakistan to PIN: 042-160183-LS2-181.
References
- Angadi , S.V. and Entz , M.H. 2002 . Water relations of standard height and dwarf sunflower cultivars . Crop Science , 42 : 152 – 159 .
- Bohm , W. 1979 . Methods for Studying Root Systems , New York : Springer-Verlag .
- Chimenti , C.A. , Pearson , J. and Hall , A.J. 2002 . Osmotic adjustment and yield maintenance under drought in sunflower . Field Crop Research , 75 : 235 – 246 .
- Chimenti , C.A. , Marcantonio , M. and Hall , A.J. 2006 . Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases . Field Crop Research , 95 : 305 – 315 .
- Entz , M.H. , Gross , K.G. and Fowler , D.B. 1992 . Root growth and soil-water extraction by winter and spring wheat . Canadian Journal of Plant Science , 72 : 1109 – 1120 .
- Kage , H. and Ehlers , W. 1996 . Does transport of water to roots limit water uptake of field crops? . Zeitschrift für Pflanzenernährung und Bodenkunde , 159 : 583 – 590 .
- Kage , H. , Kochler , M. and Stützel , H. 2004 . Root growth and dry matter partitioning of cauliflower under drought stress conditions: measurement and simulation . European Journal of Agronomy , 20 : 379 – 394 .
- Kamoshita , A. , Rodriguez , R. , Yamauchi , A. and Wade , L.J. 2004 . Genotypic variation in response of rainfed lowland rice to prolonged drought and rewatering . Plant Production Science , 7 : 406 – 420 .
- Kramer , P.J. 1983 . Cell water relations . In : Water Relations of Plants (pp. 25 – 26 ). Academic Press , Oxford, , England .
- Ludlow , M.M. , Santamaria , J.M and Fukai , S. 1990 . Contribution of osmotic adjustment to grain yield in Sorghum bicolor (L.) Moench under water-limited conditions. II. Water stress after anthesis . Australian Journal of Agricultural Research , 41 : 67 – 78 .
- Merah , O. 2001 . Potential importance of water status traits for durum wheat improvement under Mediterranean conditions . The Journal of Agricultural Science , 137 : 139 – 145 .
- Meyer , R.F. and &Boyer , J.S. 1981 . Osmoregulation, solute distribution, and growth in soybean seedlings having low water potentials . Planta , 151 : 482 – 489 .
- Moinuddin and Khanna-Chopra , R. 2004 . Osmotic adjustment in chickpea in relation to seed yield and yield parameters . Crop Scence , 44 : 449 – 455 .
- MSTAT-C Development Team 1989 . MSTAT User's Guide: A Microcomputer Program for the Design, Management and Analysis of Agronomic Research Experiments . 1st edition . Michigan State University , East Lansing, ML .
- Ogawa , A. , Kawashima , C. and Yamauchi , A. 2005 . Sugar accumulation along the seminal root axis, as affected by osmotic stress in maize: A possible physiological basis for plastic lateral root development . Plant Production Science , 8 : 173 – 180 .
- Serraj , R. and Sinclair , T.R. 2002 . Osmolyte accumulation: can it really help increase crop yield under drought conditions? . Plant, Cell and Environment , 25 : 333 – 341 .
- Subbarao , G.V. , Ghauhan , Y.S. and Johansen , C. 2000 . Patterns of osmotic adjustment in pigeonpea – its importance as a mechanism of drought resistance . European Journal of Agronomy , 12 : 239 – 249 .
- Tangpremsri , T. , Fukai , S. and Fischer , K.S. 1995 . Growth and yield of sorghum lines extracted from a population for differences in osmotic adjustment . Australian Journal of Agricultural Research , 46 : 61 – 74 .
- Thomas , H. and Evan , C. 1989 . Effects of divergent selection for osmotic adjustment on water relations and growth of plants of Lolium perenne . Annals of Botany , 64 : 581 – 587 .