Abstract
A technique developed at the Department of Plant Biotechnology EVIKA of the Estonian Research Institute of Agriculture to multiply disease-free potato seed tubers involves growing plantlets in vitro in plastic rolls on peat before transplanting them to the field. The effects of the method of multiplication, variety, and experimental year on tuber yield were investigated. Plants cultured in vitro from micro-plants were compared with plants grown from tip- and stem-cuttings, and truncated plants that had served as a source of the mentioned cuttings. Two late-maturing potato varieties, Ants and Vigri, were used. The multiplication method, variety, and environmental factors significantly affected the number of tubers per plant and average weight of a tuber. Whereas plants cultured in vitro consistently produced substantially greater numbers of tubers and they were the right size to serve as seed tubers, plants from tip- and stem-cuttings produced fewer tubers and the size of full-grown tubers was too large for seed-production. Truncated plants fell between the two. For seed-potato growing perspectives, the plants from in vitro and truncated plants are the most applicable. Growing tip-and stem-cuttings, the shorter growth period to achieve suitable-sized seed tubers should be adapted. Differences between multiplication methods were smaller in the case of Vigri, which tends to produce more shoots. It is concluded that multiplication methods that result in more shoots per plant also lead to more and smaller tubers. Environmental conditions affected both the number and weight: the dry year, when conditions for growth were adverse, reduced the effect of other factors on the number of tubers whereas near-optimal growth conditions intensified the effect of those factors on tuber weight.
Introduction
To derive maximum value from disease-free plants, it is necessary to multiply them rapidly. Several methods are available to multiply meristem cultures of potato, of which the following three are the most common: micro tubers, mini tubers, and tubers produced in vivo.
Micro tubers (0.02–0.7 g, 3–10 mm in diameter) are produced entirely in vitro by changing the nutrient medium and/or external conditions. The number of micro tubers is usually limited to only one per micro plant or explant (Hussey & Stacey, Citation1984; Estrada & Dodds, Citation1986; Garner & Blake, Citation1989; Struik & Lommen, Citation1990; Nowak & Asiedu, Citation1992).
Mini tubers (0.1–10 g, 5–25 mm in diameter) are produced from explants cultured in vitro under semi in vivo conditions. Mini tubers can be produced all the year round in glasshouses at high densities using a culture medium. The number of mini tubers produced varies from two to more than 10 per plant, depending on the production method (Struik & Lommen, Citation1990; Ranalli et al., Citation1994).
Tubers produced in vivo are multiplied under field conditions, with emphasis on clonal selection; such production is not considered a rapid multiplication technique.
Earlier, production of seed tubers in Estonia was based either on multiplication in vitro or on growing plants in a greenhouse in pots, which ensured two harvests a year (Rosenberg, Citation1981). The aim of continuing research has been to create a simple, effective, widely applicable, and more energy-efficient but less labour-intensive method, which would also be environment-friendly. In the current technology, created in the Department of Plant Biotechnology EVIKA of the Estonian Research Institute of Agriculture, first-generation seed tubers are produced in the field by transplanting plants raised on peat in plastic rolls from plants cultured in vitro or by repetitive multiplication using tip- and stem-cuttings, and truncated plants (plants that have served as sources of the cuttings). For seed-production purposes, the tubers usually have to be harvested before full maturity to avoid excessive size of the tubers. Repetitive multiplication in plastic rolls followed by the open field production of the first generation of tubers has proved cheaper and simpler than production in vitro or in greenhouses (Rosenberg, Citation1981; Rosenberg & Kotkas, Citation1986; Kotkas, Citation1987; Rosenberg et al., Citation1988; Kotkas &, Särekanno, Citation1996; Kotkas & Rosenberg, Citation1999).
In the past, we had investigated the factors that influence the efficiency of multiplication in vitro (Rosenberg &, Kotkas, Citation1984; Rosenberg et al., Citation1997 ,Citation2005; Kotkas & Rosenberg, Citation1999) and in greenhouses in plastic rolls (Kotkas, Citation1987; Särekanno Citation2000; Kotkas & Särekanno, Citation2000 ,Citation2001) including composition of the multiplication medium in vitro, fertilizers added to the peat, and use of growth regulators to promote rooting of cuttings in plastic rolls (Kotkas & Särekanno, Citation1996 ,Citation2001). However, not enough attention has been given to factors that influence growth of plants raised from meristem culture in the field, and when the dynamics of potato vines and tuber yields were investigated in the field, the plants had been raised from tubers (Tooming et al., Citation1978, Van Loon, Citation1987, Eremeev, Citation2007; Eremeev et al., Citation2008) or from plantlets cultured in vitro (Lommen, Citation1999) and not by the methods used in this study.
Accordingly, the objective of the present research was to study, under field conditions, growth and tuber formation in plants that had been propagated using different kinds of propagules. Results of some aspects of this study, namely leaf area index and its dependence on the method of multiplication, variety, and environmental conditions, have been already published (Särekanno et al., Citation2008, in press). This paper discusses the effect of the same three factors on the number of tubers per plant and average weight of a tuber with emphasis on the multiplication method, especially differences between the methods created by EVIKA, which are based on repetitive multiplication in plastic rolls and multiplication of explants in vitro.
Materials and methods
Greenhouse and field trials were carried out during the growing season of 2005, 2006, and 2007 in EVIKA in Saku (59.3°N, 24.7°E). Two local late-maturing potato varieties, namely Ants and Vigri, both bred for Estonian conditions at the Jõgeva Plant Breeding Institute, were chosen, and were multiplied by four different methods: (i) explants in vitro, (ii) tips of shoots, (iii) pieces of stems, and (iv) truncated plants. All were grown in plastic rolls before they were transplanted to the field.
Multiplication methods and pre-growth under greenhouse conditions
Treatment 1
Explants in vitro. The plants were multiplied from micro-cuttings (1–1.5 cm in length, with 1 leaf) in test tubes (in vitro) on artificial media. The micro-cuttings were rooted and grown in vitro for 3 weeks after which, in the second half of May, the plants were transferred to plastic rolls filled with peat and kept under greenhouse conditions for 2–3 weeks.
Treatment 2
Tips of shoots or tip cuttings in plastic rolls. Plants grown from micro-cuttings in vitro were transplanted to plastic rolls filled with peat in the second half of April and grown under greenhouse conditions for 2–3 weeks. In the second half of May, shoot tips (1.5–2.0 cm in length, with leaves) were cut with simple scissors, the bottom ends treated with a rooting agent, and the excised pieces planted in plastic rolls filled with peat. The plants were grown in the rolls for 2 weeks (including 5–6 days required for the cuttings to strike roots).
Treatment 3
Pieces of stems or stem cuttings on plastic rolls. Pieces of stems (1.5–2.0 cm in length, with 1 leaf) were cut from the same plants, the tips of which had been used for Treatment 2. The bottom ends of the pieces were treated with rooting agent and the cuttings were planted in plastic rolls filled with peat. The plants were grown in the rolls for 2 weeks (including 5–6 days required for the cuttings to strike roots).
Treatment 4
Truncated plants grown in plastic rolls. The plants that served as the source of both tip- and stem-cuttings were retained in the plastic rolls filled with peat and allowed to grow for another 2 weeks. During that time, new shoots began to develop from axial buds of the source plants.
A week before transplanting to the field, all the plants were moved outdoors for acclimatization.
Field trials
The field trials were conducted near Saku (59.3°N, 24.7°E) in a sandy loam soil (Sceletic Regosol, WRB).
In the first half of June, the plants growing in plastic rolls were transferred to the field. Each plant was transplanted individually by hand. The spacing was 20×70 cm. The experimental layout used randomized block design with 32 plots divided into four replications. Each plot was 10.5 m2. Routine agro-technological practices for seed potato were followed.
The field trial was discontinued in the first few days of September each year because the intention was not to allow tubers to grow any further, although the plants had not completed their vegetative growth by then.
Measurements
The dynamics of growth were measured 13–14 times (at intervals of 5–7 days), depending on the year and the planting and harvesting times. Two weeks after transplanting to the field, 10 control plants of each variety from each multiplication method were marked and these were left to grow until the last harvest. The control plants were used for measuring some indicators of the growth stage before every sampling, and sample plants were chosen on the basis of these indicators. Plant height and the number of shoots per plant served as indicators at the early growth stage. Later, when the shoots had lodged, we used the distribution of shoots by their hardness (divided visually into four grades) and the sum of shoot lengths instead. Eight samples (two varieties and four multiplication methods) were examined each time, each sample consisting of four plants. While selecting the samples, plants that were next to any gap within a row were avoided. To avoid the edge-effect, the plots were protected from all sides by guard rows of the same variety.
Tubers from the sampled plants were separated, counted, and weighted.
Analysis of experimental data
For time-dependent analysis of data, calendar time was replaced with biological time expressed as cumulative thermal time in degree days. For potato, the best match between the data on experimental growth functions for different years was achieved by using cumulative daily mean temperatures above zero (Sepp, Citation1983; Kadaja & Tooming, Citation2004).
The number of tubers per plant and average weight of a tuber were analysed throughout the growing period as absolute measured values separately for each year and for each multiplication method put together as the average of both the varieties. To assess the probability of differences being due to multiplication methods, dispersion analysis was used and least significant differences (LSD05) were calculated. Since the samples were not large because of technical reasons but sampling had been more frequent, tubers number and weight were plotted using a moving average over three sampling dates (over two dates in the case of the first and the last dates) to decrease random fluctuations.
Since the number of tubers and average weight of a tuber are time-dependent, it was not possible to apply the same methods for simultaneous analysis of their absolute values during the entire growing period. Whereas the main objective of this research was to compare different methods of multiplication with the method of raising explants in vitro, the relative number of tubers and average tuber weight relative to the values of the same two variables in plants raised in vitro were also calculated. For this purpose, for every sampling date, the number of tubers per plant and average weight of a tuber in each replication of every sample were divided by the corresponding mean values calculated for the plants raised in vitro. Such ratios make the values comparable and allow the data collected throughout the growing period to be analysed. To examine whether any of the three factors (multiplication method, year, and variety) significantly influences the number of tubers and average weight of a tuber, dispersion analysis was performed with the relative values. The results of dispersion analysis are presented as the F statistic = MSmodel/MSerror (MS – mean square), the F subindexes representing the model degrees of freedom and the number of observed degrees of freedom. The influence of a factor was taken as proven if the differences were significant at p <0.05 level.
Statistica 7.0 software (Statsoft, Citation2005) was used with unsmoothed original data for dispersion analysis.
Results and discussion
Meteorological background
The experimental years differed mainly in terms of the amount and distribution of precipitation and also in terms of temperatures (). The sum of daily mean temperatures from planting to harvest was 1850 degree days in 2005, 2060 degree days in 2006, and 1670 degree days in 2007.
Table I. Average temperature, precipitation, and total radiation for the period June–August at the experimental site in Saku.
Number of tubers
In 2005, plants raised in vitro produced substantially more tubers than those grown by other methods of multiplication (LSD05>0.62) during the entire experimental period. The differences between tip- and stem-cuttings were not significant, and both methods yielded fewer tubers compared to the other two treatments (a). The number of tubers per plant kept increasing with time throughout the experiment, reaching 7.4 with the in vitro method, 4.2 with shoot tips, 4.5 with stem cuttings, and 6.5 with truncated plants.
Figure 1. The number of tubers per plant in 2005 (a), 2006 (b), and 2007 (c), plotted as a moving average over three sample times (over two in the case of the first and the last dates). Multiplication methods: MP – micro plants raised in vitro; TC – tip cuttings; SC – stem cuttings; TP – truncated plants.
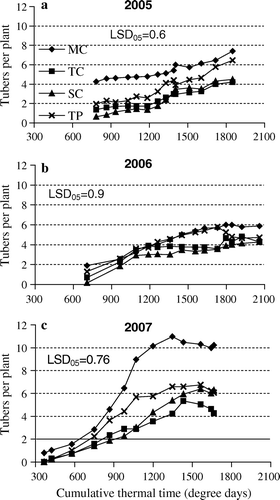
In 2006, the weather was generally unfavourable for plant growth and development (). Due to the drought as well as wide fluctuations in temperature, fewer tubers were formed than in 2005, although the ranking of treatments remained the same (LSD05>0.98). Up to the accumulated temperature of 1260 degree days (50 DAP, or days after planting), all the multiplication methods yielded fairly comparable number of tubers (b). Beyond that, the number of tubers decreased in the case of stem cuttings whereas the decline in the case of shoot tips set in 61 DAP (1450 degree days). In the case of truncated plants, the number stayed close to that in the case of plants raised in vitro up to 77 DAP (1730 degree days) and declined thereafter. Probably the extremely dry soil conditions caused undersized tubers to dry out or to degenerate. In 2006, the number of tubers did not increase markedly at the end of the growth period as was the case in 2005. The number of tubers per plant was maximum (6.0) in the plants raised in vitro 81 DAP (1800 degree days) and minimum (4.3) in the case of stem cuttings 99 DAP (2060) degree days) (b). Differences between the in vitro method and other methods were narrower in 2006 than in the other two years ().
Table II. Final differences in examined indicators between plants raised in vitro and those raised from other multiplication methods
In 2007, the weather was particularly favourable: temperature and precipitation were more uniformly distributed during the main growing period and incident solar radiation was high in August (), the month of maximum assimilation by meristem plants. Predictably, the yields that year were maximum. In all the multiplication methods, first tubers had formed by 11 DAP (420 degree days) (c). Unlike the other experimental years, in 2007 the number of tubers produced by plants raised in vitro stayed higher than that produced by plants raised by other methods throughout the growing period (LSD05>0.76). The difference widened considerably 45 DAP (980 degree days), exceeding final harvest differences of the previous two years (). Plants raised in vitro again produced the highest number (10.5 tubers per plant) and those raised from shoot tips produced the least (5.3). The maximum number was reached sooner than in previous years – 69 DAP (1430 degree days). For all multiplication methods, the number of tubers dropped slightly at the end of the experimental period.
Earlier experiments at EVIKA have shown that more stems and branches per plant are formed in the case of plants raised in vitro and truncated plants than in those raised from tip- and stem cuttings (Kotkas, Citation2003). In the present experiment, averaged over the two varieties and three experimental years, the plants raised in vitro formed 2.6 main stems per plant; truncated plants, 2.8; stem cuttings, 2.1; and tip cuttings, 1.9 (LSD05=0.4). We assume such inequality to be partly due to the weaker root system of plants raised from tip- and stem- cuttings since rooting itself claims one week of their sojourn in the greenhouse. Our earlier experiments have also shown that plants raised in vitro and truncated plants form tubers earlier. This observation, taken together with results of the present experiment, indicates a connection between the number of tubers and the number of stems – a connection that has also been noticed in potato plants multiplied from tubers: the greater the number of stems, the greater the number of tubers per plant (He Wei, Citation1997).
Average weight of a tuber
In 2005, significant differences in the average weight of a tuber between multiplication methods became apparent 37 DAP (1030) degree days) (a). During the rest of the experimental period, mean tuber weight was significantly smaller in plants raised in vitro than in those raised by other methods (LSD05>12.9). The greatest increase in tuber weight was recorded between 65 and 71 DAP (1510–1610 degree days), reaching 24.6 g for stem cuttings but remaining 2.7 g for truncated plants. Mean tuber weight continued to increase with time until the final harvest, reaching 50.2 g in the plants raised in vitro, 153.2 g for tip cuttings, 132.0 g for stem cuttings, and 86.9 g for truncated plants (a).
Figure 2. Average weight of a tuber in 2005 (a), 2006 (b), and 2007 (c), plotted as a moving average over three sample times (over two in the case of the first and the last dates). Multiplication methods: MP – micro plants raised in vitro; TC – tip cuttings; SC – stem cuttings; TP – truncated plants.
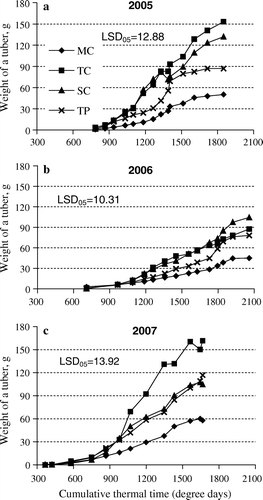
In the unfavourable conditions of 2006, mean tuber weight was lower than that in 2005 (b). Differences between multiplication methods as compared to the values reached in plants raised in vitro appeared 46 DAP (1190 degree days) for tip- and stem-cuttings and 81 DAP (1800 degree days) for truncated plants (LSD05>10.3). The average weight of a tuber increased most markedly between 81 and 84 DAP (1800–1840 degree days), the most (11.4 g) in the case of stem cuttings and the least (5.3 g) in the case of plants raised in vitro. As in 2005, mean tuber weight continued to increase with time until the final harvest. The highest value (104.4 g) was recorded in the case of stem cuttings, followed, in that order, by tip cuttings (87.5 g), truncated plants (77.9 g), and plants raised in vitro (45.0 g). And also as observed in 2005 for the number of tubers, differences between multiplication methods were the smallest in 2006 ().
In the very favourable year 2007, mean tuber weight increased faster than it did in the previous two years (c). Differences between the plants raised in vitro and those raised by other methods of multiplication were already noticeable 45 DAP (980 degree days) (LSD05>13.9). However, after that point, average tuber weight in plants raised in vitro did not increase as quickly as that in other treatments. The increase was the most marked between 57 and 64 DAP (1200–1350 degree days), being 23.0 g for tip cuttings and 8.1 g for plants raised in vitro. Mean tuber weight at the final harvest was higher than that in previous years, being 161.7 g for tip cuttings and 57.8 g for plants raised in vitro ().
The higher mean weights recorded in tip- and stem-cuttings are a logical outcome of fewer tubers per plant recorded in those two methods: the greater the number of tubers per plant, the lower the mean tuber weight and vice versa. Results of the three experimental years proved that plants grown from tip- and stem-cuttings formed tubers of the size particularly appropriate for seed tubers (20–60 g) one to more than two weeks earlier than truncated plants or those raised in vitro did. These differences should be considered in selecting a suitable date for harvest because although plants raised from tip- and stem-cuttings produce fewer tubers than those raised by the other methods do, the tubers are produced sooner, which lowers the chances of seed potato being infected, especially in regions where excessively moist weather towards the end of potato's growth period contributes to spread of diseases.
The weight of tubers also depends on the growth and development of leaves and branches (Caldiz et al., Citation2001). Our earlier research (Särekanno et al., Citation2008, in press) showed that leaf area index was higher in plants raised from tip- and stem-cuttings, being inversely correlated with the number of tubers. Such inverse connection now also reveal between the number of tubers and the mean weight of a tuber.
The influence of multiplication method
To test whether the number of tubers and mean tuber weight are influenced by the method of multiplication, we compared the experimental data from the two varieties and three years. Differences between multiplication methods were determined relative to the values recorded in plants raised in vitro (), a method that allows simultaneous use of data for the entire experimental period. The methods did influence the two parameters significantly (number of tubers: F 3, 1242=99.77, p <0.05; mean tuber weight F 3, 1238=59.31, p <0.05).
Figure 3. Differences between multiplication methods in the number of tubers per plant (a) and average weight of a tuber (b) determined relative to in vitro micro plants multiplication method over three experimental years and two varieties. Multiplication methods: MP – micro plants raised in vitro; TC – tip cuttings; SC – stem cuttings; TP – truncated plants. Different letters indicate significant differences (p < 0.05) between multiplication methods. Vertical bars denote 0.95 confidence intervals.
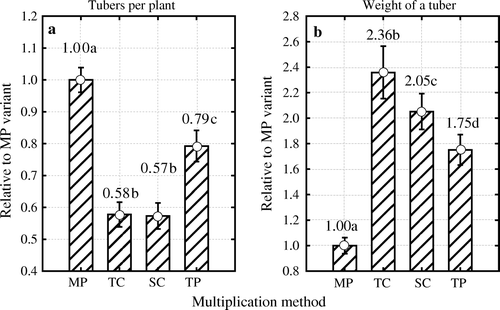
In relative terms, plants raised in vitro produced considerably more tubers than those raised by other multiplication methods. The number of tubers produced by plants raised from tip-and stem-cuttings was only 42–43% of that produced by plants raised by in vitro, although the first-mentioned two methods did not differ significantly between themselves (a).
Similarly, in relative terms, plants raised by all other multiplication methods produced heavier tubers than plants raised in vitro: plants raised from tip cuttings 2.36 times heavier, those produced from stem cuttings 2.05 times heavier and those produced by truncated plants 1.75 times heavier (b). All multiplication methods differed significantly between themselves.
In summary, the suitability of all tested multiplication methods for seed potato production was confirmed. The technology of multiplication of meristem plants from tip- and stem-cuttings and truncated plants in plastic rolls is practical and more convenient than multiplication directly from in vitro micro-cuttings under sterile laboratory conditions. Although by the in vitro micro plants multiplication method the most suitable size of the tubers and the highest number of tubers is usually achieved, the simplicity, energy- and time sparing of the EVIKA methods speaks in their favor.
The influence of year
To decide whether the number of tubers and average tuber weight are influenced by the environment, we compared the experimental data from the two varieties and all four multiplication methods for the entire growth period (). Differences between years were again determined relative to the values recorded in plants raised in vitro. Results of dispersion analysis (the number of tubers: F 2, 1243=37.92, p <0.05; average tuber weight: F 2, 1239=8.35, p <0.05) proved the significant influence of weather parameters of every year.
Figure 4. Differences between years in relative number of tubers per plant (a) and average weight of tuber (b), calculated relative to micro plants raised in vitro (MP), over all multiplication methods and two varieties. Different letters indicate significant differences (p < 0.05) between years. Vertical bars denote 0.95 confidence intervals.
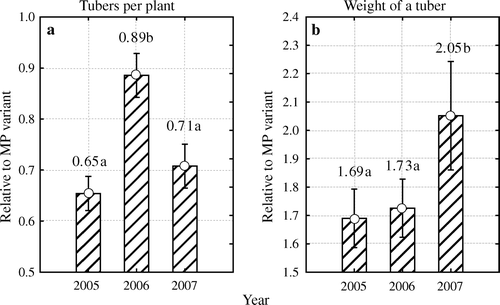
In relative scale, the difference between the multiplication methods in terms of the number of tubers was large in 2005 and 2007 but much smaller in 2006, the year in which the weather had been unfavourable (a). The difference in terms of average weight of a tuber was also large, and especially so in 2007, the year in which the weather had been particularly favourable (b).
In all our experiments, the plants were about 10–15 cm tall when planting out. However, acclimatization to field conditions proceeded differently in different years. In the dry and hot 2006, plant growth was suppressed after field planting and the plants suffered throughout the growth period (transplanting shock). Plants raised in vitro suffered the most, their leaf area index remained smaller than that of plants raised by other multiplication methods (Särekanno et al., Citation2008, in press), the average weight of a tuber they produced was the lowest, and the lead they had over plants raised by other methods in terms of the number of tubers was the smallest in three years ( and ). Lommen (Citation1999) also reported that transplanting shock can be severe for plants raised in vitro, especially under suboptimal conditions.
The influence of variety
To explain the influence of variety on the number of tubers and average weight of a tuber, we compared the relative values of these two parameters in plants raised by the three methods to those recorded in plants raised in vitro, pooling the experimental data for all three years and all multiplication methods for the entire growth period (). The difference between varieties also proved significant according to dispersion analysis (the number of tubers: F 1, 1244=5.20, p <0.05; average tuber weight: F 1, 1240=10.61, p <0.05).
Figure 5. Differences in the number of tubers per plant (a) and average weight of tubers (b) calculated relative to micro plants in vitro (MP) and averaged over different years and multiplication methods, depending on the variety. Different letters indicate significant differences (p < 0.05) between varieties. Vertical bars denote 0.95 confidence intervals.
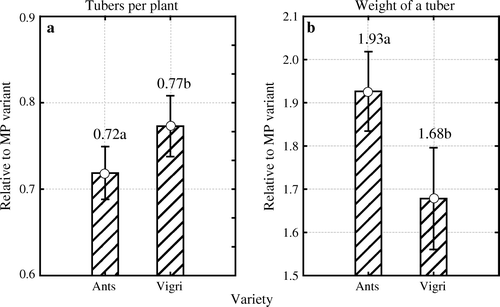
Variety Ants produced relatively fewer tubers (0.72 relative to the absolute value of 1.0) than variety Vigri (0.77). This means that when compared to other multiplication methods, plants of variety Ants raised in vitro produced fewer tubers per plant than those produced by variety Vigri (a).
With respect to tuber weight, the results were the exact opposite, with variety Ants producing heavier tubers (b). We can conclude that variety Vigri, producing more stems (2.8 main stems per plant on average over all multiplication methods and all years), experienced smaller differences in tuber weight between plants raised in vitro and those raised by other methods than those experienced by variety Ants, which produced fewer stems (1.8 main stems per plant).
Conclusion
The results of this analysis assure the robustness of multiplication methods on plastic rolls using truncated plants, stem- and tip-cuttings in combination as an alternative to multiplication via in vitro micro plants.
Acknowledgements
The experiments on which the present study was based were made within the Target Research Project no. 0442528 S03 and grants no. 6132 and 6092 of Estonian Science Foundation. The authors are grateful to Mrs. Luule Kurimu and Mrs. Erika Vesik for generous technical assistance in sample processing.
References
- Caldiz , D.O. , Fernandez , L.V. and Struik , P.C. 2001 . Physiological age index: a new, simple and reliable index to assess the physiological age of seed potato tubers based on haulm killing date and length of the incubation period . Field Crops Research , 69 : 69 – 79 .
- Eremeev , V ., 2007 . The influence of thermal shock and pre-sprouting of seed potatoes on formation of some yield structure elements PhD thesis Tartu, , Estonia 126 .
- Eremeev , V. , Lõhmus , A. , Lääniste , P. , Jõudu , J. , Talgre , L. and Lauringson , E. 2008 . Influence of seed potato pre-planting treatments on leaf area and tuber yield formation . Acta Agriculturæ Scandinavica, Section B-Soil & Plant Science , 48 : 35 – 42 .
- Estrada , R.P. and Dodds , J. H. 1986 . Induction of in vitro tubers in a range of potato genotypes . Plant Cell, Tissue and Organ Culture , 7 : 3 – 10 .
- Garner , N. and Blake , J. 1989 . The induction and development of potato microtubers in vitro on media free of growth regulating substances . Annals of Botany , 63 : 663 – 674 .
- He Wei 1997 Agronomic and ecological studies on the potato (Solanum tuberosum L.) in Southwest China. Seed and crop management . PhD thesis Wageningen Agricultural University Wageningen, , The Netherlands 13 .
- Hussey , G. and Stacey , N.J. 1984 . Factors affecting the formation of in vitro tubers of potato (Solanum tuberosum L.) . Annals of Botany , 53 : 565 – 578 .
- Kadaja , J. and Tooming , H. 2004 . Potato production model based on principle of maximum plant productivity . Agricultural and Forest Meteorology , 127 : 17 – 33 .
- Kotkas , K . 1987 . Kartuli meristeemtaimede paljundamine. [Propagation of potato meristem plants] . Teaduse soovitused tootmisele Tallinn, , Estonia 12 15 . [In Estonian]
- Kotkas , K Särekanno , M 1996 An effective and environmentally safe potato multiplication technology created in Research Centre EVIKA . Abstracts of 13th Triennial Conference of EAPR Veldhoven, , The Netherlands 198 199 .
- Kotkas , K. and Rosenberg , V. 1999 . Disease eradication and propagation of the initial seed potato material in Estonia . Potato Research , 42 : 577 – 583 .
- Kotkas , K. and Särekanno , M. 2000 . The influence of plant propagation method and planting density on the productivity of potato meristem plants . Transactions of the Estonian Academic Agricultural Society, (Tartu Estonia) , 11 : 25 – 29 .
- Kotkas , K. and Särekanno , M. 2001 . The productivity of potato meristem plants depending on pre-planting treatment . Transactions of the Estonian Academic Agricultural Society (Tartu, Estonia) , 14 : 105 – 109 .
- Kotkas , K . 2003 Influence of culture medium composition and preservation conditions on in vitro preservation of potato varieties by means of meristem plants and on subsequent influence in field Tartu, , Estonia PhD thesis 108 .
- Lommen , W.J.M. 1999 . Causes for low tuber yields of transplants from in vitro potato plantlets of early cultivars after field planting . Agricultural Science , 134 : 152 – 168 .
- Nowak , J. and Asiedu , S.K. 1992 . Gelling agent and light effects on in vitro tuberization of potato cultivars . American Potato , 69 : 461 – 670 .
- Ranalli , P. , Bassi , F. , Ruaro , G. , del Re , P. , Di Candilo , M. and Mandolino , G. 1994 . Microtuber and minituber production and field performance compared with normal tubers . Potato Research , 37 : 383 – 391 .
- Rosenberg , V . 1981 . Sposob polutsenie posadosnovo materjala kartofelja i sreda dlja razmnozenija regenerirovannōh rastenii . SU Autoritunnistus nr. 1025373 . [In Russian]
- Rosenberg , V Kotkas , K . 1984 . Sposobō uskorennovo razmnozenija rastenii kartofelja . Sposob nautsnōx trudov. LIII EMMTUI Zastsita rastenii , Tallinn, , Estonia 89 97 [In Russian]
- Rosenberg , V Kotkas , K . 1986 . Sposob razmnozenija posadosnovo materiala kartofelja v kulture tkani . SU Autoritunnistus nr. 15013118 . [In Russian]
- Rosenberg , V ., Kiisk J ., Kotkas , K Sarnet , V . 1988 . Sposob posadki rastenii-regenerantov kartofelja . SU Autoritunnistus nr. 1678255 . [In Russian]
- Rosenberg , V ., Kotkas , K Särekanno , M 1997 Brasov, , Romania . Review about the research and production of seed potato in Estonia . Proceedings of the International Potato Symposium 10 13 .
- Rosenberg , V. , Kotkas , K. and Särekanno , M. 2005 . The number of tubers per plant and tuber's weight formation dynamics of potato meristem plants . Transactions of Estonian Agricultural University (Tartu, Estonia) , 220 : 72 – 74 .
- Särekanno , M . 2000 The productivity of disease-free potato plants . Transactions of the Jõgeva PBI III. ‘Plant breeding and seed production’. (Jõgeva, Estonia) , 167 175 .
- Särekanno , M ., Kadaja , J ., Kotkas , K ., Rosenberg , V ., Vasar , V ., Ojarand , A & Eremeev , V . 2008 . Leaf area index development of potato meristem plants derived trough different multiplication methods and grown under field conditions . Acta Agriculturæ Scandinavica, Section B-Soil & Plant Science , in press .
- Sepp , J . 1983 . Eksperimental'noe opredelenie funktsij rosta kartofelja. Experimentally determined growth functions for potato . In : H . Tooming P . Karing , Agroklimaticheskie Uslovija i Produktivnost’ sel'skohozjaistvennyh kul'tur. [Agroclimatic conditions and crops productivity]. Trudy VNIISHM Proceedings of All-Union Research Institute of Agricultural Meteorology , . 11. Gidrometeoizdat (Leningrad), 36–40. [In Russian]
- Statsoft 2005 . Statistica 7,0 . Copyright 1984–2005. Tulka, OK, USA .
- Struik , P.C Lommen , W.J.M . 1990 . Production, storage and use of micro- and minitubers . Proceedings of the 11th Triennial Conference of EAPR, Edinburgh , UK , 122 133 .
- Tooming , H.G ., Mäetalu , H.I ., Kyjva , P.H ., Tammets , T.H Rajg , H.G . 1978 . Programmirovanie maksimal'nyh urožaev kartofelja. [Programming of maximum potato yield] . Vestnik sel'skohozjajstvennyh nauk (Reports of Agricultural Sciences) , 2 257 , 110 117 . In Russian
- Van Loon , C.D. 1987 . Effect of physiological age on growth vigour of seed potatoes of two cultivars. 4. Influence of storage period and storage temperature on growth and yield in the field . Potato Research , 30 : 441 – 450 .