Abstract
In recreational areas, such as parks and golf courses, plants like white clover are considered as weeds. In an attempt to identify biocontrol agents that can be used to control growth of clover, a number of bacterial isolates were studied. Two approaches were considered: soil treatment for suppression of Rhizobium leguminosarum, a symbiotic bacteria providing clover with nitrogen, and direct suppression by leaf-spray treatment. Selected bacterial isolates were first screened against R. leguminosarum in a dual culture. Some of the tested isolates significantly inhibited the growth of R. leguminosarum. Soil inoculation of these isolates had a significant growth-reducing effect on clover seedlings. Leaf-spray treatment of bacteria had a significant impact on clover growth. Depending on the plant growth stage, this effect can reach up to 86% reduction in clover shoot dry weight. Different temperatures did not influence the effect on clover. The growth-reducing effect of bacteria was also shown on another important dicotyledonous weed, Chenopodium album.
Introduction
White clover (Trifolium repens) is considered a weed in green areas used for ornamental or recreational purposes such as parks, sports fields, and golf courses. Considerable financial resources are invested annually for the control of weeds using chemical herbicides which, with the active substance fluroxipyr, the only approved substance for suppressing dicotyledonous weeds on golf courses in Sweden, are sold annually in amounts of 45 metric tonnes (Kemikalieinspektionen, Citation2006).
Legislation at both national and local level in Scandinavian and other countries aims at reducing the use of chemical pesticides, to decrease the environmental impact of human activities, and to protect water supplies. One example is Delsjo Golf Club, Gothenburg, Sweden, which is prohibited by local authorities from using any chemical pesticide due to the course location close to a public water supply. To this end, some national golf federations, such as the Swedish Golf Federation, have taken proactive steps to meet environmental goals.
Using microorganisms to control weeds in green areas is an alternative method that may reduce costs, decrease dependence on chemicals, and lower the impact on the environment. Bioherbicides based on microorganisms can be more selective than chemical herbicides and affect only the desired species (Bolton & Elliot, Citation1989). Another advantage is the decreased chance of induction of resistance in the target species, due to a number of different mechanisms involved (Crump et al., Citation1999).
One group of bacteria that can be used as biocontrol agents against weeds are deleterious rhizobacteria, which are nonparasitic rhizobacteria colonizing plant root surfaces and able to suppress plant growth (Alström, Citation1987; Åström & Gerhardson, Citation1988; Kremer & Kennedy, Citation1996). The dependence of clover on symbiotic Rhizobium leguminosarum (Fries, Citation1973) might also be used to combat white clover. Thus, bacterial isolates with antagonistic properties towards R. leguminosarum may be useful as bioherbicides against clover. As lactic acid bacteria (LAB) are known to produce antifungal and antibacterial metabolites (Messens & De Vuyst, Citation2002; Schnürer & Magnusson, Citation2005), and are generally recognized as safe (GRAS) according to USFDA regulations (Wessels et al., Citation2004), LAB isolates were also included in this study.
The objective of the present study was to evaluate several bacterial isolates as potential biocontrol agents of white clover and other dicotyledonous weed species in turf grass. The study was based on the following hypotheses; bacteria can suppress or reduce growth of the target weeds by either a) production of metabolites that injure leaves or roots, b) preventing R. leguminosarum colonization of root surfaces, or c) other antagonistic mechanisms.
Materials and methods
Bacterial isolates, media, and culture conditions
The bacterial strains used in this study are listed in . The bacteria for the experiments were chosen by the following criteria: Pseudomonas isolates S 611, Å 112, and Å 313 have shown deleterious effects on plants in a previous study (Åström & Gerhardson, Citation1988), the rest of the Pseudomonas and LAB isolates showed deleterious effects on dicotyledonous plants in a pre-screening test.
Table I. Bacterial strains used in this study.
Strains of lactic acid bacteria (LAB) were grown on MRS agar (Oxoid Ltd, Basingstoke, England) in anaerobic jars under CO2+N2 atmosphere (GasPak system, BBL, Cockneyville, MD, USA) or in MRS broth, at 30°C for 48 h. Isolates MA 335, MA 304, and SB 17 were originally isolated from plant roots, and cultured in vegetable peptone broth (VPB, Oxoid Ltd, Basingstoke, England) at room temperature. Isolates S 611, Å 112, and Å 313 were grown in King's B medium (King et al., Citation1954) at room temperature. R. leguminosarum strain W1 was grown in yeast mannitol broth (YMB) (Somasegaran & Hoben, Citation1994) at 28°C. All strains except LAB were cultured in darkness on an orbital shaker at 120 rpm for 48 h. Bacterial cells of the isolates were obtained by centrifugation of liquid fermentate, washed, and suspended in 0.01 M MgSO4 ·7H2O.
For the colonization study, MiLAB6 was transformed with plasmid pLV100 (Sorvig et al., Citation2005) as described by Aukrust et al. (Citation1995). Transformants were grown on MRS agar containing 15 µg ml−1 chloramphenicol (MRS-C) and a single clone chosen for further characterization. The presence of the plasmid was verified by PCR with the primers CatF2 (5′-gaa agg ata tga aat tta tcc ctc tt-3′) and CatR2 (5′-tac cct atg aat tat ttg aaa ttc a-3′) using PuReTaq Ready-To-Go™ PCR Beads (GE Healthcare Life Sciences, Uppsala, Sweden) at an annealing temperature of 47°C. A small amount of one bacterial colony was used as template. The stability of the plasmid in the absence of selection was verified by repeated inoculation into MRS broth. After 50 generations, all colonies were resistant to chloramphenicol and carried the plasmid as verified by PCR.
Plant material, growth conditions, and spray treatment
Seeds of white clover variety Lena were obtained from Svalöf Weibull AB, Svalöv, Sweden. Chenopodium album seeds were obtained from Herbiseed, Twyford, UK. Seeds were sown in trays filled with nonsterile peat soil containing 62% light peat, 13% black peat, and 25% sand, calcite 6 kg/m3, dolomite 2 kg/m3 and NPK 14–7–15 1.2 kg/m3; pH 6.5, organic C 19%. Four seedlings were then transplanted into a plastic pot filled with peat soil. All treatments were run in four replicates. Unless specified, plants were grown at 20–22°C in a growth chamber with a 12-h day length and approx. 60–70% relative humidity (RH) and watered with tap water when needed. All the spray treatments, except in the colonization experiment, were done with an all-glass TLC Reagent Sprayer (Kebo, Stockholm, Sweden) using an electrical compressor. In general, plants were sprayed at 2nd-to-3rd leaf stage with 5 ml of bacterial culture, media, or other solutions per pot.
Antagonistic effects of bacterial isolates against R. leguminosarum in dual cultures
The antagonistic activity of different strains () was tested against R. leguminosarum strain W1 (Carlsson, Citation2000) on Yeast Mannitol Agar (YMA) in order to look for effects that can disturb and/or hinder establishment of nodules on clover roots. The Pseudomonas isolates were chosen due to their growth-reducing capability shown on other plant species (Åström & Gerhardson, Citation1988). Four different strains of Lactobacillus species isolated from plant material were also included in the study. Cells of W1 were obtained by centrifugation of a liquid culture in YMB, washed, and suspended in 0.01 M MgSO4 ·7H2O to a suitable density. One hundred µl of the suspension were spread on the surface of agar plates. The plates were left to dry for 30 min, after which four holes were made in each plate. Holes were made with a 6-mm hole punch. Aliquots (80 µl) of the fermentate of the strains to be tested were then added to the wells and the plates were incubated at 28°C for 72 h. Culture media were used as controls and the treatments were run in triplicate.
Co-inoculation of R. leguminosarum and tested isolates in clover seedlings: Sand experiments
White clover seeds were sown in plastic trays filled with sand at 20°C in a growth chamber and watered with ¼ strength Hoagland's No. 2 Basal Salt solution (Sigma-Aldrich, USA). Two-week-old seedlings were transplanted into plastic pots filled with sand, four seedlings/pot, and left to establish for two days. Each pot was then inoculated with 20 ml of strain W1 (1×107 cfu/ml) and 20 ml of the strain to be tested. Clover seedlings were grown for two weeks after which the shoots were cut and their fresh weight determined. Culture media were used as controls; all treatments were run in triplicate.
Co-inoculation of R. leguminosarum and the tested isolates in clover seedlings: Seed germination pouch experiments
White clover seeds were surface sterilized in 10% sodium perchlorite solution amended with 0.01% Tween 20 and rinsed three times with sterile distilled water. Four seeds were then placed in a sterilized cyg™ seed germination pouch (Mega International, Minneapolis, MN, USA) filled with 20 ml of sterile ¼ strength Hoagland solution. Ten seeds were also placed on VPA plates to confirm sterility. The pouches were placed in a growth chamber at 20°C and watered with sterile tap water when needed. One-week-old seedlings were inoculated with 100 µl each of W1 (4×109 cfu ml−1) directly onto the root surface using a pipette. One week later, each seedling received 100 µl of the tested strains (4×109 to 5×109 cfu ml−1). All treatments were run in four growth pouches. Root and shoot fresh weights were measured after ten days.
The effect of LAB as spray treatment
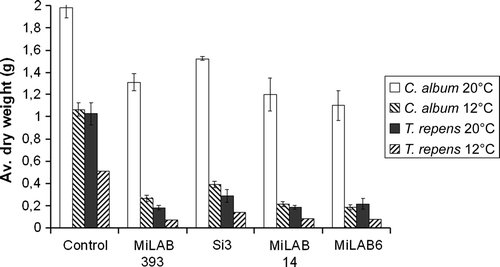
LAB cultures were prepared and sprayed onto clover plants. The pH of the supernatants was for MiLAB6 = 3.72, MiLAB14 and MiLAB393 = 3.82, and for Si3 = 4.12. To investigate if temperature influences the phytotoxic effect of LAB, the four strains were screened against white clover and C. album at 12 and 20°C.
Soil inoculation and spray treatment of Poa annua by MiLAB6
A turf material of Poa annua was obtained from Viksjö golf club (Järfälla, Sweden). The grass was cut into pieces to fit into 8×8 cm pots filled with a bottom layer of peat mix and a top layer of sand. Pots were then inoculated with 10 ml of undiluted two-fold- or three-fold-diluted culture of MiLAB6. In the second experiment, the pots were sprayed twice with the same volume and dilutions as in the soil-inoculation experiment; both experiments also included untreated controls. The experiments were done as four replicates and repeated twice. The pots were checked daily for any visible reaction and finally read after three weeks of soil inoculation or two weeks after the last spray treatment in the second experiment as described below.
Colonization and survival of MiLAB6 in clover plants
MiLAB6 (pLV100) was grown for 48 h in MRS broth without chloramphenicol. Cells from two 200-ml portions of the culture were washed twice by centrifugation and suspended in 0.01 M MgSO4 ·7H2O and 25% MRS in 0.01 M MgSO4 ·7H2O. Finally, the cells were suspended in 200 ml of the respective solution.
Immediately before spraying, all leafs of five-week-old white clover plants were cut to simulate mowing before weed treatment. Five plants in one pot (four replicates) were sprayed with five ml of A = 10 mM MgSO4 ·7H2O, B = overnight culture in MRS, C = cells suspended in 10 mM MgSO4 ·7H2O, and D = cells suspended in 25% MRS in 0.01 M MgSO4 ·7H2O. The number of cfu ml−1 for the respective treatment, as determined by plating on MRS-C, were A = 0, B = 1.2×109, C = 1.1×109, and D = 1.5×109. The plants were incubated at 20°C, 70% RH, and 12 h light per 24 h.
Three time points were chosen; two hours, seven days, and 21 days after spraying, one plant from each pot was removed. The recording after two hours were done in order to measure the number of living bacteria stuck to leaves after inoculation. To determine the total number of lactic acid bacteria and number of MiLAB6 (pLV100) per g fresh weight, plants were ground in a mortar in 10 ml of MgSO4 ·7H2O followed by serial dilution and spreading on MRS, and MRS-C respectively.
Statistical analysis
Data were analysed by ANOVA and treatment means were separated by Bonferroni test (P < 0.05) using the Statistica software (CitationStatSoft, USA). Error bars in all figures are±standard error of mean.
Results and discussion
Dual culture
Several bacterial isolates significantly reduced the radial growth of strain W1 in YMA. The four LAB strains gave inhibition growth zones with a diameter of 10–40 mm for strain W1 (). MiLAB6 was the most active strain against W1. Sterile filtered supernatant, as well as the washed cells, could also inhibit growth of W1, but to a lesser extent than the culture (). The bacterial strains S 611, Å 112, Å 313, and MiLAB6, all showing a good activity against W1, were selected for further testing below.
Table II. Antagonistic activity of strains S 611, Å 112, Å 313, and MiLAB6 in dual cultures against W1. Washed cells and supernatant from a MiLAB6 culture were also tested. Inhibition of radial growth was measured in mm.
Co-inoculation of R. leguminosarum and the tested isolates in clover seedlings: Sand experiments
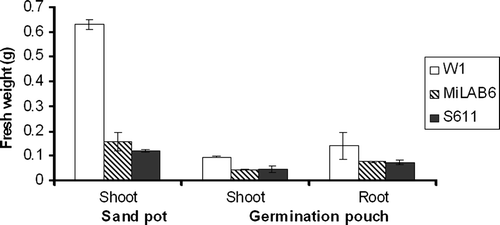
Inoculation of clover seedlings with strains S 611, Å 112, Å 313, and MiLAB6 in combination with strain W1 negatively affected the plant growth, as indicated by the significant reduction in shoot fresh weight (). Necrosis and eventually plant death was observed after a few days of inoculation. Control plants inoculated with strain W1 were healthy and small nodules were observed on the root system. The strains MiLAB6 and S 611 were chosen for further testing in germination pouches.
Figure 1. Inhibition of clover seedlings grown in sand pots and germination pouches by co-inoculation of strain W1 and tested bacteria. W1 = control inoculation with strain W1. Error bars are ± standard error of mean. Data were analysed by ANOVA and treatment means were separated by Bonferroni test (P < 0.05).
Co-inoculation of R. leguminosarum and the tested isolates in clover seedlings: Seed germination pouch experiments
In this experiment, clover plants were first inoculated with strain W1 in order to colonize of the roots before antagonists were added. Strains MiLAB6 and S 611 significantly inhibited clover growth in terms of reduction in shoot and root fresh weights (). In addition, fewer nodules were observed in plants treated with these strains than in plants inoculated with W1 alone (data not shown).
In the co-inoculation experiments both of the selected isolates significantly reduced growth of clover grown in sand. Deleterious rhizobacteria such as S 611 can also interfere with colonization of legumes by R. leguminosarum and reduce nitrogen content of plants (Berggren et al., Citation2005). LAB have occasionally been isolated from soil, but it seems that the concentrations are low, as these bacteria need to be enriched before isolation (Chen et al., Citation2005; Yanagida et al., Citation2005, Citation2006). These results indicate that LAB strains might be less suitable for use as soil bioherbicides.
The effect of overnight cultures of LAB as spray treatment
As the LAB strains might be less suitable for soil inoculation we investigated the possibility of using these strains as a bioherbicide applied as leaf spray. Acetic acid is used for killing weeds in gardens (Young, Citation2004); therefore it might be possible to use overnight cultures of LAB strains producing lactic acid as leaf spray.
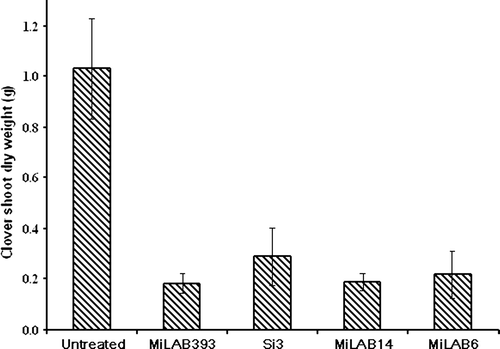
The spray treatment of LAB strains MiLAB-6, -14, -393, and Si3 significantly inhibited the growth of white clover plants. The LAB strains reduced shoot dry weight by 72–82% ().
Figure 2. Results of leaf spraying with cultures of MiLAB-6, -14, and -393 and Si3 against white clover plants. Error bars are ± standard error of mean. Data were analysed by ANOVA and treatment means were separated by Bonferroni test (P < 0.05).
The influence of temperature on the performance of LAB strains was tested in white clover and C. album at 12 and 20°C (). In general, temperature did not influence the phytotoxic effect of LAB strains on clover plants when grown at 12 and 20°C. When C. album plants were tested at 12°C, up to 82% reduction in dry weight was achieved by strain MiLAB6, whereas for C. album plants grown at 20°C, the same isolate reduced the dry weight by 44%.
Soil inoculation and spray treatment of Poa annua by MiLAB6
MiLAB6 did not injure P. annua plants when inoculated into the soil (results not shown), but when the fermentate was sprayed on the leaves of P. annua plants, leaf tips were damaged. However, P. annua plants recover very rapidly and at 12 days after inoculation the plants had recovered totally (results not shown).
Colonization and survival of MiLAB6 in clover plants
To test if the biological activity could be linked to the bacterial cells themselves or to metabolites in the media various combinations of cells, buffer, and spent media was used as detailed in the Material and methods section. Results are recorded in four groups: A: buffer; B: bacteria and spent media; C: bacteria and buffer; D: bacteria and diluted media. After spraying, the number of MiLAB6 (pLV100) detected were for B = 1.8×107 cfu g−1, C = 4.5×107 cfu g−1, and D = 8.7×107 cfu g−1, all fresh weight. No bacteria were detected on the control. Thus, any epiphytic flora present on the plants were below the detection level at the time of spraying (approximately 40 cfu/g). After 7 days, the number of bacteria had declined 100–1000-fold in all treatments. At day 21, the number had decreased another 100-fold, suggesting that MiLAB6 (pLV100) does not colonize the plants under these conditions. Thus, most likely the effects of spraying plants with MiLAB6 are due to metabolites already formed in the culture supernatant and therefore a long-lasting effect might not be expected.
The effect of the isolates tested showed a potential for use as bioherbicides; however, LAB seem not to be suitable for use under the conditions used in the experiments. The results from our experiments suggest another approach for further tests, such as testing deleterious rhizobacteria in field trials against white clover. This approach has been successfully used earlier, using several microorganisms as spray treatment to control the growth of different weed species under both greenhouse and field conditions (Weissmann & Gerhardson, Citation2001). Bacterial cells of strain MiLAB6 were not toxic to the clover plants when sprayed on the leaf surface. This can be due to the nonphytopathogenic nature of LAB or due to the fact that MiLAB6 was unable to colonize the clover leaves. The activity of LAB against weeds is probably metabolite(s) dependent, which was also noticed in other weed-biocontrol bacteria (Weissmann & Gerhardson, Citation2001). These results indicate that use of LAB to control white clover in golf courses should be possible.
Acknowledgements
MASE (Microbial Activity for a Sound Environment) is a research program financed by MISTRA (Swedish Foundation for Strategic Environmental Research) and industrial partners. The golf project is also kindly funded by the Swedish Golf Federation. DOM (Domestication of Microorganisms) is a research program financed by MISTRA. Isolates were kindly provided by Lantmännen BioAgri AB and Georg Carlsson (SLU). We thank Cecilia Berglund for technical assistance in the colonization study. We also thank Christopher Folkeson Welch for fruitful discussions and critical reading of the manuscript.
References
- Alström , S. 1987 . Factors associated with detrimental effects of rhizobacteria on plant growth . Plants and Soil , 102 : 3 – 9 .
- Aukrust , T.W. , Brurberg , M.B. and Nes , I.F. 1995 . Transformation of Lactobacillus by electroporation . Methods in Molecular Biology , 47 : 201 – 208 .
- Berggren , I. , Alström , S , Van Vuurde , J.W.L. and Mårtensson , A. 2005 . Rhizoplane colonization of peas by Rhizobium leguminosarum bv. viceae and deleterious Pseudomonas putida . FEMS Microbiology Ecology , 5 : 71 – 78 .
- Bolton , H. and Elliott , L.F. 1989 . Toxin production by a rhizobacterial Pseudomonas spp. that inhibits wheat growth . Plant and Soil , 114 : 269 – 278 .
- Carlsson , G. 2000 . Variation in Rhizobium leguminosarum bv. trifolii populations in nodules of three Trifolium species cultivated together in the field . Master Thesis: Swedish University of Agricultural Sciences, Department of Agricultural Research for Northern Sweden. Umeå ,
- Chen , Y.S. , Yanagida , F. and Shinohara , T. 2005 . Isolation and identification of lactic acid bacteria from soil using an enrichment procedure . Letters of Applied Microbiology , 4 : 195 – 200 .
- Crump , N.S. , Ash , G.J. , & Fagan , R.J. 1999 . The development of an Australian Bioherbicide . In A.C . Bishop , M . Boersma , & C.D . Bames 12th Australian Week Conference , 235 237 .
- Fries , N. 1973 . Mineralnäringen . In: Biologi volym 3, Fysiologisk Botanik 146 . Uppsala, , Sweden : Almqvist & Wiksell (in Swedish) .
- Kemikalieinspektionen 2006 . http://www.kemi.se/upload/Trycksaker/Pdf/Statistik/forsalda_bkm_2006.pdf (accessed 26 February 2009)
- King , E.O. , Ward , M.K. and &. Rainey , D.E. 1954 . Two simple media for demonstration of pyocyanin and fluorescein . Journal of Laboratory and Clinical Medicine , 44 : 301 – 307 .
- Kremer , R.J. and Kennedy , A.C. 1996 . Rhizobacteria as Biocontrol Agents of Weeds . Weed Technology , 10 : 601 – 609 .
- Magnusson , J. and Schnürer , J. 2001 . Lactobacillus coryniformis subsp. coryniformis Strain Si3 Produces a Broad-Spectrum Proteinaceous Antifungal Compound . Applied and Environmental Microbiology , 67 : 1 – 5 .
- Magnusson , J. , Ström , K. , Roos , S. , Sjögren , J. and Schnürer , J. 2003 . Broad and complex antifungal activity among environmental isolates of lactic acid bacteria . FEMS Microbiology Letters , 219 : 129 – 135 .
- Messens , W. and De Vuyst , L. 2002 . Inhibitory substances produced by Lactobacilli isolated from sourdoughs–a review . International Journal of Food Microbiology , 72 : 31 – 43 .
- Schnürer , J. and Magnusson , J. 2005 . Antifungal lactic acid bacteria as biopreservatives . Trends in Food Science & Technology , 16 : 70 – 78 .
- Somasegaran , P. and Hoben , H.J. 1994 . “ Counting rhizobia by a plant infection method ” . In Handbook for Rhizobia , Edited by: Somasegaran , P. and Hoben , H.J. 58 – 77 . New York, , USA : Springer-Verlag .
- Sorvig , E. , Skaugen , M. , Naterstad , K. , Eijsink , V.G.H. and Axelsson , L. 2005 . Plasmid p256 from Lactobacillus plantarum represents a new type of replicon in lactic acid bacteria and contains a toxin-antitoxin-like plasmid maintenance system . Microbiology , 151 : 421 – 431 .
- Statsoft, Inc . 2005 . Statistica 7.0 copyright 1984–2005 . Tulsa, OK, USA , 716 .
- Ström , K. , Sjögren , J. , Broberg , A. and Schnürer , J. 2002 . Lactobacillus plantarum MiLAB393 produces the antifungal cyclic dipeptides cyclo(L-Phe_L-Pro) and cyclo(L-Phe_trans-4-OH-L-Pro) and 3-Phenyllactic acid . Applied and Environmental Microbiology , 68 : 4322 – 4327 .
- Weissmann , R. and Gerhardson , B. 2001 . Selective plant growth suppression by shoot application of soil bacteria . Plant and Soil , 234 : 159 – 170 .
- Wessels , S. , Axelsson , L. , Bech Hansen , E. , De Vuyst , L. , Laulund , S. , Lahteenmaki , L. , Lindgren , S. , Mollet , B. , Salminen , S. and von Wright , A. 2004 . The lactic acid bacteria, the food chain, and their regulation . Trends in Food Science & Technology , 15 : 498 – 505 .
- Yanagida , F. , Chen , Y.-S. and Shinohara , T. 2005 . Isolation and characterization of lactic acid bacteria from soils in vineyards . Journal of General and Applied Microbiology , 51 : 313 – 318 .
- Yanagida , F. , Chen , Y.-S. and Shinohara , T. 2006 . Searching for bacteriocin-producing lactic acid bacteria in soil . Journal of General and Applied Microbiology , 52 : 21 – 28 .
- Young , S.L. 2004 . Natural product herbicides for control of annual vegetation along roadsides . Weed Technology , 18 : 580 – 587 .
- Åström , B. and Gerhardson , B. 1988 . Differential reactions of wheat and pea genotypes to root inoculation with growth-affecting rhizosphere bacteria . Plant and Soil , 109 : 263 – 269 .