Abstract
Nitrogen management is a major concern in high-yielding cotton (Gossypium hirsutum L.)-production systems. Objectives of this study were to investigate cotton leaf photosynthesis, plant growth, canopy spectral reflectance, and lint-yield responses to N application rate and to determine plant N uptake, partitioning, and use efficiency in Mississippi Delta, USA. Treatments included four N rates of 0, 56, 112, and 168 kg N ha−1. Increased N rate significantly affected cotton leaf photosynthetic rate, leaf area index, N concentrations of leaves and fruit, plant N uptake, and N-use efficiency, but had less effect on N partitioning among the plant tissues. Lint-yield response to N rate depended on soil N level and experimental year. Cotton plant shoot N concentration and N uptake significantly and linearly correlated with the selected ratios (R 715/R 405, r 2=0.65*** and R 795/R 755, r 2=0.70***, respectively) of canopy reflectance measured during the squaring and fruiting of plant development. Proper management of N application in cotton based on soil N analysis and plant N status could improve cotton N-use efficiency and lint yield. Remote-sensing algorithms, developed from canopy reflectance ratios in this study, may be used to estimate N concentration in cotton shoots and plant N uptake and help producers make cotton N-management decisions during the growing season.
Introduction
The indeterminate growth habit of cotton (Gossypium hirsutum L.) dramatically affects its response to nitrogen (N) fertilizer supply (Reddy et al., Citation1997). Therefore, N management is an important practice in high-yielding cotton-production systems (Gerik et al., Citation1998). Studies have shown both N deficiency and excess of N supply can negatively affect cotton plant growth and development, boll retention, and lint yield (Gerik et al., Citation1998; Reddy et al., Citation2004). Insufficient N supply often affects several facets of cotton growth and developmental processes, resulting in a reduced leaf area index (LAI) (Fernandez et al., Citation1996; Zhao & Oosterhuis, Citation2000). In addition, insufficient N leads to low leaf chlorophyll concentration, photosynthetic rate, and biomass production (Zhao & Oosterhuis, Citation2000), resulting in reduced lint yield and poor fibre quality (Heagle et al., Citation1999; Reddy et al., Citation2004). However, the relationship between lint yield of irrigated cotton and N-application rate is not always linear in commercial cotton production (Wood et al., Citation1992; Chua et al., Citation2003). When N fertilizer rate reaches a given amount, a further increase in N application may not increase, and may potentially decrease, cotton lint yield (Hutmacher et al., Citation2004) due to excessive vegetative growth and thus result in increased fruit abscission. Excessive N application increases not only input cost for fertilizers, pesticides, plant-growth regulators, and defoliation chemicals (Roberts et al., Citation1996), but also the potential for groundwater contamination (Jaynes et al., Citation2001). Therefore, more accurate application of N fertilizer based on plant N requirement, soil type, and soil N availability to plants can improve cotton yield, increase N-use efficiency (NUE), reduce farm inputs, increase economic net return of agricultural systems, and minimize the negative impacts of N losses on the environment.
The increase in cotton lint yield in recent years has been attributed to increased photo-assimilate partitioning to reproductive organs rather than to vegetative organs (Pettigrew & Gerik, Citation2007). Nutrients, especially N, play a vital role in directing carbohydrates partitioning to reproductive organs. Greater partitioning of nutrients during later reproductive growth (from boll development to boll opening) was related to higher yields and improved fibre quality of G. hirsutum and G. barbadense L. as compared with G. arborium L. (Wahid et al., Citation2003). Nitrogen partitioning to various organs also plays an essential role in determining crop growth and yield. Nitrogen deficiency decreases shoot growth and results in decreased shoot:root ratio due to root-growth enhancement (Fernandez et al., Citation1996).
The timing, amount, and method of N supply also play a major role in determining N partitioning to various organs in cotton. Partitioning of N by cotton plants varies with N input and irrigation method (Janat, Citation2004). Although Boquet and Breitenbeck (Citation2000) reported that maximum N uptake in upland cotton was between 49 and 71 days after planting, little is known about cotton biomass N partitioning, fertilizer N recovery, and NUE in Mississippi Delta. Better understanding of fertilizer N recovery and N partitioning among plant tissues would help growers improve cotton N management and profits.
Studies have demonstrated that remote sensing is a powerful tool to detect crop growth, environmental stresses, and yields. Leaf- or canopy-reflectance measurements in cotton can be used to assess plant N status (Fridgen & Varco, Citation2004; Zhao et al., Citation2005 ,Citation2007). Nitrogen deficiency causes a decrease in leaf chlorophyll content, resulting in an increase in leaf reflectance around 550- and/or 710-nm spectrum (Buscaglia & Varco, Citation2002; Carter & Estep, Citation2002; Zhao et al., Citation2005). Buscaglia and Varco (Citation2002) and Zhao et al. (Citation2005) investigated the relationships between cotton leaf N concentration and leaf reflectance or reflectance ratios. They found that some typical reflectance ratios could be used to estimate cotton leaf N concentration. However, it is unclear if within-season N uptake by cotton correlates with canopy reflectance or reflectance ratios.
A 2-year field experiment was conducted with irrigated cotton in the mid-south region of the USA to better understand cotton plant N uptake and partitioning, NUE, and canopy reflectance as affected by N-application rate. The specific objectives of this study were to determine (i) the effects of N fertilizer rate on N concentrations and N accumulation in leaves, stems, and fruits, (ii) plant NUE, and (iii) the relationships between canopy reflectance ratios and shoot N concentration or accumulated N in above-ground tissues.
Materials and methods
Experimental conditions and treatments
Experiment was conducted on a fine, smectitic, nonacid, thermic Vertic Epiaquept (Leeper silt clay loam) soil at the R.R. Foil Plant Science Research Center, Mississippi State University, Mississippi State, MS. Seeds of cotton cv. NUCOTN 33B, a mid-maturity cultivar, were sown on 14 May 2001 and 24 May 2002. Soil N, P, K, and organic matter (OM) contents were determined in the top 50 cm soil depth prior to cotton seeding in both years at the Mississippi State University Soil Testing Laboratory. Rows were spaced 1 m apart and oriented in an east-west direction. Seedlings were thinned to a density of nine plants per metre row at the second true-leaf stage. Dates of first square (FS) and first flower (FF) stages were on 24 June [41 days after sowing (DAS)] and 17 July (64 DAS), respectively, in 2001; and 28 June (35 DAS) and 19 July (56 DAS), respectively, in 2002.
The experiment was conducted in two adjacent fields with previous cotton crop to eliminate the effects of the first year's fertilization on the second-year N treatments. Four N-rate treatments were (1) 0 N applied during the growing seasons (0-N); (2) 56 kg N ha−1 applied at the 2nd true leaf stage (56-N); (3) 112 kg N ha−1 split (56 and 56 kg N ha−1) applied at the 2nd true leaf stage and at the FS stage (112-N); and (4) 168 kg N ha−1 split applied as 56 kg N ha−1 at the 2nd true leaf stage and 112 kg N ha−1 at the FS stage (168-N). Liquid N fertilizer of N solution and suspensions, containing 32% N (NSOL, Mississippi Chem. Corp., Yazoo City, MS), was injected beside each row. The experiment was designed as a randomized complete block with five (2001) or six (2002) replications. Plot size was 8 m wide by 15 m long with eight rows per plot.
During the experiment, annual accumulated heat units in 2001 (1778 °C day) and in 2002 (1900 °C day) at the experimental location were higher than the long-term average (1634 °C day) over a 28 year period (1973–2000), and total annual precipitation was slightly higher (6%) than the long-term average of 1444 mm (). Furrow irrigation was applied mid-season (June to August) based on regional cotton production recommendations to avoid water-deficit effects.
Table I. Monthly accumulated heat units, total monthly precipitation, and long-term (1973–2000) means at the experimental site, Mississippi State, Mississippi in 2001 and 2002.
Measurements
A LI-6400 portable photosynthesis system (LI-COR Inc., Lincoln, NE) was used to measure leaf net photosynthetic rate (Pn) on five uppermost, fully expanded mainstem leaves from five plants in the first three replicates at different growth stages between 1000 and 1200 h central DST (Daylight Saving Time). When measuring leaf Pn, the photosynthetically active radiation (PAR), provided by a 6400-02 LED light source, was set to 1500 mol m−2 s−1, temperature inside the leaf cuvette was set to 30 °C, relative humidity was adjusted to near ambient level (60 to 70%), and leaf chamber CO2 concentration was set to 360 mol mol−1.
During the growing season, all the plants in a 1 m section of row from each plot either from row 2 or row 7 were harvested at five sampling dates, corresponding to 28, 43, 52, 67, and 84 DAS in 2001 and at 34, 45, 52, 69, and 83 DAS in 2002. Plants were immediately transported to the laboratory and separated into leaves, stems (stem + petioles), and fruits (squares + flowers + bolls). After measurements of leaf area using a LI-3100 area meter (LI-COR Inc., Lincoln, NE), the separated plant parts were dried in a forced air oven at 70oC for 72 h, weighed, and ground for analysis of N concentrations (Nelson & Sommers, Citation1972). Plant accumulated N was estimated using the sum of the tissue N concentration multiplying their dry matter of various plant parts. Finally, when all bolls were opened, seedcotton in four middle rows of each plot was harvested mechanically. A sub-sample of 500 gram seedcotton from each plot was ginned to calculate lint percentage (i.e., lint weight ÷ seedcotton weight ×100). Lint yield was determined according to seedcotton yield obtained from mechanical harvest and lint percentage.
Canopy reflectance was measured within one day of the sampling date for plant-growth analysis. Reflectance data were collected between 1100 and 1300 h DST from all plots using a portable ASD FieldSpec FR spectroradiometer (Analytical Spectral Devices Inc., Boulder, CO). Three hyperspectral measurements were made at wavelengths ranging from 350 to 2500 nm with a 1 nm sampling interval. When collecting canopy spectral reflectance data, the distance between the optical head of the spectroradiometer and the plant canopy was 2 m and the optical head was held perpendicular to land surface throughout the season. After optimization of the ASD instrument, a Spectralon white panel (Labsphere, Inc., Sutton, NH) was used to obtain reference signal prior to taking three canopy-reflectance measurements from each plot. The canopy reflectance was computed as the ratio of canopy radiance to the radiance from the white reference panel. Reflectance values in three wavelength ranges (i.e., 350–399, 1350–1449, and 1750–1969 nm) were omitted from data analysis due to erratic data because of atmospheric water absorption.
The three spectral reflectance measurements in each plot at each measuring date were averaged and mean values used in data analysis. Reflectance data were averaged over 10 nm intervals to reduce the amount of data for analysis. Because reflectance values measured at the seedling stage (28 DAS in 2001 and 34 DAS in 2002) were mainly attributed to the amount of bare soil, these data were omitted when determining relationships between plant N and canopy reflectance. Except for the first measurement date, data of canopy reflectance as well as above-ground tissue N concentration and N accumulation in the other four sampling dates were pooled across years, N-rate treatments, and measuring dates. According to Zhao et al. (Citation2005), coefficients of determination (r 2) were calculated and used to evaluate linear relationships of entire tissue N concentrations and amount of plant shoot-accumulated N with reflectance at 10 nm wavebands. The reflectance values at 405 and 795 nm (R 405 and R 795), having greatest r 2-values with N concentration and the accumulated N, respectively, were used as the numerators, and the reflectance values at all other wavebands (R i ) as denominators in order to calculate reflectance ratios (R 405/R i and R 795/R i ), and the r 2-values of the reflectance ratios with the tissue N concentration and accumulated N were further determined. The best reflectance ratios, which had the largest r 2-value with plant N concentration or amount of accumulated N, were selected using the methods of Zhao et al. (Citation2005). Linear regression of the reflectance ratios and tissue N concentration or plant accumulated N was employed to develop remote-sensing algorithms.
To estimate plant N uptake and NUE, values for plant accumulated N, fertilizer N uptake, fertilizer N recovery, and fertilizer NUE were calculated based on tissue N content and total dry matter at the last sampling date (i.e., 82 DAS in 2001 and 84 DAS in 2002) and lint yield at the final harvest. More specifically, these N parameters were estimated using the following formulae:
Data analysis
Data were analysed using SAS MIXED procedures and Fisher Least Significant Difference (LSD) tests at P=0.05 probability level (SAS Institute, Citation2002) were employed to distinguish among treatments for lint yield, plant accumulated N, fertilizer N uptake and recovery, and fertilizer NUE. Correlation analysis was used to select optimum reflectance ratios that have greatest r 2-value with tissue N concentration or plant accumulated N. Data of the N concentration and accumulated N were plotted against the corresponding reflectance ratios, and linear regression analyses were also carried out.
Results
Leaf photosynthetic rate
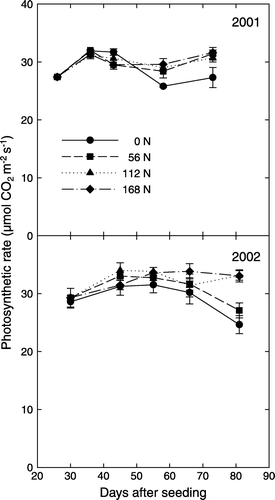
Cotton leaf Pn was low in early growth stage (before FS), reached maximum between FS and FF stages, and then levelled off or declined as plants aged (). In 2001, the 56- and 112-N treatments did not differ from the 168-N treated plants in leaf Pn throughout the growing season. Starting from 50 DAS, leaf Pn of the 0-N-treated plants was significantly less than that of other treatments. At 73 DAS, the 0-N-treated plants had a 15% less leaf Pn (27 mol m−2 s−1) compared with the mean of the other three treatments (31 mol m−2 s−1). In 2002, both the 0-N and 56-N treatments had much less leaf Pn than did the 168-N treatment during flowering and fruiting (). At 81 DAS, leaf Pn of the 168-N-treated plants was 33 mol m−2 s−1; while leaf Pn of the 0-N- and 56-N-treated plants were 25 and 27 mol m−2 s−1, respectively.
Above-ground biomass
Above-ground biomass (ABM) of cotton plants was low before square stage and then increased rapidly during squaring and fruiting (). Plant ABM did not differ among the N treatments before the FF. The 56-N and 112-N treatments did not differ from the 168-N treatment in ABM at any sampling date, but the 0-N treatment had significantly (P<0.05) less ABM than did the 168-N treatment after the FF. On the final sampling date, the 0-N treatment had 20% less ABM in 2001 and 41% less ABM in 2002, as compared with the 168-N treatment ().
Table II. Above-ground biomass accumulation (kg ha−1) of cotton plants during squaring and fruiting as affected by the four N fertilizer treatments of 0, 56, 112, and 168 kg N ha−1 in 2001 and 2002. Each value is the mean of five (2001) or six (2002) replications.
Tissue N concentration
Nitrogen concentration differed greatly among leaves, stems, and fruits (). Leaf N concentration was highly affected by year, plant-growth stage, and N fertilizer rate. Leaf N differences among the N treatments were somewhat greater in 2002 than in 2001. Leaf N level was typically low in seedling stage, reached a maximum between 40 and 50 DAS, and then slowly declined as the plants matured (). The 168-N treatment in 2001 had significantly greater leaf N concentration than did other N treatments.
Figure 2. Nitrogen concentrations of leaves (dashed lines), stems (solid lines), and fruits (dotted lines) during plant growth as affected by N-application-rate treatments of 0 N (circle), 56 N (square), 112 N (triangle), and 168 N (diamond).
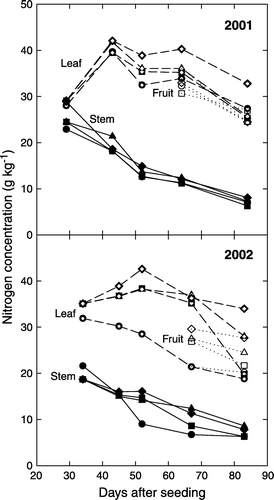
Stem N concentrations were less than the leaf N concentrations and the values declined linearly throughout the growing season. Fruit N concentration was comparable to leaf N; however, the differences in fruit N concentration among the N rate treatments were smaller than in leaf N ().
N accumulation
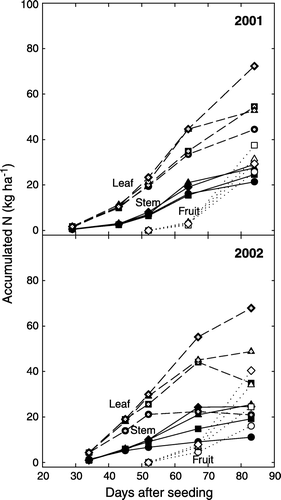
From FS through peak flower stage (35–67 DAS), N accumulation in leaves and stems increased rapidly and leaves were a major N sink among the plant tissues. Starting from 70 DAS, N accumulation in fruits rapidly increased (). Around 85 DAS, fruit accumulated more N than did stems. The 0-N treatment had a 24% reduction in LAI (data not shown), but only a 16% reduction in leaf N concentration as compared with the 168-N treatment at 84 DAS in 2001. In 2002, LAI and leaf N concentration of the 0-N treatment decreased 53 and 45%, respectively, compared with the 168-N treatment at 83 DAS. The maximum shoot N-accumulation rates occurred between 65 and 84 DAS for the 56-, 112-, and 168-N treatment and were 2.1, 2.5, and 3.0 kg ha−1 d−1, respectively.
Relationships between plant N concentration/accumulation and canopy reflectance ratios
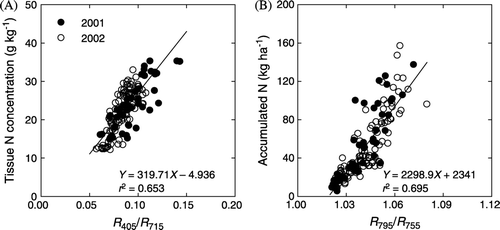
When calculating correlations of the N concentration and N accumulation with the reflectance values at each waveband across years, N treatments, and measurement dates (n=176), we found significant correlation between cotton canopy reflectance at 405 nm and shoot N concentration (r 2 =0.52***) and reflectance at 795 nm and amount of accumulated N in the above-ground tissues (r 2=0.35***). Correlation analysis of reflectance ratio data sets with the N parameters found shoot N concentration and accumulated N were highly related to R 405/R 715 (r 2=0.65***) and R 795/R 755 (r 2=0.70***), respectively (). The reflectance ratio provided further improvement in linear relationships between canopy reflectance and either the tissue N concentration or accumulated N, as compared with single-waveband reflectance.
Figure 4. Relationships between (A) N concentration of above-ground plant tissues and ratio of canopy reflectance at 715 and 405 nm (R 715/R 405) and (B) accumulated N and reflectance ratio of R 795/R 755. Linear regression equations are based on data pooled across years, N treatments, and measuring dates (P<0.001, n=176).
Plant N partitioning
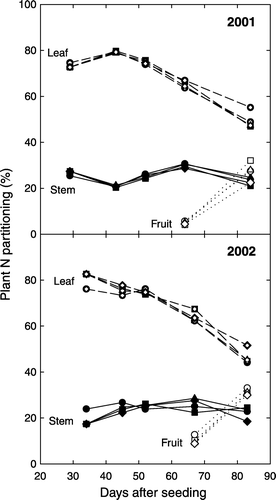
Nitrogen rate had little effect on N partitioning in plant tissues (). Leaves were a major N sink from 30 to 84 DAS and leaf N declined as plants aged (). At the FS stage, about 80% of total plant N was partitioned to leaves, while leaf N was between 46 and 50% of total accumulated N in ABM at 83 DAS. In contrast, stem N changed little during plant growth, ranging from 20 to 30% of total accumulated N in above-ground tissues in both years. Fruit N rapidly increased as plant growth progressed. At 67 (2001) to 69 (2002) DAS, fruit N accounted for only 5 to 10% of total accumulated N of all the tissues; however, the fruit N fraction reached 28–31% about three weeks after FF (83 DAS), averaged across the N treatments (). Therefore, fruit is the major N sink during boll development. Fruit development requires more N, resulting in a rapid decline in leaf N concentration ().
Lint yield, fertilizer N recovery, and nitrogen-use efficiency (NUE)
Overall, both year and N rate significantly (P<0.01) affected lint yield and the interactive effect of year×N rate on yield also was significant (P<0.001) (). Year significantly affected accumulated N (P<0.05), N uptake (P<0.01), and fertilizer N recovery (P<0.05), but did not affect fertilizer NUE. Nitrogen-application rate had significant effects on the accumulated N (P<0.001), N fertilizer uptake (P<0.05), and fertilizer NUE (P<0.001), but not fertilizer N recovery. There were no interactions between year and N rate in any of these variables.
Table III. Significance (P-values) of each source of variation for cotton lint yield (kg ha−1), above-ground tissue accumulated N (kg ha−1), fertilizer N uptake (kg N ha−1) at 84 days after seeding, fertilizer N recovery (%), and fertilizer N-use efficiency (NUE) (kg lint kg−1 N application).
When comparisons were made among N treatments within each year, lint yield did not differ in 2001 (). However, in 2002 the 0-N and 56-N treatments had significantly (P<0.05) lower lint yield than did the 168-N treatment and lint yield did not differ significantly between the 112-N and the 168-N treatments. These results suggest that 112 kg N ha−1 was optimal for lint yield in the present study. The difference in lint-yield response to N rate between the two experimental years was probably associated with soil N level and organic matter because soil P and K were similar in 2001 and 2002 (data not shown). Soil NO3-N contents in the top 50 cm soil depth at cotton planting was 15 mg kg−1 in 2001 and 11 mg kg−1 in 2002. Plant-accumulated N and fertilizer N uptake at 83 DAS increased with the increase in N rate, while the effect of N rate on fertilizer N recovery did not differ significantly across N rates. Fertilizer NUE, defined as lint yield divided by the amount of fertilizer N application, decreased sharply as N rate increased. Averaged across years, the fertilizer NUE of the 56-, 112-, and 168-N treatments was 23, 13, and 9 kg lint kg−1 N, respectively ().
Table IV. Cotton lint yield, above-ground tissue accumulated N, fertilizer N uptake 83 days after seeding, fertilizer N recovery, and fertilizer N-use efficiency (NUE) as affected by N-application rate and year. Data are means±S.D. of four (2001) or five (2002) replications.
Discussion
The present study found no difference among the N treatments in ABM production during cotton early growth, but low N rate significantly reduced ABM during the latter part of the growing season (). Less ABM of the low-N-treated plants during fruiting probably resulted from reductions in LAI (Zhao et al., Citation2007) and leaf photosynthesis rate (). Studies have shown that N is directly or indirectly involved in leaf photosynthesis (Zhao & Oosterhuis, Citation2000; Milroy & Bange, Citation2003). The response of cotton leaf photosynthesis to leaf N status has been reported by Milroy and Bange (Citation2003). The overall shape of the relationship between Pn and N concentration of individual leaves can be described by an exponential rise to a maximum when leaf N reaches a certain level (Milroy & Bange, Citation2003). Similar to other field crops (Ding et al., Citation2005), cotton bolls were a dominant N sink during fruit development, and decreased cotton leaf Pn during reproductive growth under N limitation in the present study was related to increased N partitioned to fruits ().
In the present study, the seasonal pattern of N concentration in leaves collected from the entire plant canopy as affected by N rate is consistent with that in the uppermost, fully expanded leaves reported by Zhao et al. (Citation2005). Leaf N concentration was most highly dependent on both plant-growth stage and N fertilizer rate (). In contrast, changes in stem N concentrations among the N treatments were much less than leaf N concentrations. During fruit development, fruit N concentration rapidly increased, while leaf and stem N concentrations declined (), indicating fruits were a strong N sink during fruit development of cotton. Unruh and Silvertooth (Citation1996) similarly reported that leaves were a major N sink until 1500 heat units were accumulated, after which the developing fruits were a major sink for N.
Boquet and Breitenbeck (Citation2000) reported that cotton maximum N uptake occurred between 49 and 71 DAS and cotton receiving 84 and 168 kg N ha−1 had N-uptake rates of 2.9 and 4.3 kg ha−1 d−1, respectively. In the present study, we found maximum shoot N accumulation (i.e., N uptake) rates occurred later in the season, between FF (65 DAS) and 3 weeks after FF (84 DAS), and the maximum N-uptake rates for the 56-N, 112-N, and 168-N treatments were 2.1, 2.5, and 3.0 kg ha−1 d−1, respectively. The differences of our results from the report of Boquet and Breitenbeck (Citation2000) in cotton peak N-uptake time and uptake rate may be associated with differences in soil properties, crop genotype, or experimental location. Both studies indicate that cotton plant N uptake is closely associated with N rate.
Nitrogen rate mainly influenced N accumulations in leaves and fruits; however, N rate had little effect on stem N accumulation (). Less leaf N accumulation for the low-N treatment was strongly associated with both reduced LAI (Zhao et al., Citation2007) and low leaf N concentration (). For example, the 0-N treatment had a 24% reduction in LAI (data not shown), but only 16% less leaf N concentration than in the 168-N treatment at 84 DAS in 2001. At 83 DAS in 2002, LAI and leaf N concentration of the 0-N treatment decreased by 53 and 45%, respectively, compared with the 168-N treatment. Cotton plant N accumulation and the amount of N required to produce a given amount of lint has varied in different studies. Halevy and Kramer (Citation1986) reported that total N accumulation in above-ground plant tissues ranged from 10 to 12 kg N per 100 kg lint under moderate yields of 1200–1500 kg lint ha−1. Mullins and Burmester (Citation1990) concluded that cotton varieties commonly grown in the southeastern USA remove an average of 18 kg N per100 kg lint. More recently, Unruh and Silvertooth (Citation1996) observed an average of 12 kg N per 100 kg lint, with 205 kg N ha−1 accumulated in above-ground plant tissues. Our results indicate that N accumulation in above-ground plant tissues at physiological cutout stage (82–84 DAS) was lower than that in previous studies reported by Mullins and Burmester (Citation1990) and Unruh and Silvertooth (Citation1996). In the present study, cotton plant N uptake ranged from 110 to 130 kg N ha−1 for the lint yield of 1400–1500 kg ha−1, equivalent to 7.9–8.7 kg N per 100 kg lint (). Therefore, there is the potential to improve NUE in model cotton-production system. Great variation and lack of difference among the N treatments in fertilizer recovery suggest that the cultivar used in the current study may be unable to utilize the additional available N in the soil for growth and yield. This may be attributed to the low N requirement of this genotype or limited root density for extracting N from soils.
Janat (Citation2004) used urea as N source and reported surface-irrigated cotton at physiological maturity partitioned 57% of accumulated N in the fruiting forms, 34% in the leaves, and 9% in the stems. We found that at three weeks after FF, the percentages of accumulated N in fruit, leaves, and stems were approximately 30, 47, and 23%, respectively. The final partitioning of N recorded by Janat (Citation2004) to various plant components was different from that recorded in the current study. This may be attributed to the differences in source, method of N supply, method of tissue sampling, and specific sampling date. Our last sampling date was three weeks after FF rather than maturity to avoid leaf-abscission effects on the measured variables. The partitioning of nutrients and photosynthate to developing fruit has a large effect on the amount and duration of vegetative growth in cotton (Gerik et al., Citation1994).
Previous studies have documented a linear relationship between cotton leaf N concentration and leaf/canopy reflectance ratios (Zhao et al., Citation2005), but it is unclear if canopy reflectance ratios can be used to estimate total shoot N concentration and N uptake. Our results indicate that shoot N concentration and accumulated N were highly correlated with R 405/R 715 and R 795/R 755, respectively (). Therefore, cotton shoot N concentrations and accumulated N (or N uptake) during growth may be quickly and nondestructively estimated using the remote-sensing algorithms (i.e., canopy reflectance ratios calibrations) developed in this study.
Acknowledgements
This research was funded in part by the National Aeronautical and Space Administration through the GeoResources Institute at Mississippi State University, MS and USDA-UV-B monitoring Program at Colorado State University, Fort Collins, CO. We express our appreciation to D. Brand (Senior Research Associate), K. Gourley (Biological Science Technician), A.R. Mohammed (Graduate Assistant) and S. Koti for technical support and Drs Darrin Dodds, Frank Matta, and Jack McCarty for helpful suggestions. Contribution from the Department of Plant and Soil Sciences, Mississippi State University, Mississippi Agricultural and Forestry Experiment Station, paper no. J-11331. Mention of specific trade or product names does not imply endorsement or preferential treatment by the United States Department of Agriculture to the exclusion of any other product that may be suitable.
References
- Boquet , D.J. and Breitenbeck , G.A. 2000 . Nitrogen rate effect on partitioning of nitrogen and dry matter by cotton . Crop Science , 40 : 1685 – 1693 .
- Buscaglia , H.J. and Varco , J.J. 2002 . Early detection of cotton leaf nitrogen status using leaf reflectance . Journal of Plant Nutrition , 25 : 2067 – 2080 .
- Carter , G.A. and Estep , L. 2002 . “ General spectral characteristics of leaf reflectance responses to plant stress and their manifestation at the landscape scale ” . In From laboratory spectroscopy to remotely sensed spectra of terrestrial ecosystems , Edited by: Muttiah , R.S. 271 – 293 . Dordrecht, the Netherlands : Kluwer Academic Publishers .
- Chua , T.T. , Bronson , K.F. , Booker , J.D. , Keeling , J.W. , Mosier , A.R. , Bordovsky , J.P. , Lascano , R.J. , Green , C.J. and Segarra , E. 2003 . In-season nitrogen status sensing in irrigated cotton. I. Yields and nitrogen-15 recovery . Soil Science Society of America Journal , 67 : 1428 – 1438 .
- Ding , L. , Wang , K.J. , Jiang , G.M. , Biswas , D.K. , Xu , H. , Li , L.F. and Li , Y.H. 2005 . Effect of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years . Annals of Botany , 96 : 925 – 930 .
- Fernandez , C.J. , McInnes , K.J. and Cothren , J.T. 1996 . Water status and leaf area production in water- and nitrogen-stressed cotton . Crop Science , 36 : 1224 – 1233 .
- Fridgen , J.L. and Varco , J.J. 2004 . Dependency of cotton leaf nitrogen, chlorophyll, and reflectance on nitrogen and potassium availability . Agronomy Journal , 96 : 63 – 69 .
- Gerik , T.J. , Jackson , B.S. , Stockle , C.O. and Rosenthal , W.D. 1994 . Plant nitrogen status and boll load of cotton . Agronomy Journal , 86 : 514 – 518 .
- Gerik , T.J. , Oosterhuis , D.M. and Torbert , H.A. 1998 . Managing cotton nitrogen supply . Advances in Agronomy , 64 : 115 – 147 .
- Halevy , J. and Kramer , O. 1986 . Nitrogen fertilizer management of cotton grown under drip irrigation in a grumusol . Irrigation Science , 7 : 63 – 72 .
- Heagle , A.S. , Miller , J.E. , Booker , F.L. and Pursley , W.A. 1999 . Ozone stress, carbon dioxide enrichment, and nitrogen fertility interactions in cotton . Crop Science , 39 : 731 – 741 .
- Hutmacher , R.B. , Travis , R.L. , Rains , D.W. , Vargas , R.N. , Roberts , B.A. , Weir , B.L. , Wright , S.D. , Munk , D.S. , Marsh , B.H. , Keeley , M.P. , Fritschi , F.B. , Munier , D.J. , Nichols , R.L. and Delgado , R. 2004 . Response of recent Acala cotton varieties to variable nitrogen rates in the San Joaquin Valley of California . Agronomy Journal , 96 : 48 – 62 .
- Janat , M. 2004 . Assessment of nitrogen content, uptake partitioning and recovery by cotton crop grown under surface irrigation and drip fertigation by using isotopic technique . Communications in Soil Science and Plant Analysis , 35 : 2515 – 2535 .
- Jaynes , D.B. , Colvin , T.S. , Karlen , D.L. , Cambardella , C.A. and Meek , D.W. 2001 . Nitrate loss in subsurface drainage as affected by nitrogen fertilizer rate . Journal of Environmental Quality , 30 : 1305 – 1314 .
- Milroy , S.P. and Bange , M.P. 2003 . Nitrogen and light responses of cotton photosynthesis and implications for crop growth . Crop Science , 43 : 904 – 913 .
- Mullins , G.L. and Burmester , C.H. 1990 . Dry matter, nitrogen, phosphorus and potassium accumulation by four cotton varieties . Agronomy Journal , 82 : 729 – 736 .
- Nelson , D.W. and Sommers , L.E. 1972 . A simple digestion procedure for estimation of total nitrogen in soil and sediments . Journal of Environmental Quality , 1 : 423 – 425 .
- Pettigrew , T.W. and Gerik , T.J. 2007 . Cotton leaf photosynthesis and carbon metabolism . Advances in Agronomy , 94 : 209 – 236 .
- Reddy , K.R. , Hodges , H.F. and McKinion , J.M. 1997 . Crop modeling and applications: A cotton example . Advances in Agronomy , 59 : 225 – 290 .
- Reddy , K.R. , Koti , S. , Davidonis , G.H. and Reddy , V.R. 2004 . Interactive effects of carbon dioxide and nitrogen nutrition on cotton growth, development, yield, and fiber quality . Agronomy Journal , 96 : 1148 – 1157 .
- Roberts , B.A. , Curley , R.G. , Kerby , T.A. Wright , S.D. ,& Mayfield , W.D. 1996 . Defoliation, harvest and ginning 305 323 . In S.J. Hake , T.A. Kerby , & K.D. Hake , Cotton production manual . Publication 3352 . University of California, Division of Agriculture and Natural Resources , Oakland, CA, , USA .
- SAS Institute 2002 . SAS User Guide: Statistics . SAS Institute Inc., Cary, NC, USA .
- Unruh , B.L. and Silvertooth , J.L. 1996 . Comparisons between an Upland and a Pima cotton cultivar: II. Nutrient uptake and partitioning . Agronomy Journal , 88 : 589 – 595 .
- Wahid , A. , Bukhari , S. and Rasul , E. 2003 . Inter-specific differences in cotton for nutrient partitioning from subtending leaves to reproductive parts at various developmental stages: consequences for fruit growth and yield . Biologia Plantarum , 47 : 379 – 385 .
- Wood , C.W. , Tracy , P.W. , Reeves , D.W. and Edmisten , K.L. 1992 . Determination of cotton nitrogen status with a hand-held chlorophyll meter . Journal of Plant Nutrition , 15 : 1435 – 1448 .
- Zhao , D. Oosterhuis , D.M. 2000 . Nitrogen application effect on leaf photosynthesis, nonstructural carbohydrate concentrations and yield of field-grown cotton . In D.M. Oosterhuis Proceedings of the 2000 Arkansas Cotton Research Meeting . AAES Special Report 198 , 69 71 .
- Zhao , D. , Reddy , K.R. , Kakani , V.G. , Read , J.J. and Koti , S. 2005 . Selection of optimum reflectance ratios for estimating leaf nitrogen and chlorophyll concentrations of field-grown cotton . Agronomy Journal , 97 : 89 – 98 .
- Zhao , D. , Reddy , K.R. , Kakani , V.G. , Read , J.J. and Koti , S. 2007 . Canopy reflectance in cotton for growth assessment and lint yield prediction . European Journal of Agronomy , 26 : 335 – 344 .