Abstract
The discovery of induced pluripotent stem cells (iPSCs) has diversified approaches to studies of human diseases. iPSCs can be used in regenerative medicine and for the analysis of the pathogenesis of hereditary diseases. They can also be applied to research on immune disorders, including the influence of genetic factors on autoimmune diseases in the human system. Some immune cells, such as dendritic cells and macrophages, can be differentiated from iPSCs. Thus, immune disorders caused by defects in the innate immune system can be studied with that approach. We propose that biological mechanisms of genetic risks could be examined by mutating or modifying disease-susceptibility genes in iPSCs by genome editing. Studies using human iPSCs are also expected to elucidate the underlying pathogenesis of immunological diseases and new approaches to drug discovery.
Keywords:
1. Introduction
Clinical immunology focuses on investigations of human immunological diseases. This pursuit often requires intensive analysis of human subjects. However, this approach is relatively difficult compared with the use of experimental animals. As discussed below, the emergence of induced pluripotent stem cells (iPSCs) solves some parts of these problems in human studies and opens a new horizon for disease research in humans. iPSCs are characterized by their enormous proliferation capacity and the capability to differentiate into all cell types. In 1981, Evans and Martin [Citation1,Citation2] established mouse embryonic stem (ES) cells, and Thomson [Citation3] succeeded in establishing human ES cells in 1998. However, since it is necessary to use surplus embryos (pre-implantation embryos) or in vitro fertilized eggs to prepare human ES cells, ethical problems can arise. In 2006, Yamanaka successfully generated pluripotent stem cells by introducing four genes (Oct3 4, Sox2, Klf4, c-Myc) into mouse somatic cells (fibroblasts), naming them iPSCs [Citation4]. He also established iPSCs in humans in 2007 [Citation5]. iPSCs can be maintained in an undifferentiated state with pluripotency, form colonies, and proliferate without limit. Thereafter, the possibility of human research using pluripotent stem cells greatly expanded, and the ethical problems associated with ES cells were largely overcome. Currently, applications for human disease research, drug screening, and regenerative medicine using human iPSCs are being developed.
2. Necessity of human research in clinical immunology
Conventional autoimmune disease research has been conducted by combining research on experimental animal models with limited studies of human samples. For example, experimental animal models of autoimmune disorders include spontaneous disease onset mice (MRL/lpr mice) as a model of systemic lupus erythematosus (SLE) and SKG mice as a model of inflammatory arthritis. Moreover, induced disorders in mice include collagen-induced arthritis and use of topical imiquimod to generate SLE in model mice. There are several advantages to the use of murine models. For example, it is easier to conduct research that is a direct application of basic immunology. Moreover, interventions such as genetic modification make it possible to conduct experiments with clear causality. In addition, it is easy to examine internal and lymphoid organs, and experiments utilizing genetic and environmental backgrounds can be performed. Of course, studies can be repeated at will. The studies of experimental animals often provide important notice of the human diseases and therapies. For example, the importance of T cells in the pathogenesis of myositis and the effectiveness of T cell suppression therapy were reported for myosin light chain- immunized mice, a mouse model of myositis [Citation6]. Based on these observations in mice, T-cell targeted therapy, using abatacept, is currently being conducted against human inflammatory myopathy.
On the other hand, there are many differences between murine and human immune systems, and it has been pointed out that mouse disease models and human autoimmune diseases differ greatly, especially in their etiology. Therefore, research on human autoimmune disorders requires the use of human specimens. Common methodology requires the analysis of specimens (peripheral blood, tissues, etc.) obtained from patients after the onset of the disease followed by comparisons with healthy controls. Although this methodology is comparatively easy to be performed, the results are generally affected by various environmental factors. Furthermore, the specimens are usually obtained at the end stage of disease development, and ongoing disease processes can modify the characteristics of the specimens apart from essential pathological events. Therefore, when the researcher finds a statistically significant difference in factor X between patients and healthy controls, it is difficult to determine whether factor X is actually causative or just a result of ongoing disease.
Human specimens are expected to have many biases due to differences in individuals. A sufficient number of patient-derived specimens are required to obtain robust results, which can be difficult to achieve especially when the disease is rare. Additional problems with human subjects include heterogenous genetic and environmental backgrounds and ethical problems of human live specimen collection (repetitive specimen collection, or invasive specimen collection from internal organs or need for large numbers of cells). These problems pose limitations on human disease research. Genetic research is a promising methodology to solve the above problems. Large-scale genetic studies have made significant progress in demonstrating many causal genetic factors of human disease [Citation7]. On the other hand, further research on the biological effects and pathological roles of these genetic factors is required. Human iPSCs are a promising research tool and enable human disease research using human samples [Citation8].
3. Analysis of immune diseases using human iPSCs
Studies of genetic diseases caused by a single genetic mutation have been conducted using patient-derived iPSCs. In these cases, it is possible to explain the cause of the disease through the action of a single mutated gene. Therefore, the significance of the studies and the use of mutant-iPSCs are clear. For example, recovery of α1 antitrypsin production in iPSC-derived hepatocytes was reported through use of iPSCs derived from α1 antitrypsin-deficient patients after restoring the wild-type gene by genome editing [Citation9]. Chronic granulomatous disease (CGD) is a disease characterized by bacterial infections from an early age. CGD is associated with functional deterioration in neutrophils. Genetically, mutations in NADPH oxidase have been confirmed, suggesting an impairment of the bactericidal action mediated by reactive oxygen species (ROS) in neutrophils. iPSCs generated from CGD patients showed the presence of an impaired oxidative burst in neutrophils. Thus, a key mechanism of CGD pathogenesis was reproduced in an iPSC system. After the genetic mutation was restored to wild-type using genome editing technology, abnormality of neutrophils was improved [Citation10]. In the case of genetic diseases caused by a single genetic mutation, it is possible to analyze the disease pathogenesis by using disease-specific iPSCs. And, these disease-specific iPSC-derived cells can be used to conduct drug screening. The possible advantages of conducting research on human immune diseases by focusing on iPSCs are summarized below.
3.1. Analysis of human specimens
Studies using human cells and tissues are indispensable for the study of human diseases. However, collecting necessary and sufficient samples is often difficult. Human research is often associated with ethical problems. Therefore, in the study of autoimmune diseases, experiments are often limited to obtaining readily available specimens, such as serum and peripheral blood. Studies using tissues biopsy specimens, such as skin and lymph nodes, are also performed to obtain important information regarding immunological responses. On the other hand, it is usually difficult to collect specimens from internal organs, where pathological conditions are developing. Also, sampling and collection of large amounts of specimens may be undesirable because of ethical problems. Using several kinds of cells differentiated from human iPSCs enables repetitive analysis of a large number of human cells. In autoimmune diseases, susceptibility and abnormality in their target organs are also considered to be important research subjects. By using iPSC-derived cells, experiments using tissue-specific cells, such as muscle and kidney, can be performed.
3.2. Experiments with unbiased background
Patient populations are significantly affected by their heterogeneous genetic backgrounds, environmental factors, aging, and treatments. These heterogeneous effects contribute to the difficulty of analyzing human specimens. In addition, it is generally impossible to examine specimens before the onset of symptoms. Experiments using human iPSCs enable investigators to exclude or at least minimize the influences of these factors. In particular, it is possible to investigate the direct biological effects of genetic factors with little or no environmental influences. These benefits allow investigations to reveal the biological function of risk genes of autoimmune diseases identified by genome-wide association study (GWAS).
3.3. Cell differentiation processes
With iPSCs, it is possible to study stages in cell differentiation. In autoimmune diseases, immune cell differentiation is sometimes dysregulated. By observing immune cell differentiation with iPSCs, a cryptic pathologic process could be determined.
3.4. Functional studies of disease-susceptibility genes by genome-edited iPSCs
After identifying a suspect gene in the autoimmune disease process, additional functional analyses are required to characterize the actual roles of the gene in view of its pathogenesis. Biological phenotypes can be studied by analyzing mutant genes in iPSCs. Genome editing with iPSCs is now possible [Citation9,Citation10]. For example, a disease-associated gene mutation can be introduced into iPSCs, and analysis of these cells can reveal the biological mechanisms of genetic susceptibility. With such a strategy, it will be possible to obtain more informative and reliable results than with studies of cell lines.
3.5. Drug discovery
It is possible to culture and differentiate large numbers of iPSCs that can be used for drug screening. By differentiating iPSCs into immune cells, a system reflecting the pathogenesis of autoimmune diseases can be established, and this system could be a promising tool for screening drugs. Identification of novel drug discovery targets is expected in such a system. It could also be applied to drug toxicity testing.
4. iPSC-based study of pathogenesis of immune diseases
Studies of immune diseases primarily focus on immune cells; however, cells from target organs, such as kidney and muscle, can be analyzed. Although iPSCs can be differentiated into various types of cells, including immune cells, the present repertoire of immune cells into which they can be differentiated is limited (see ). Differentiation of iPSCs into dendritic cells and macrophages is possible, and these cells have the capacity to present antigens and produce cytokines [Citation11–14]. Auto-inflammatory diseases are characterized by repetitive or persistent inflammation due to abnormalities of the innate immune system. There are autoinflammatory diseases in which genetic mutations are associated with the regulation of inflammatory cytokine secretions [Citation15]. It is thought that enhancement of IL-1β production via dysregulation of the inflammasome plays an important role in the pathogenesis of autoinflammation. By using disease-specific iPSCs having these genetic features, it is likely that innate immune cells with pathological features can be reproduced. Similar analysis is also possible for diseases such as adult onset Still’s disease, which is a kind of the autoinflammatory diseases.
Table 1. Summary of immune cells derived from IPSCs.
In autoimmune diseases, immune responses by the acquired immune system to autoantigens play an important role in pathogenesis. However, no protocol has yet been established for the differentiation of iPSCs into CD4+T cells and B cells, which play pivotal roles in the acquired immune system. It is speculated that the microenvironments in the thymus and bone marrow are essential for the differentiation and maturation of these cells. However, even in autoimmune disorders, the cells of the innate immune system play a certain role in the pathological condition. For example, in rheumatoid arthritis, inflammatory cytokine production by macrophages is an important pathological mechanism. In SLE, an excessive autoantigen load from dead cells is probably a cause of autoimmunity. Thus, the function of phagocytic cells is a major research focus of studies of SLE. In addition, excessive cytokine production and antigen presentation by dendritic cells are thought to lead to the activation of the acquired immune system in SLE [Citation16]. Therefore, it is likely that some pathological conditions of the autoimmune diseases can be analyzed by using iPSC-derived innate immune cells.
Another important and inevitable question is whether it is possible to study multifactorial diseases using iPSCs. Almost all autoimmune diseases are likely multifactorial. Even though the contribution of various factors differs for each disease, it is thought that a combination of genetic factors and environmental factors precipitates the disease. For example, SLE is a multifactorial disease, and about 50 causal genetic loci have been reported thus far. Interestingly, many of these genes play important roles in immune cells, which suggest a close relationship between genetic and immunological mechanisms in its pathogenesis [Citation16]. The usefulness of iPSCs is being debated in studies of multifactorial diseases because its significance is not as clear as monogenic disease, such as hereditary diseases.
In the studies using human specimens, it could be possible to obtain a robust conclusion by analyzing a large number of specimens. In iPSC research, comparison between patient-derived and healthy control-derived iPSCs could be performed by preparing disease-specific iPSC lines from many patients. Disease-specific iPSC banks for difficult diseases are now being prepared. However, the establishment of iPSCs from many patients is expensive and labor-intensive. In addition, various iPSC lines derived from the same specimens should be considered, as this approach could reduce confounding variables. For example, genome-edited iPSCs could clarify the biological functions of disease-susceptibility genes in autoimmune diseases in the same genetic and environmental background. The causal relationships linking a genetic background and phenotype of autoimmune diseases remain unclear at present; however, by using iPSCs as a research tool, it is possible to analyze causal relationships and to identify novel drug targets.
5. Conclusions
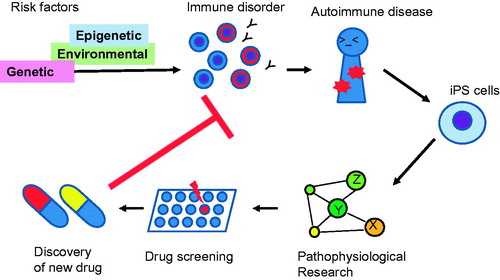
iPSC-based studies of immune diseases are summarized in . It is important to establish a suitable iPSC-based system for analyzing immune disorders, and experimental methods are now under development. iPSC experimental systems based on the causality of the diseases can also be developed into drug discovery and screening systems. Therefore, iPSC research is a promising approach for elucidating disease pathogenesis and discovery of new drugs. The use of iPSCs will open a new horizon for clinical immunology.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156.
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638.
- Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult fibroblasts by defined factors. Cell. 2007;131:861–872.
- Hasegawa H, Kawahata K, Mizoguchi F, et al. Direct suppression of autoaggressive CD8+ T cells with CD80/86 blockade in CD8+ T cell-mediated polymyositis models of mice. Clin Exp Rheumatol. 2017;35:593–597.
- Yamamoto K, Okada Y, Suzuki A, et al. Genetics of rheumatoid arthritis in Asia–present and future. Nat Rev Rheumatol. 2015;11:375–379.
- Natsumoto B, Shoda H, Fujio K, et al. Investigation of the pathogenesis of autoimmune diseases by iPS cells. Jpn J Clin Immun. 2017;40:48–53.
- Yusa K, Rashid ST, Strick-Marchand H, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cell. Nature. 2011;478:391–394.
- Flynn R, Grundmann A, Renz P, et al. CRISPR-mediated genotypic and phenotypic correction of a chronic granulomatous disease mutation in human iPS cells. Exp Hematol. 2015;43:838–848.
- Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34 + CD43 + CD45 progenitors. J Clin Invest. 2009;119:2818–2829.
- Senju S, Haruta M, Matsumura K, et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–883.
- Masakatsu D, Niwa A, Tanaka T, et al. Rubust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions. Pros One. 2013;8:e59243.
- Iizuka-Koga M, Asashima H, Ando M, et al. Functional analysis of dendritic cells generated from t-ipscs from CD4+ T cell clones of Sjögren's syndrome. Stem Cell Rep. 2017;8:1155–1163.
- McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144.
- Bentham J, Morris DL, Cunninghame Graham DS, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47:1457–1464.
- van Wilgenburg B, Browne C, Vowles J, et al. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One. 2013;8:e71098.
- Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res. 2010;156:147–154.
- Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283.
- Kitayama S, Zhang R, Liu T-Y, et al. Cellular adjuvant properties, direct cytotoxicity of re-differentiated Vα24 invariant NKT-like cells from human induced pluripotent stem cells. Stem Cell Rep. 2016;6:213–227.
- Yamada D, Iyoda T, Vizcardo R, et al. Efficient regeneration of human Vα24+ invariant natural killer T cells and their anti-tumor activity in vivo. Stem Cells. 2016;34:2852–2860.
- Nishimra T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126.
- Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Sell. 2013;12:31–36.