Abstract
Osteoporosis is a global public health problem thought to be caused by an imbalance in bone metabolism. We examined in this study the 40% ethanol fraction of HP-20 resin in combination with a hot-water adzuki extract (EtEx.40) for its effect on osteoblast and osteoclast differentiation. EtEx.40-treated murine preosteoblast MC3T3-E1 cells exhibited significantly elevated alkaline phosphatase activity and mineralization. EtEx.40 facilitated osteoblast differentiation by up-regulating such osteoblast differentiation-related molecules as runt-related transcription factor 2, distal-less homeobox 5, and osterix via p38 mitogen-activated protein kinase. EtEx.40 also suppressed the formation of large tartrate-resistant acid phosphatase-positive multinucleated cells in RAW264.7 cells that had been stimulated with the receptor activator of the nuclear factor κB ligand/macrophage colony-stimulating factor. EtEx.40 significantly inhibited NF-κB activation, thus reducing the expression of such downstream molecules as c-Fos and NFATc1. Our findings suggest that EtEx.40 could be used to maintain bone mass.
Graphical Abstract
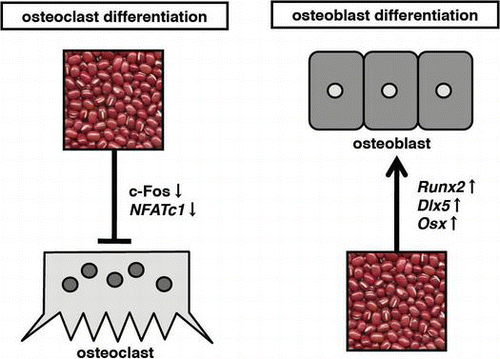
We prepared a 40% ethanol fraction of HP-20 resin treated with a hot water extract of adzuki (EtEx.40). EtEx.40 regulates osteoblast and osteoclast differentiation.
Bone metabolic balance (bone resorption and bone formation) is maintained by osteoclasts and osteoblasts.Citation1) Aging is accompanied by disruption in bone metabolism leading to osteoporosis; the risk is particularly high in women who tend to lose bone after menopause. Aging-associated degeneration of the osteoblast function also reduces bone mass, as does malnutrition or under-nutrition (a deficiency of micronutrients and macronutrients). Osteoporosis is therefore widely recognized as a major global public health problem.
Such anti-osteoporosis drugs as bisphosphonates, ipriflavone, alendronate, and raloxifeneCitation2–Citation4) have been developed, and many chemical components of food regulate bone metabolism.Citation5–Citation7) Nutritional factors are critically important in bone homeostasisCitation8,Citation9) and may offer effective adjuvant therapies for preventing or treating bone loss.
Adzuki beans (Vigna angularis) are mainly produced and consumed in East Asia. Confectioners boil adzuki beans in a cooker; a by-product of this process is a hot-water extract (HWEA). This HWEA contains such chemical compounds as catechins and saponins which are well-known bioactive ingredients.Citation10–Citation13) We have previously reported that the 40% ethanol-eluted fraction (EtEx.40) of HWEA and HP-20 resin possessed antitumor,Citation14–Citation17) antioxidative,Citation18) anti-metastatic,Citation18) and anti-diabetic activities,Citation19,Citation20) lowered the serum cholesterol levelsCitation21) and enhanced melanogenesis.Citation22) EtEx.40 has also suppressed antigen-mediated degranulation in rat basophilic leukemia RBL-2H3 cells.Citation23) The color of HWEA originates from (+)-catechin. Choi et al.Citation24) and Nakamura et al.Citation25) have reported that (+)-catechin and green tea catechin regulated osteoblast and osteoclast differentiation. Since we had previously isolated (+)-catechin in EtEx.40,Citation23), we believed that this extract might also be useful as dietary support for regulating bone metabolism. We demonstrate here that EtEx.40 regulated osteoblast and osteoclast differentiation and explore the mechanisms by which this regulation occurred.
Materials and methods
Preparation of the test materials
Adzuki beans (V. angularis; 2.5 kg) harvested in Tokachi, Hokkaido, Japan, were boiled to obtain HWEA (15 L). This was concentrated 100-fold by a plate heat exchanger (Izumi Food Machinery Co., Hyogo, Japan). Concentrated HWEA (30 mL) was separated by open-column chromatography on Diaion HP-20 (Mitsubishi Chemical Co., Tokyo, Japan) with a column size of 50 φ × 300 mm and a resin wet volume of 400 g. Stepwise elution was performed with 5 L of distilled water respectively followed by 40, 60, and 80% ethanol (WEx, EtEx.40, EtEx.60, and EtEx.80). The fractions were evaporated to dryness in vacuo. Each sample was dissolved in dimethylsulfoxide (DMSO) to a final concentration of 5–50 μg/mL directly prior to alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase (TRAP) assays. With the exception of EtEx.40, the column eluates had only weak effects on osteoblast and osteoclast differentiation (data not shown); we thus focused our study on EtEx.40.
Reagents
Eagle’s α-minimal essential medium (α-MEM), Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Life Technologies (Carlsbad, CA, USA). The LabAssay™ALP kit was obtained from Wako Pure Chemical Industries (Osaka, Japan). The TRAP & ALP assay kit and TRAP & ALP double-stain kit were acquired from Takara Bio (Ohtsu, Japan). Antibodies to p44/42 mitogen-activated protein kinase (MAPK; extracellular signal-regulated kinase, ERK), phospho-p44/42 MAPK (Thr202/Tyr204; p-ERK), p38 MAPK (p38), phospho-p38 MAPK (p-p38), phospho-Smad1/5/8, and the nuclear factor κB (NF-κB) pathway sampler kit were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies to runt-related transcription factor 2 (Runx2) and Osterix (Osx) were obtained from Abcam (Cambridge, MA, USA), and the antibody to distal-less homeobox 5 (Dlx5) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The MC3T3-E1 murine preosteoblast cell line was purchased from RIKEN Cell Bank (Tsukuba, Ibaraki, Japan) and the RAW264.7 murine osteoclast-like cell line, from the American Type Culture Collection (VA, USA). The MC3T3-E1 cells were cultured in phenol red-free α-MEM supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The RAW264.7 cells were cultured in phenol red-free DMEM supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Cell treatment
MC3T3-E1 cells were seeded in 24-well plates (1 × 105 cells/mL, 500 μL/well; Nunc, Roskilde, Denmark) and cultured for 24 h in phenol red-free α-MEM supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. After pre-culturing, the medium was replaced with an osteoblast differentiation medium (ODM) supplemented with 10% FBS, 10 mm β-glycerophosphate (Sigma, St. Louis, USA), 10 mm HEPES (pH 6.8), and L-ascorbic acid in phenol red-free α-MEM. Various concentrations of the extract were added. Spent ODM was replaced with a fresh medium and extract every 3 d. The extracts were diluted with DMSO, and the control wells only received equivalent amounts of the solvent (DMSO).
RAW264.7 cells were seeded in 24-well plates (1 × 105 cells/mL, 500 μL/well; Nunc) and cultured for 24 h in phenol red-free DMEM supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Various concentrations of the extracts were added, and culturing was continued for 2 h. The receptor activator of the NF-κB ligand (RANKL; 50 ng/mL, Oriental Yeast Co., Tokyo, Japan) and the macrophage colony-stimulating factor (M-CSF; 10 ng/mL, BioVision Research Products, Mountain View, CA, USA) were added to induce osteoclast differentiation. The culture medium was replaced every 3 d.
ALP activity and staining
We used ALP activity and staining to measure osteoblast differentiation in the MC3T3-E1 cells cultured for 6 d in ODM and ODM + EtEx.40 (5, 10, and 20 μg/mL). To assess the ALP activity, the treated cells were washed twice with PBS, lysed with 200 μL of a lysis buffer (10 mm Tris–HCl, 1 mm MgCl2, 0.5% Triton-X, adjusted to pH 6.8), and incubated on ice for 5 min. The cell lysate was sonicated for 1 min and centrifuged at 1000 × g and 4 °C for 10 min. ALP activity was measured by the spectrophotometric method, using a LabAssay™ALP kit (Wako). The absorbance at 405 nm was measured with an MTP-600F microplate reader (Corona Electric Co., Hitachinaka, Japan). The treated cells were washed twice with PBS and fixed for 10 min with 70% EtOH at room temperature for ALP staining with the TRACP & ALP double-staining kit.
Alizarin red staining
To detect calcification during differentiation, the ODM-treated, ODM + EtEx.40 (10–20 μg/mL), and untreated MC3T3-E1 cells were washed twice with PBS and fixed for 10 min with 500 μL of ice-cold 70% ethanol. The fixed cells were stained with 500 μL of an Alizarin red S solution (Sigma, St. Louis, MO, USA).
TRAP activity and TRAP staining
RAW 264.7 cells were stimulated with sRANKL (50 ng/mL)/M-CSF (10 ng/mL) in the presence or absence of EtEx.40 (5–50 μg/mL). After 6 d, the cells were fixed with ice-cold 70% EtOH. To measure osteoclast differentiation, we examined the TRAP activity and staining with the TRACP & ALP assay kit (Takara Bio) and the TRACP & ALP double-staining kit (Takara Bio) according to the manufacturer’s protocols.
Western blotting
To prepare the cell lysates, MC3T3-E1 and/or RAW264.7 cells were washed twice with PBS and harvested. The cell pellet was resuspended in the RIPA buffer containing 25 × a Complete® and Phosphatase Inhibitor Cocktail® (Roche). The protein content was measured with a DC protein assay kit (Bio Rad, Hercules, CA, USA). Each whole cell lysate was resuspended in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer containing 2% 2-mercaptoethanol and boiled at 98 °C for 5 min. The protein from each lysate (20 μg) was separated by SDS-PAGE in 12% polyacrylamide gel and electroblotted on to a PVDF membrane (GE Healthcare, Piscataway, NJ, USA). After blocking for 1 h in 5% non-fat milk in TPBS (PBS and 0.1% Tween 20), the membrane was incubated overnight at 4 °C with various primary antibodies. The membrane was then washed thrice with TPBS, incubated at room temperature with the horseradish peroxidase-conjugated secondary antibody, and then washed thrice with TPBS. Protein bands were detected with the enhanced ECL™ Prime western blotting detection reagent (GE Healthcare) and a Davinch-Chemi chemiluminescence detector (Wako).
Quantitative real-time PCR
To measure the Runx2, Osx, and Dlx5 expression after treating with ODM + EtEx.40 for 3 d, total RNA was extracted with Trizol containing phenol/guanidinium isocyanate (Life Technologies) and DNase I. cDNA was generated with the PrimeScript™ reagent kit (Takara Bio). Quantitative real time-PCR (Thermal Cycler Dice® TP800) was performed with a SYBR® Premix Taq™ II kit (Takara Bio) and the following primer sets: Runx2-sense, 5ʹ-GACGTGCCCAGGCGTATTTC-3ʹ and Runx2-antisense, 5ʹ-AAGGTGGCTGGGTAGTGCATTC-3ʹ; Osx-sense, 5ʹ-TCTCCATCTGCCTGACTCCT-3ʹ and Osx-antisense, 5ʹ-AGCGTATGGCTTCTTTGTGC-3ʹ; Dlx5-sense, 5ʹ-GCTCTAGAGCAGCTCAATCAATTCCCACCT-3ʹ and Dlx5-antisense, 5ʹ-GCTCTAGAGCAAGAGAAAGTAGCCCATCTA-3ʹ; GAPDH-sense, 5ʹ-GCACCGTCAAGGCTGAGAAC-3ʹ and GAPDH-antisense, 5ʹ-TGGTGAAGACGCCAGTGGA-3ʹ. GAPDH was used as an internal control.
Luciferase reporter assay
The pGL4.32 firefly luciferase gene [luc2P/NF-κB-RE/Hygro] (Promega, Madison, WI, USA; 0.5 μg) and pRLSV40 (Promega, 0.1 μg) were co-transfected into RAW 264.7 cells with FUGENE®6 (Promega). The luciferase activity was measured 24 h after treating with RANKL/M-CSF in the presence or absence of EtEx.40 (20 μg/mL) using a Dual-Glo luciferase assay system (Promega).
Statistical analysis
All data were analyzed with the Mac statistical analysis software package for Macintosh, version 2.0 (Esumi Co., Tokyo, Japan). All data are expressed as the mean ± standard error (SE). A statistical analysis was performed by a one-way analysis of variance (ANOVA) and Tukey-Kramer test. p-values < 0.05 were considered statistically significant.
Results and discussion
EtEx.40 enhanced ODM-stimulated osteoblast differentiation
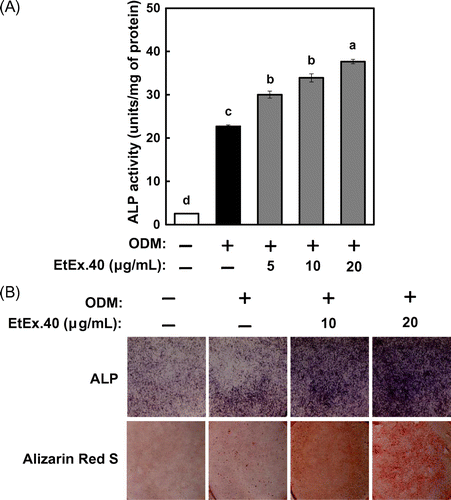
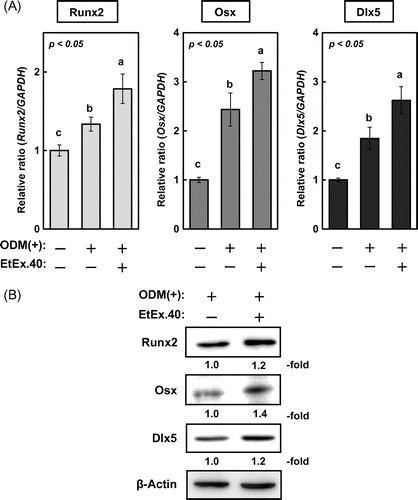
We examined ALP activity and ALP staining as a marker of osteoblast differentiation in EtEx.40-treated murine preosteoblast MC3T3-E1 cells. Fig. (A) shows that the ALP activity was elevated by ODM stimulation and increased further with EtEx.40 in a concentration-dependent manner (Fig. (A) and (B) upper panel). EtEx.40 at 20 μg/mL enhanced the ALP activity by approximately 165% in comparison to the ODM-stimulated cells. We also measured the mineralization in ODM and ODM + EtEx.40-treated MC3T3-E1 cells by Alizarin red O staining. EtEx.40 augmented the formation of mineralized nodules on the MC3T3-E1 cells. EtEx.40 thus enhanced ODM-stimulated osteoblast differentiation.
Fig. 1. Effects of EtEx.40 on ODM-stimulated MC3T3-E1 cell differentiation.
Note: (A) MC3T3-E1 cells were cultured for 6 d in an osteoblast differentiation medium (ODM) with EtEx.40. The ALP activity was assayed by spectrophotometry, using a LabAssay™ALP kit. Means not sharing a common letter in a column are significantly different at the p < 0.05 level. A statistical analysis was performed by ANOVA and the Tukey–Kramer test. (B) MC3T3-E1 cells were incubated in the presence of EtEx.40 (10 or 20 μg/mL) or a vehicle (DMSO) for 6 or 20 d. ALP staining results are shown in the upper panel, and mineralization in the MC3T3-E1 cells stained with an Alizarin Red O solution are shown in the lower panel.
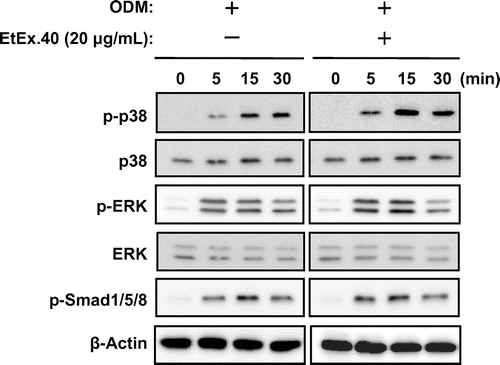
EtEx.40 up-regulates p38 MAPK in ODM-stimulated osteoblasts
To investigate the molecular mechanisms underlying the EtEx.40-mediated enhancement of osteoblast differentiation in ODM-stimulated MC3T3-E1 cells, we examined changes in the expression of Runx2, Dlx5, and Osx. Lee et al. have reported that Dlx5 specifically regulated Runx2 type II expression.Citation26) Ulsamaer et al. have also reported that BMP-2 facilitated Osx expression in osteoblasts via activation of Dlx5 expression by p38 MAPK.Citation27) The upregulation of these molecules by EtEx.40 therefore enhanced the activity of the Dlx5/Runx2/Osx pathway. These genes were significantly upregulated in the ODM + EtEx.40-treated cells vs. the ODM-treated cells (Fig. (A)), and the protein expression was accordingly increased (Fig. (B)). Various signal molecules are activated in ODM-stimulated MC3T3-E1 cells so we also examined the activation of Smad1/5/8, ERK, and p38 MAPK.Citation28,Citation29) There were no changes in Smad1/5/8 and ERK activation in the ODM- and ODM + EtEx.40-treated cells; however, the level of p-p38 MAPK in the ODM + EtEx.40-treated MC3T3-E1 cells was higher than that in the ODM-treated cells (Fig. ). p38 MAPK has regulated osteoblast differentiation through Dlx5 and Osx expression.Citation27,Citation30) Therefore, the EtEx.40-induced p38 MAPK activation enhanced osteoblast differentiation.
Fig. 2. mRNA and protein expression of osteoblast differentiation-related molecules in MC3T3-E1 cells treated with EtEx.40.
Note: (A) mRNA expression of Runx2, Osx, and Dlx5 in ODM plus EtEx.40 (20 μg/mL) or a vehicle (DMSO) for 3 d was assessed by using real-time PCR. Data are expressed as the mean ± SE (n = 9; p < 0.05). (B) Runx2, Osx, and Dlx5 expression in ODM-stimulated osteoblastogenesis. A representative blot from three independent experiments is shown.
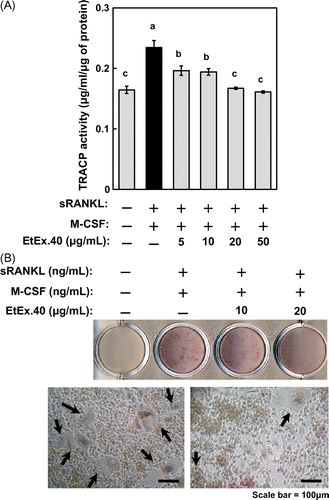
EtEx.40 attenuated RANKL/M-CSF-co-stimulated osteoclast differentiation
Next, to determine the effect of EtEx.40 on bone resorption, RAW264.7 cells were stimulated for 6 d with RANKL/M-CSF; the TRAP activity and TRAP staining were assessed as markers of osteoclastogenesis. Fig. (A) shows that the TRAP activity was suppressed by EtEx.40 in a concentration-dependent manner at 5–50 μg/mL (Fig. (A)). EtEx.40 also inhibited the formation of large TRAP-positive multinucleated cells at 5–20 μg/mL, but had no effect on the growth of RAW264.7 cells (Fig. (B)). These data indicate that EtEx.40 inhibited osteoclast differentiation.
Fig. 4. EtEx.40 inhibition of sRANKL/M-CSF-stimulated RAW264.7 cell differentiation.
Note: Cells were assessed for their TRAP activity (A) and TRAP staining (B). The TRAP activity and TRAP staining were evaluated by using the TRACP & ALP assay kit and TRACP & ALP staining kits. (A) Data are expressed as the mean ± SE (n = 9; p < 0.05). (B) The upper photo shows TRAP staining in RANKL-stimulated RAW264.7 cells in the presence or absence of EtEx.40 (20 μg/mL). The lower photos (ii) show the microscopic aspects of (i). Arrows indicate large TRAP-positive multinucleated cells (B-ii). The scale bar shows 100 μm.
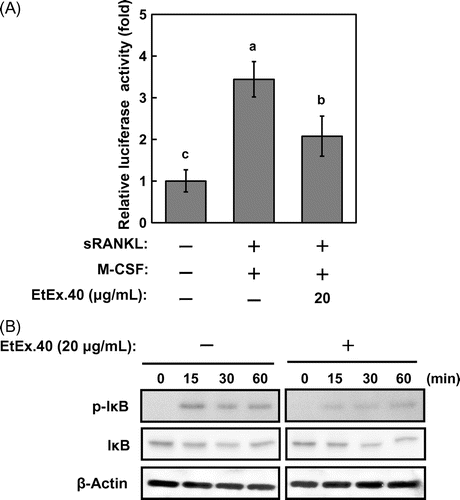
EtEx.40 inactivates NF-κB signaling in RANKL/M-CSF-stimulated osteoclasts
To characterize the mechanisms by which EtEx.40 inhibited RANKL/M-CSF-stimulated osteoclastogenesis, we examined the c-Fos and NFATc1 expression, as these are well-known differentiation-related molecules. c-Fos-deficient mice do not exhibit osteoclast formation and thus present an osteopetrotic phenotype.Citation31) Fig. shows that the expression of c-Fos and NFATc1 in RAW264.7 cells treated with RANKL/M-CSF + EtEx.40 was suppressed in comparison to the cells treated with RANKL/M-CSF. Upon binding of RANKL to RANK, such intracellular signaling molecules as MAPKs [ERK, p38 MAPK, and stress-activated protein kinases/Jun amino-terminal kinases (JNK)], Akt, and NF-κB were activated in RAW 264.7 cells. NFATc1 expression regulates the NF-κB and c-Fos pathways in RANKL-stimulated osteoclasts.Citation32,Citation33) p50/p52 NF-κB double-knockout mice have shown severe osteopetrosis.Citation34) Thus, NF-κB signaling plays a critical role in osteoclast development. We examined the activation of NF-κB signaling using a luciferase reporter assay and western blotting. EtEx.40 suppressed the transcriptional activity by approximately 44% of the untreated control (Fig. (A)). IκB activation was also down-regulated roughly 50% by EtEx.40 (Fig. (B)). We found no changes in MAPKs and Akt in the RANKL/M-CSF + EtEx.40-treated RAW264.7 cells (data not shown). Lee et al. have reported that luteolin suppressed osteoclast differentiation by inactivating ATF2 which is a downstream effector of p38 MAPK. We must therefore examine the effect of the downstream molecules of MAPKs in RANKL/M-CSF + EtEx.40-treated RAW264.7 cells. These results raise the possibility that EtEx.40 would not affect the RANKL-induced RANK/TRAF6/TAK1/MAPKs pathways for osteoclastogenesis. We also suggest that the down-regulation of c-Fos expression by EtEx.40 was not due to MAPK activation. M-CSF activates c-Fos and ERK, but not NF-κB and NFATc1 in RANKL-stimulated osteoclasts.Citation35) The EtEx.40 suppression of NFATc1 expression was mainly due to the direct inactivation of NF-κB and down-regulation of c-Fos expression.
Fig. 5. EtEx.40 suppression of the sRANKL/M-CSF-stimulated expression of c-Fos and NFATc1 in RAW264.7 cells.
Note: A representative blot from three independent experiments is shown.
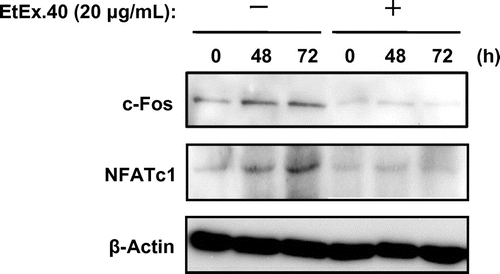
Fig. 6. EtEx.40 attenuation of the sRANKL/M-CSF-stimulated transcriptional activity of NF-κB in RAW264.7 cells.
Note: (A) RAW264.7 cells transfected with the NF-κB-luciferase plasmid and pRLSV40 were pre-treated with EtEx.40 (20 μg/mL) or the vehicle (DMSO) for 2 h and were then stimulated with sRANKL (50 g/mL)/M-CSF (10 ng/mL) for 24 h. The cells were lysed, and the luciferase activity was measured with a luciferase reporter assay system. Data are expressed as the mean ± SE (n = 12, p < 0.05). (B) RAW264.7 cells were pre-treated with EtEx.40 (20 μg/mL) or the vehicle (DMSO) for 2 h and then stimulated with sRANKL (50 ng/mL)/M-CSF (10 ng/mL) for the indicated times. Phosphorylated Iκ-B and IκB were detected with specific antibodies.
Our findings suggest that EtEx.40 could be used to treat senile osteoporosis and postmenopausal osteoporosis. We must identify the active compound in EtEx.40. We have isolated six compounds from EtEx.40 (Fig. ), the effects of which in osteoblast or osteoclast differentiation models are being studied in our laboratory to identify the active compound. Lee et al. have reported that the consumption of such a legume as adzuki improved the levels of bone markers in ovariectomized rats.Citation36) Further studies will verify the effect of EtEx.40 on osteoporosis in animal models.
References
- Manolagas SC. Endocr. Rev. 2000;21:115–137.
- Liel Y, Castel H, Bonneh DY. Osteoporos. Int. 2003;14:490–495.
- Zhang X, Li SW, Wu JF, Dong CL, Zheng CX, Zhang YP, Du J. Endocrinol. 2010;26:76–80.
- Iannirri T, Rosini S, Lodi D, Frediani B, Rottigni V, Palmieri B. Am. J. Ther. 2012;19:228–246.
- Tsuji-Naito K. Bioorg. Med. Chem. 2008;16:9176–9183.
- Yamaguchi M. J. Health Sci. 2008;54:356–369.
- Lee JH, Jin H, Shim HN, Kim HN, Ha H, Lee ZH. Mol. Pharmacol. 2010;77:17–25.
- Levis S, Lagari VS. Curr. Osteoporos. Rep. 2012;10:296–302.
- Yamaguchi M. Mol. Cell. Biochem. 2012;366:201–221.
- Hirao K, Yumoto H, Nakanishi T, Mukai K, Takahashi K, Takegawa D, Matsuo T. Life Sci. 2010;86:654–660.
- Takahashi S, Hori K, Hokari M, Gotoh T, Sugiyama T. Biomed. Res. 2010;31:155–159.
- Tamura S, Yoshihira K, Fujiwara K, Murakami N. Bioorg. Med. Chem. Lett. 2010;20:2299–2302.
- Zhang W, Popovich DG. J. Agric. Food Chem. 2010;58:5315–5319.
- Itoh T, Itoh Y, Mizutani M, Fujishiro K, Furuichi Y, Komiya T, Hibasami H. Nippon Shokuhin Kagaku Kougaku Kaishi. 2002;49:339–344. Japanese.
- Itoh T, Itoh Y, Mizutani M, Fujishiro K, Furuichi Y, Komiya T, Hibasami H. J. Nutr. Sci. Vitaminol. 2004;50:295–299.
- Itoh T, Itoh Y, Hibasami H, Katsuzaki H, Imai K, Furuichi Y, Komiya T. Nippon Shokuhin Kagaku Kougaku Kaishi. 2005;52:339–344. Japanese.
- Itoh T, Itoh Y, Hibasami H, Katsuzaki H, Imai K, Furuichi Y, Komiya T. Nippon Eiyo Shokuryo Gakkaishi. 2005;58:281–287.
- Itoh T, Umekawa H, Furuichi Y. Biosci. Biotechnol. Biochem. 2005;69:448–454.
- Itoh T, Kita N, Kurokawa Y, Kobayashi M, Horio F, Furuichi Y. Biosci. Biotechnol. Biochem. 2004;68:2421–2426.
- Itoh T, Kobayashi M, Horio F, Furuichi Y. Nutrition. 2009;25:134–141.
- Itoh T, Furuich Y. Nutrition. 2009;25:873–882.
- Itoh T, Furuichi Y. Biosci. Biotechnol. Biochem. 2005;69:873–882.
- Itoh T, Hori Y, Atsumi T, Toriizuka K, Nakamura M, Maeyama T, Ando M, Tsukamasa Y, Ida Y, Furuichi Y. Phytother. Res. 2013;26:1003–1011.
- Choi EM, Hwang JK. Biol. Pharm. Bull. 2003;26:523–526.
- Nakamura H, Ukai T, Yoshimura A, Kozuka Y, Yoshioka H, Yoshinaga Y, Abe Y, Hara Y. J. Periodontal Res. 2010;45:23–30.
- Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS, Wozney JM, Chi XZ, Bae SC, Choi KY, Cho JY, Choi JY, Ryoo HM. J. Biol. Chem. 2005;280:35579–35587.
- Ulsamer A, Ortuno MJ, Ruiz S, Susperregui AR, Osses N, Rosa JL, Ventura F. J. Biol. Chem. 2008;283:3816–3826.
- Cao X, Chen D. Gene. 2005;357:1–8.
- Dijke PT. Curr. Med. Res. Opin. 2006;22:S7–S11.
- Wang X, Goh CH, Li B. Endocrinology. 2007;148:1629–1637.
- Johnson RS, Spiegelman BM, Papaioannou V. Cell. 1992;71:577–586.
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Dev. Cell. 2002;3:889–901.
- Nakashima T, Hayashi M, Takayanagi H. Trends Endocrinol. Metab. 2012;23:582–590.
- Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. J. Biol. Chem. 2007;282:18245–18253.
- Hodge JM, Collier FM, Pavlos NJ, Kirkland MA, Nicholson GC. PLoS ONE. 2011;e21462. doi:10.1371/journal.pone.0021462
- Lee SH, Jin N, Paik DJ, Kim DY, Chung IM, Park Y. Nutr. Res. 2011;31:397–403.