Abstract
This study investigated the production of ethanol from unutilized branches pruned from pear trees by steam explosion pretreatment. Steam pressures of 25, 35, and 45 atm were applied for 5 min, followed by enzymatic saccharification of the extracted residues with cellulase (Cellic CTec2). High glucose recoveries, of 93.3, 99.7, and 87.1%, of the total sugar derived from the cellulose were obtained from water- and methanol-extracted residues after steam explosion at 25, 35, and 45 atm, respectively. These values corresponded to 34.9, 34.3, and 27.1 g of glucose per 100 g of dry steam-exploded branches. Simultaneous saccharification and fermentation experiments were done on water-extracted residues and water- and methanol-extracted residues by Kluyveromyces marxianus NBRC 1777. An overall highest theoretical ethanol yield of 76% of the total sugar derived from cellulose was achieved when 100 g/L of water- and methanol-washed residues from 35 atm-exploded pear branches was used as substrate.
Graphical Abstract
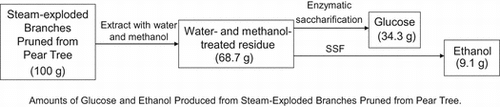
Amounts of glucose and ethanol produced from steam-exploded branches pruned from pear tree.
Ethanol is an important biofuel that can partially replace gasoline. Recently, extensive research has been carried out to enable the establishment of a bio-refinery process, which uses unutilized lignocellulosic biomass such as hardwood,Citation1−Citation3) softwood,Citation4−Citation6) and herbaceous plants,Citation7,8) but current technologies for ethanol production from lignocellulosic biomass are impractical due to high transportation costs. Irrespective of the ethanol production process, the mass of feedstock required necessitates transportation costs that increase as farm-to-plant distances increase.Citation9)
Branches pruned from apple and pear trees are a by-product of fruit cultivation and are abundant sources of lignocellulosic biomass. The Agriculture, Forestry, and Fisheries Technology Support Center of the Fruit Tree Research Institute of Tokushima Prefecture has estimated that 1650 tons of pruned pear branches containing 53% moisture were discharged during 2010–2011 in the prefecture. Large quantities of this material are not used, and hence accumulate on farms. Since pear plantation farms are located in urban areas, it should be possible to limit the transportation and collection costs of this otherwise unused biomass. Advanced utilization of this material as a carbon resource holds promise for the bioproduction of ethanol.
The main components of lignocellulosic biomass are cellulose, hemicellulose, and lignin. Because a hard network of lignin surrounds the cellulose, it is necessary to decompose the lignin by an environmentally friendly pretreatment method prior to enzymatic conversion of cellulose to glucose. Over the years, various pretreatment methods have been developed for lignocellulosic biomass, including physical,Citation10) chemical,Citation11,12) hydrothermal,Citation13,14) hydrothermal–chemical,Citation15,16) and hydrothermal–biologicaCitation7,Citation15) methods. Steam explosion is a hydrothermal pretreatment method for lignocellulosic biomass that uses high pressure and high temperature steam without the addition of any chemicals to cause autohydrolysis, defibriation, and delignification. This process has several advantages over conventional chemical treatments; it eliminates the use of toxic substances such as strong acids and alkalis, chemical-tolerant equipment, waste processing systems, and high preliminary feedstock volume. Therefore, many studies on unutilized plant biomass pretreatment using steam explosion have been done recently.Citation17−Citation21)
The main objective of this study was to investigate steam explosion pretreatment of the branches pruned from pear trees (BPPT) for bioethanol production via simultaneous saccharification and fermentation (SSF). For this purpose, optimal steam explosion pretreatment conditions were investigated for enzymatic saccharification and microbial conversion of hydrolyzates to ethanol by SSF. Removal of inhibitors of fermentation from pretreated BPPT by water and methanol extraction methods was investigated, and the final amount of ethanol produced was evaluated.
Materials and methods
Branches pruned from pear trees
Raw 1-, 5-, 10-, and 20-year-old pruned pear (Pyrus pyrifolia cv. Kousui) branches (containing bark) supplied by the Agriculture, Forestry, Fisheries Technology Support Center of the Fruit Tree Research Institute (Tokushima, Japan) were cut into 1–2 cm chips.
Steam explosion
Pretreatment of the BPPT was conducted in a steam explosion apparatus (Japan Chemical Engineering and Machinery Co., Ltd., Osaka, Japan). The reactor was charged with 100 g (dry matter) of feedstock per batch. Saturated steam from the boiler was allowed to enter the reactor to heat the bagasse at controlled pressures of 25, 35, and 45 atm. Each pressure level was maintained for 5 min, and then the reactor was depressurized instantly. The exploded BPPT was recovered in a cyclone and cooled to room temperature.
Sugars and phenol compounds and lignin analysis of the raw BPPT
Reducing sugar components in the raw BPPT were determined with a sulfuric acid (H2SO4) hydrolyzate of the branches by the standards of the Technical Association of the Pulp and Paper Industry. Subsequently, each hydrolyzate was analyzed by the reducing sugar analysis system (Prominence series with a post-column derivatization system using arginine, Shimadzu, Kyoto, Japan) and a fluorescence detector (RF-20Axs, Shimadzu). For phenol compounds containing lignin, 1 g of dried pear branches was added to 15 mL of 72% (w/v) H2SO4 and this was kept at room temperature for 4 h. The residue was placed in a 100-mL conical flask, washed with 560 mL of distilled water, and autoclaved for 1 h. The insoluble material was washed with distilled water and heat dried at 105 °C to a constant weight, and its mass was recorded. All analytical determinations were performed in duplicate, and the means were calculated.
Component analysis of steam-exploded BPPT
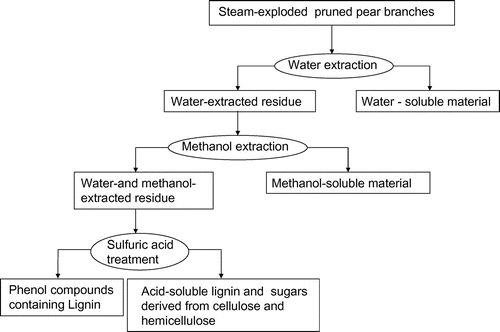
Component analysis of steam-exploded BPPT was done using distilled water and methanol as extraction solvents (Fig. ). Asada et al.Citation1) and Sasaki et al.Citation17,22) also analyzed samples using this extraction method. Water-soluble material, methanol-soluble material, phenol compounds containing lignin, acid-soluble lignin, and sugars derived from cellulose and hemicellulose were determined as follows: five grams of the dry steam-exploded pear branches was added to 100 mL of distilled water and incubated for 24 h at room temperature. Solid and liquid materials were separated by filtration and the filtrate was recovered from the liquid, concentrated, dried, and weighed (water-soluble material). This water-washed residue was treated at room temperature for 24 h in a Soxhlet extractor by 150 mL of methanol to dissolve the methanol-soluble material. After concentration and drying of the extract, the methanol-soluble material was weighed. The water- and methanol-extracted residues consisted of holocellulose and phenol compounds containing lignin. About Fifteen milliliters of 72% (w/v) H2SO4 was added to this residue (1 g) and this was kept at room temperature for 4 h. The residue was then placed in a 100-mL conical flask, washed with 560 mL of distilled water, and autoclaved for 1 h. The insoluble material was washed with distilled water, and then it was heat dried at 105 °C to a constant weight, and its mass was recorded (phenol compounds containing lignin). The acid-soluble lignin in the hydrolyzed liquid was measured by UV spectrophotometry at 205 nm. The sugars derived from cellulose and hemicellulose in the hydrolysis were analyzed by HPLC equipped with a refractive index detector with a HPX-87H column (Bio-Rad, Richmond, CA) at 50 °C with 5.0 mM H2SO4 as the mobile phase at 0.6 mL/min.
Enzymatic hydrolysis
The steam-exploded BPPT was enzymatically hydrolyzed with Cellic CTec 2 (produced by Trichoderma reesei) purchased from Novozymes Japan (Chiba, Japan). Enzymatic hydrolysis was done using a 10 mL of 0.05 M sodium phosphoric acid buffer (pH 5.0) at 50 °C on a rotary shaker at 140 rpm over 72 h. The substrate concentration and enzyme loadings were 50 g/L and 20 mg enzyme protein/g of substrate, respectively. The supernatant was centrifuged to remove solid wastes and was analyzed for glucose. All enzymatic hydrolysis experiments were done in duplicate and the means were calculated.
The enzymatic saccharification rate was calculated by the following equation:
Micro-organisms and inoculum cultivation
Kluyveromyces marxianus NBRC 1777 (purchased from the National Institute of Technology and Evaluation, Biological Resource Center, NBRC, Chiba, Japan) was used to produce ethanol from the hydrolyzate of the BPPT. The yeast strain was subcultured every 4 weeks. The strain was precultured in 100 mL of medium in a 300-mL flask at 30 °C for 24 h by a shaking incubator at 60 rpm. The preculture medium contained 20 g/L glucose, 10 g/L peptone, and 10 g/L yeast extract. The cells were harvested by centrifugation (2000 g), rinsed thoroughly with sterile distilled water, centrifuged again, and then resuspended in sterile distilled water.
Ethanol fermentation of the steam-exploded pear branches by SSF
The medium for SSF (10 mL) contained various initial concentrations (dry matter) of steam-exploded pear branches, or water-extracted residue, or water- and methanol-extracted residue, as substrate. This SSF nutrient medium (10 mL) also contained 10 g/L peptone, 10 g/L yeast extract, 0.1 M sodium acetate buffer (pH 5.0), Cellic CTec2 (20 mg enzyme protein/g of substrate), and 0.25 g/L yeast inoculum. The steam-exploded pear branches, nutrient medium, and buffer were autoclaved (121 °C for 20 min), and the enzyme solution was added after sterilization with a 0.22-μm filter. Cultivation was carried out in a test tube with 10 mL of the medium by an orbital shaker at 150 rpm at 45 °C. Aliquots were collected and assayed for ethanol content. The all fermentation experiments were performed in duplicate, and the means were calculated. The theoretical yield of ethanol production was calculated by the following equation:
Determination of residual glucose and ethanol produced
The residual glucose and D-lactic acid concentrations in the fermentation medium were determined by the mutarotase GOD method (Glucose C-II test, Wako Pure Chemicals, Osaka, Japan), and analyzed by HPLC by a refractive index detector and a Bio-Rad HPX-87H column at 50 °C with a 5.0 mM H2SO4 mobile phase at a flow rate of 0.6 mL/min.
Results and discussion
Composition analysis of raw and steam-exploded BPPT
To evaluate the quality of the substrate for fermentation, the chemical composition of the raw 1-, 5-, 10-, and 20-year-old pruned pear branches was determined (Table ). The glucose contents of the 1-, 5-, 10-, and 20-year-old pruned pear branches were 37.6, 37.0, 38.9, and 43.6%, respectively. Though more glucose was found in the 10- and 20-year-old pruned pear branches, the 1-year-old pruned pear branches were studied because of their relative agricultural abundance.
Table 1. Chemical composition (%) of raw pruned pear branches.
Steam explosion was used to break down the lignin network in the pear branches and to increase the accessibility of the enzymes to cellulose. Asada et al. reported in 2011 that the maximum amount of glucose after enzymatic saccharification, 598 mg of glucose/g of dry aspen chopsticks (10 cm long and 0.5–0.7 cm wide), was obtained using steam-exploded aspen chopsticks treated at a steam pressure of 25 atm and steaming time of 5 min. Moreover, Yamashita et al. reported in 2010 that using Japanese cedar chips (3–5 cm long and 0.5–1 cm wide), the maximum production of glucose after enzymatic saccharification was 326 mg of glucose/g of dry Japanese cedar chips. Based on these results, steam explosion conditions, i.e. steam pressure 25 atm, 35 atm, and 45 atm and steaming time 5 min, were chosen. Table shows the chemical composition of the steam-exploded pear branches (steam pressures of 25, 35, and 45 atm were applied for 5 min) separated by the extraction method as illustrated in Fig. . After steam explosion at 25, 35, and 45 atm, 13.8, 10.9, and 11.4%, respectively, of the material was water soluble and contained sugars derived from holocellulose and other sugar decomposition materials (organic acids, furfural, etc.). In general, hemicellulose hydrolyzes more readily than cellulose.Citation23,24) Hence, water-soluble sugars from the branches exploded at 25 atm may have been composed predominantly of sugar derived from xylan. On the other hand, the steam-exploded branches at above 35 atm gave rise to smaller quantities of water-soluble sugars and more methanol-soluble materials. Sugars derived from xylan can decompose into water-insoluble materials such as furfural under higher pressure.Citation25) Since sugars derived from cellulose decreased with increasing steam pressure (34.4% for 35 atm and 31.1% for 45 atm), glucose may have been liberated and decomposed to water-soluble hydroxymethylfurfural (5-HMF).Citation18,24,26) Thus, the production and decomposition of sugars occurred in the steam-exploded pear branches. Generally, these compounds (fran derivatives) are known as fermentation inhibitors,Citation27) and, it is necessary to remove these products from the fermentation substrates. With increased production of sugars and greater degradation of biomass, the amount of sugars derived from the cellulose decreased from 37.4% at 25 atm to 31.1% at 45 atm. The amount of methanol-soluble material also increased with steam pressure. Steam pressures of 25 and 45 atm resulted in 14.9% and 22.4% of methanol-soluble materials, respectively, and it probably contained low molecular weight lignin.Citation27−Citation29) Maximum reduction of lignin was observed at a steam pressure of 25 atm (29.1%). At higher pressures, a gradual increase was observed owing to the combination of lignin with low molecular weight lignin (methanol-soluble material)Citation28,30,31) and water-soluble material.Citation28,30,31)
Table 2. Chemical composition (%) of steam-exploded pruned pear branches (1-year old) at various steam pressures (steaming duration 5 min).
Enzymatic saccharification of steam-exploded BPPT
The effect of steam explosion on the enzymatic saccharification of BPPT was studied at a substrate concentration of 50 g/L (Fig. ). A water- and methanol-extracted residue was used as substrate because many reports indicated that water- and methanol-soluble fermentation inhibitors, phenol compounds derived from lignin, inhibit this enzyme reaction.Citation32−Citation34) Steam pressures of 25, 35, and 45 atm at a steaming time of 5 min were investigated. The glucose concentration after 72 h of saccharification increased following all three steam explosion treatments, resulting in 24.9 g/L of detectable glucose at steam pressures of 25 atm, 25.6 g/L at 35 atm, and 21.0 g/L at 45 atm. These values corresponded to 349, 343, and 271 mg of glucose/g of the dry steam-exploded pear branch, respectively. The substrates contained 37.4, 34.4, and 31.1% of the sugars derived from cellulose, respectively (Table ). The enzymatic saccharification rates were 93.3% for 25 atm, 99.7% for 35 atm, and 87.1% for 45 atm. The decreased rate of saccharification in the extracts pretreated with steam at 45 atm can be explained by the presence of lignin (a combination of lignin with low molecular weight lignin and water-soluble material under “Composition analysis of raw and steam exploded BPPT”) that reduces the access of the enzyme to cellulose. Donohoe et al.Citation35) have reported on lignin on the cell wall of biomass after dilute acid treatment, which act as a physical barrier preventing enzyme access to the carbohydrate fraction of the biomass during enzymatic hydrolysis, and Rahikainen et al.Citation36) studied the interaction of cellulases with softwood lignin.
Ethanol production by SSF with steam-exploded BPPT
Since K. marxianus ferments ethanol at temperatures close to the optimum for enzymatic hydrolysis (between 38 and 45 °C), the cost of cooling after sterilizing and the risk of contamination in the industrial process have been reduced.Citation37) Many researchers produce ethanol using K. marxianus recently.Citation38−Citation40)
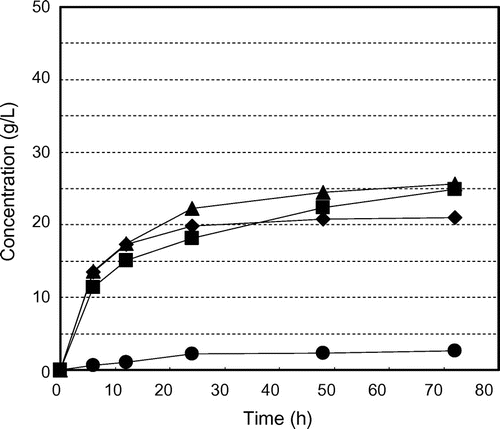
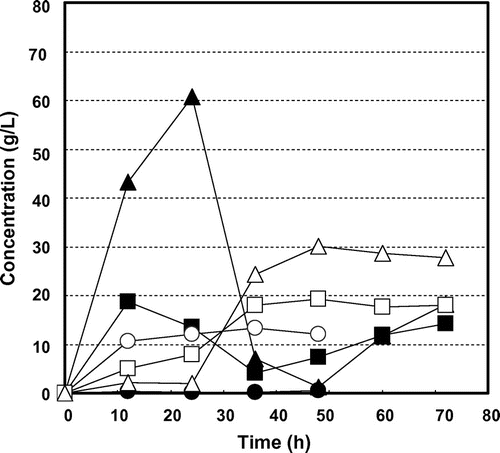
Fig. shows the time courses of glucose production and consumption and ethanol production by K. marxianus NBRC 1777 by steam-exploded BPPT as substrate (100 g/L). Glucose production was observed for every steam pressure condition but there was no glucose consumption or ethanol production. This indicates the presence of fermentation inhibition materials in steam-exploded pear branches. Klinke et al.Citation41) Palmqvist and Hahn-Hägerdal,Citation24) and Larsson et al.Citation42) have reported pretreatment processes (high temperature pretreatment and acid pretreatment of biomass) that generate fermentation inhibitors such as sugar- and lignin-derived materials, organic acids, fran derivatives, and phenol compounds. Fig. shows the time courses of glucose production and consumption and of ethanol production by residues of steam-exploded BPPT as substrates after water extraction of inhibitors (100 g/L). Ethanol production was unfettered, resulting in 13.4 g/L for 25 atm at 70 h, 13.4 g/L for 35 atm at 60 h, and 11.4 g/L for 45 atm at 48 h. This corresponds to 116, 119, and 102 mg of ethanol from 1 g of dry steam-exploded BPPT, respectively. Asada et al.Citation1) also reported that when water-extracted residue (aspen wood chopsticks) after steam explosion (steam pressure 20 atm, steaming time 5 min) was used as ethanol fermentation substrate, 27.2 g/L (initial substrate concentration 100 g/L) of ethanol was attained, but no ethanol production was observed when steam-exploded wood, that is, no extraction after steam explosion, was used. Fermentation was progressively delayed with decreased pressures of steam explosion. Fermentation started at 24 h for 45 atm, 38 h for 35 atm, and 50 h for 25 atm, indicating that substrates contain water-insoluble fermentation inhibitors that decreased access to and uptake of glucose for the fermentative organism. Thus, washing the steam-exploded residue with water only might not be sufficient. While the substrate concentration increased to 150 g/L, there was no glucose consumption or ethanol production, indicating the presence of fermentation inhibitors and strong fermentation inhibitors (results not shown). Fig. shows the time courses of glucose production and consumption and ethanol production by water- and methanol-washed residues of steam-exploded BPPT as substrates (100 g/L). All of the fermentation starting times were expedited, especially that for the pear branches exploded at 35 and 45 atm. For the pear branches exploded at 25 atm, ethanol was produced more slowly than under the other conditions. These results indicate that the 25 atm-exploded branches were not optimal for use as a substrate for ethanol production. Inhibitory compounds cannot dissolve in methanol and remain in the material. Based on all of these results, the most effective treatment pressure and inhibitor extraction method was 35 atm with water and methanol extraction. Under these treatments, the substrate concentrations increased to 150 g/L and 250 g/L. Fig. shows the time courses of glucose consumption and ethanol production by various initial concentrations of substrate (100, 150, and 250 g/L water- and methanol-treated residue of the steam-exploded BPPT at 35 atm). The maximum ethanol concentrations achieved were 13.3 g/L from 100 g/L of substrate, 19.3 g/L from 150 g/L of substrate, and 30.0 g/L from 250 g/L of substrate, corresponding to 133, 129, and 120 mg of ethanol from 1 g of dry substrate, respectively. These values corresponded to 91.2, 88.6, and 82.4 mg of ethanol per 1 g of dry steam-exploded branches. From these results, a maximum ethanol yield of 76% of theoretical was obtained from 1-year-old pruned pear branches treated by steam explosion at 35 atm for 5 min with water and methanol extraction. Hence, 34.3 g of glucose by enzymatic saccharification and 9.1 g of ethanol by SSF can be produced from 100 g of steam-exploded BPPT (Fig. , steam pressure 35 atm and steaming time 5 min).
Fig. 3. Residual glucose concentration and ethanol production profiles for steam-exploded BPPT (100 g/L) by Kluyveromyces marxianus NBRC 1777. Symbols: ● glucose at steam presure 25 atm, ○ ethanol at steam pressure 25 atm, ■ glucose at steam pressure 35 atm, □ ethanol at steam pressure 35 atm, ▲ glucose at steam pressure 45 atm, and △ ethanol at steam pressure 45 atm.
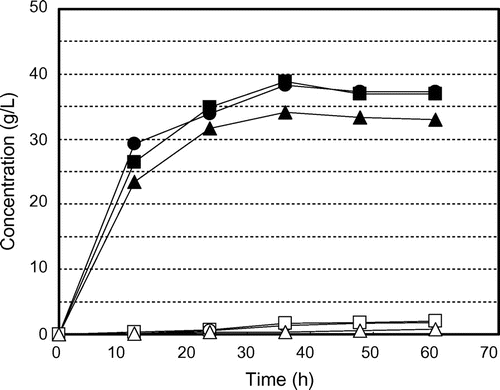
Fig. 4. Residual glucose concentrations and ethanol production profiles for water-extracted residue of steam-exploded BPPT (100 g/L) by Kluyveromyces marxianus NBRC 1777. Symbols: ● glucose at steam pressure 25 atm, ○ ethanol at steam pressure 25 atm, ■ glucose at steam pressure 35 atm, □ ethanol at steam pressure 35 atm, ▲ glucose at steam pressure 45 atm, and △ ethanol at steam pressure 45 atm.
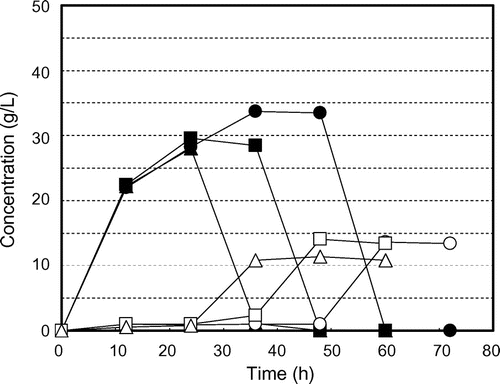
Fig. 5. Residual glucose concentrations and ethanol production profiles for water- and methanol-extracted residue of steam-exploded BPPT (100 g/L) by Kluyveromyces marxianus NBRC 1777. Symbols: ● glucose at steam presure 25 atm, ○ ethanol at steam pressure 25 atm, ■ glucose at steam pressure 35 atm, □ ethanol at steam pressure 35 atm, ▲ glucose at steam pressure 45 atm, and △ ethanol at steam pressure 45 atm.
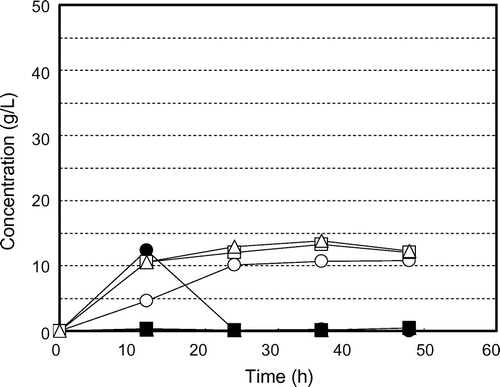
Fig. 6. Residual glucose concentrations and ethanol production profiles for various initial concentrations of substrate (water-and methanol-extracted residues of steam-exploded BPPT, steam pressure 35 atm) by Kluyveromyces marxianus NBRC 1777. Symbols: ● glucose at 100 g/L, ○ ethanol at 100 g/L, ■ glucose at 150 g/L, □ ethanol at 150 g/L, ▲ glucose at 250 g/L, and △ ethanol at 250 g/L.
Conclusions
This study indicated that BPPT can be used for the production of ethanol, a biofuel, and that significant improvements in sugar yields are possible when these branches are pretreated by steam explosion. Furthermore, it was clarified that washing a steam-exploded sample with water and methanol effectively removes inhibitors of fermentation. The best theoretical ethanol yield of 76% of total sugar derived from cellulose was obtained when 100 g/L of water- and methanol-washed residues from 35 atm-exploded pear branches was used as substrate. To the best of our knowledge, this is the first report on component analysis of BPPT, coupled with enzymatic saccharification, and of ethanol production from these branches. Although BPPT have smaller amounts of glucose (37.6%, 1-year old, Table ) than that of other woody resources (almost all stems), they are promising resources for biofuel in terms of cost-effectiveness in that pear plantation farms are located in urban areas. Future studies can focus on net energy obtained, and the costs associated with industrial scale processing.
Funding
Part of this study was funded by a Grant-in-Aid for Young Scientists (B) (No. 21750159) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Shorai Foundation for Science and Technology, and the Sumitomo Foundation.
References
- Asada C, Kita A, Sasaki C, Nakamura Y. Ethanol production from disposable aspen chopsticks using delignification pretreatments. Carbohydr. Polym. 2011;85:196–200.
- Wang JZ, Zhu JY, Zalesny RS Jr, Chen KF. Ethanol production from poplar wood through enzymatic saccharification and fermentation by dilute acid and SPORL pretreatments. Fuel. 2012;95:606–614.
- Sassner P, Martensson CG, Galbe M, Zacchi G. Steam pretreatment of H(2)SO(4)-impregnated Salix for the production of bioethanol. Bioresour. Technol. 2008;99:137–145.
- Asada C, Sasaki C, Uto Y, Sakafuji J, Nakamura Y. Effect of steam explosion pretreatment with ultra-high temperature and pressure on effective utilization of softwood biomass. Biochem. Eng. J. 2012;60:25–29.
- Baba Y, Tanabe T, Shirai N, Watanabe T, Honda Y, Watanabe T. Pretreatment of Japanese cedar wood by white rot fungi and ethanolysis for bioethanol production. Biomass Bioenerg. 2011;35:320–324.
- Yamashita Y, Sasaki C, Nakamura Y. Effective enzyme saccharification and ethanol production from Japanese cedar using various pretreatment methods. J. Biosci. Bioeng. 2010;110:79–86.
- Sasaki C, Takada R, Watanabe T, Honda Y, Karita S, Nakamura Y, Watanabe T. Surface carbohydrate analysis and bioethanol production of sugarcane bagasse pretreated with the white rot fungus, Ceriporiopsis subvermispora and microwave hydrothermolysis. Bioresour. Technol. 2011;102:9942–9946.
- Sun ZY, Tan YQ, Iwanaga T, Sho T, Kida K. Production of fuel ethanol from bamboo by concentrated sulfuric acid hydrolysis followed by continuous ethanol fermentation. Bioresour. Technol. 2011;102:10929–10935.
- Leboreiro J, Hilally AK. Biomass transportation model and optimum plant size for the production of ethanol. Bioresour. Technol. 2011;102:2712–2723.
- Silva AS, Inoue H, Endo T, Yano S, Bon EPS. Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010;101:7402–7409.
- Muhammad N, Man Z, Bustam MA, Mutalib MIA, Wilfired CD, Rafiq S. Dissolution and delignification of bamboo biomass using amino acid-based ionic liquid. Appl. Biochem. Biotechnol. 2011;165:998–1009.
- Aita GA. Enzyme hydrolysis and ethanol fermentation of dilute ammonia pretreated energy cane. Bioresour. Technol. 2011;102:4444–4448.
- Zhao Y, Lu WJ, Wang HT, Yang JL. Fermentable hexose production from corn stalks and wheat straw with combined supercritical and subcritical hydrothermal technology. Bioresour. Technol. 2009;100:5884–5889.
- Phaiboomsilpa N, Yamauchi K, Lu X, Saka S. Two-step hydrolysis of Japanese cedar as treated by semi-flow hot-compressed water. J. Wood Sci. 2010;56:331–338.
- Verma P, Watanabe T, Honda Y, Watanabe T. Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H2O2. Bioresour. Technol. 2011;102:3941–3945.
- Rocha GJM, Goncalves AR, Oliveira BR, Olivares EG, Rossell CEV. Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind. Crop Prod. 2012;35:274–279.
- Sasaki C, Okumura R, Asakawa A, Asada C, Nakamura Y. Effects of washing with water on enzymatic saccharification and d-lactic acid production from steam-exploded sugarcane bagasse. J. Mater. Waste Manag. 2012;14:234–240.
- Boluda-Aguilar M, García-Vidal L, González-Castañeda FP, López-Gómez A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010;101:3506–3513.
- Ballesteros I, Ballesteros M, Cara C, Saez E, Castro E, Manzanares P, Negro MJ, Oliva JM. Effect of water extraction on sugars recovery from steam exploded olive tree pruning. Bioresour. Technol. 2011;102:6611–6616.
- Asada C, Asakawa A, Sasaki C, Nakamaura Y. Characterization of the steam-exploded spent Shiitake mushroom medium and its efficient conversion to ethanol. Bioresour. Technol. 2011;102:10052–10056.
- Horn SJ, Eijsink VGH. Enzymatic hydrolysis of steam exploded hardwood using short processing times. Biosci. Biotechnol. Biochem. 2010;74:1157–1163.
- Sasaki C, Wanaka M, Takagi H, Tamura S, Asada C, Nakamura Y. Evaluation of epoxy resins synthesized from steam-exploded bamboo lignin. Ind. Crop Prod. 2013;43:757–761.
- Ando H, Sakaki T, Kokusho T, Shibata M, Uemura Y, Hatate Y. Decomposition behavior of plant biomass in hot-compressed water. Ind. Eng. Chem. Res. 2000;39:3688–3693.
- Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000;74:25–33.
- Dunlop AP. Furfural formation and behavior. Ind. Eng. Chem. 1948;40:204–209.
- Ulbricht RJ, Sharon J, Thomas J. A review of 5-hydroxymethylfurfural HMF in parental solutions. Fundam. Appl. Toxicol. 1984;4:843–853.
- Helle S, Cameron D, Lam J, White B, Duff S. Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzyme Microb. Technol. 2003;33:786–792.
- Asada C, Nakamura Y, Kobayashi F. Chemical characteristics and ethanol fermentation of the cellulose component in autohydrolyzed bagasse. Biotechnol. Bioprocess Eng. 2005;10:346–352.
- Kobayashi F, Take H, Asada C, Nakamura Y. Methane production from steam-exploded bamboo. J. Biosci. Bioeng. 2004;97:426–428.
- Nakamura Y, Sawada T, Inoue E. Enhanced ethanol production from enzymatically treated steam-exploded rice straw using extractive fermentation. J. Chem. Technol. Biotechnol. 2001;76:879–884.
- Chua MGS, Wayman M. Characterization of autohydrolysis aspen (P. tremuloides) lignins. Part 1. Can. J. Chem. 1979;57:1141–1149.
- Kim Y, Ximenes E, Mosier NS, Ladisch MR. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb. Technol. 2011;48:408–415.
- Ximenes E, Kim Y, Mosier NS, Dien B, Ladisch MR. Inhibition of cellulases by phenols. Enzyme Microb. Technol. 2010;46:170–176.
- Ximenes E, Kim Y, Mosier NS, Dien B, Ladisch MR. Deactivation of cellulases by phenols. Enzyme Microb. Technol. 2011;48:54–60.
- Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008;101:913–925.
- Rahikainen JL, Martin-Sanpedro R, Heikkinen H, Rovio S, Marjamma K, Tamminen T, Rojas OJ, Kruus K. Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013;133:270–278.
- Babiker M, Abdel-Banat A, Hushida H, Ano A, Nonklang S, Akada R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010;85:861–867.
- Pessani NK, Atiyeh HK, Wilkins MR, Bellmer DD, Banat IM. Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresour. Technol. 2011;102:10618–10624.
- Faga BA, Wilkins MR, Banat IM. Ethanol production through simultaneous saccharification and fermentation of switchgrass using Saccharomyces cerevisiae D5A and thermotolerant Kluyveromyces marxianus IMB strains. Bioresour. Technol. 2010;101:2273–2279.
- Oda Y, Nakamura K, Shinomiya N, Ohba K. Ethanol fermentation of sugar beet thick juice diluted with crude cheese whey by the flex yeast Kluyveromyces marxianus KD-15. Biomass Bioenerg. 2010;34:1263–1266.
- Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004;66:10–26.
- Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilveblant NO. The generation of fermentation inhibitors during dilute acid hydrolysis of soft wood. Enzyme Microb. Technol. 1999;24:151–159.