Abstract
A Japanese patient with Nasu-Hakola disease was found to have a serine-to-asparagine (S39N) substitution in human DNAX-activation protein 12 (DAP12). To elucidate the functional abnormalities of mutant-type DAP12, we expressed wild-type and mutant-type recombinant DAP12 protein with Bombyx mori nucleopolyhedrovirus (BmNPV) vector, and successfully purified the respective proteins from the hemolymph of recombinant BmNPV infected B. mori larvae.
Nasu-Hakola disease (NHD; OMIM, http://omim.org/entry/221770) is a rare autosomal recessive disease characterized by lytic lesions in the bone and early onset of frontotemporal dementia.Citation1) It can be caused by recessive mutations in the DNAX-activation protein 12 (DAP12) gene on chromosome 19p13.1Citation2) or the triggering receptor expressed on myeloid cells 2 (TREM2) gene on chromosome 6p21.1.Citation3) DAP12 associates with cell-surface receptor TREM2, and the DAP12-TREM2 complex is involved in dendritic cell maturation.Citation4) The conserved aspartic acid at position 50 in the transmembrane region of DAP12 is required for this DAP12-TREM2 interaction.Citation5)
A Japanese patient with NHD was homozygous for a G116A transition that resulted in a serine-to-asparagine (S39N) substitution in DAP12.Citation6) The function of the serine at position 39 is not known. NMR studies have been conducted on the membrane protein of DAP12, which forms a dimer, but no connection between S39 and dimerization was found.Citation7) In this study, we compared the properties of the mutant-type DAP12 (S39N) protein with those of wild-type DAP12. We expressed each recombinant DAP12 (rDAP12) protein by Bombyx mori nucleopolyhedrovirus (BmNPV) vector and then purified the proteins from the hemolymph of BmNPV-infected B. mori larvae.
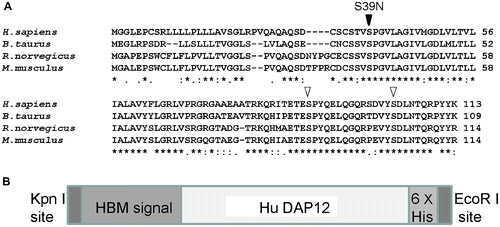
Based on the nucleotide sequences of the wild-type and mutant-type human DAP12 genes, we deduced the open reading frame (ORF) of each encoded 339 nucleotides and 113 amino acids. The molecular weight of the deduced wild-type protein (DAP12-N) was 12,179 Da, while that of the deduced mutant-type (DAP12-M) was 12,206 Da, and both proteins had a putative isoelectric point (pI) of 8.45. A search of the SMART database (http://smart.embl-heidelberg.de/) revealed that DAP12 contains a transmembrane domain from 42V to 64L. A NetPhos 2.0 (http://www.cbs.dtu.dk/services/NetPhos/) search indicated that DAP12-N has three putative serine phosphorylation sites: 39S, 89S, and 103S. In contrast, DAP12-M was predicted to have only two of these serine phosphorylation sites, 89S and 103S, because 39S was converted to asparagine (N) in the mutated type (Fig. (A), arrow). The serine at 39S in DAP12 has been found to be conserved in four mammalian species, Homo sapiens (O43914), Rattus norvegicus (Q6X9T7), Mus musculus (O54885), and Bos taurus (B2BBK0) (OrthoDB: http://cegg.unige.ch/orthodb6), suggesting that a mutation in DAP12 affects function (Fig. (A)). We also searched for a gene orthologous to DAP12 in B. mori by a tblastx search of GLEAN (http://sgp.dna.affrc.go.jp/pubdata/genomicsequences.html). No DAP12 ortholog was found in B. mori, even by merging of all the gene sets.
Fig. 1. DAP12 Protein Orthologs and Design of Recombinant BmNPV DAP12 Proteins. (A) Multiple DAP12 amino acid sequence alignment by CLUSTALW, DAP12 protein from H. sapiens (O43914), M. musculus (O54885), R. norvegicus (Q6X9T7), and B. taurus (B2BBK0). The site of the S39N Nasu-Hakola disease mutation is indicated by a black arrowhead, and S89 and S103 are indicated by white arrowheads. (B) Schematic diagram of the recombinant BmNPV DAP12 protein. Each of the sequences encoding the recombinant DAP12 proteins contains a 3′ Kpn I site following the sequences encoding the HBM signal sequence, the site where signalase-mediated cleavage occurs in the melittin peptide. The 5′ end of the sequence encodes a 6 × His tag and contains an EcoR I site. The oligonucleotides employed in this study were designed on the human DAP12 nucleotide sequence (NCBI reference sequence accession No. NM_003332).
In order to clone human DAP12, we amplified the ORF from the cDNA library of human Peripheral Blood Mononuclear Cells (PBMC).Citation8) A cDNA library was constructed from total RNA extracted from PBMCs with a SuperScript II cDNA synthesis kit (Invitrogen, Carlsbad, CA). A reverse transcription-polymerase chain reaction (RT-PCR) was run with two DAP12 gene-specific primers, 5′-GGGGGACTTGAACCCTGCAGCAGG-3′ and 5′-TCATTTGTAATACGGCCTCTGTGT-3′. DAP12-M DNA was generated by PCR with a QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), and DAP12-N DNA was amplified from the PBMC library cDNA template, with two internal complementary primers that encode for the G116A mutation 5′-AGTTGCTCTACGGTGAACCCGGGCGTGCTGGCA-3′ and 5′-TGCCAGCACGCCCGGGTTCACCGTAGAGCAACT-3′. The amplification products (wild-type and mutant-type) were cloned into pEF6/V5-His TOPO vector (Life Technologies, Carlsbad, CA), purified, and sequenced with an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
To clone the amplified DNAs into baculoviral transfer vector pBK283, which can be used for homologous recombination with a BmNPV genome, we amplified each of the human DAP12 DNAs (wild-type and mutant-type) by PCR with a Pfu Turbo DNA polymerase (Stratagene) and the following primers: 5′-GGGGTACCCCATGAAATTCTTAGTCAACGTTGCCCTTGTTTTTATGGTCGTATACATTTCTTACATCTATGCGGCCGCTCAGGCCCAGAGCGATTGCAG-3′ and 5′-GGAATTCCTCAGTGGTGGTGGTGGTGGTGTCATTTGTAATACGGCCTCT-3′.
The amplified region included the functional honey bee melittin (HBM) signal sequence and the products were secreted. Each PCR product was digested with EcoR I and Kpn I and cloned into pBK283 (Fig. (B)). Recombinant BmNPV was generated with a Bom-EX kit (Sysmex, Saitama, Japan) according to the suppliers instructions. Precise recombination in the polyhedrin gene locus was confirmed by PCR and DNA sequencing with primers. The purified recombinant virus was titrated in a plaque assay to produce high-titer stocks (2 × 107 pfu/mL). B. mori larvae (Kinshu × Showa, Ueda-Sha, Nagano, Japan) and BmN4 cells (Sysmex) maintained at 25 °C in TC-100 medium (AppliChem, Darmstadt, Germany) supplemented with 10% fetal bovine serum and Antibiotic-Antimycotic (Invitrogen) were infected with the high-titer stocks.
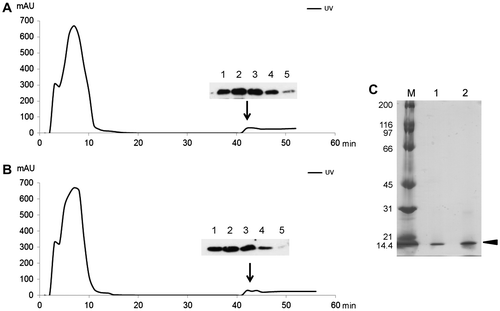
Recombinant BmNPV expressing DAP12-M or DAP12-N was assayed in BmN4 cell culture medium. The secretion of both recombinant proteins peaked at 72 h post- infection (hpi) in the culture medium (Fig. (A) and (C)), and the secretion of rDAP12 in the larval hemolymph increased to 96 hpi (Fig. (B) and (D)). Protein synthesis by BmNPV has been reported to begin 24 h after infection and to peak at 96 h.Citation9) Hence, we purified recombinant DAP12-M and DAP12-N from the larval hemolymph at 96 hpi. The HBM signal-fused DAP12 was efficiently secreted from the BmNPV-infected cells, even though DAP12 has a typical transmembrane region, and this simplified the purification of rDAP12. Briefly, hemolymph was collected from 10 BmNPV-infected larvae, and the rDAP12 proteins were purified with AKTA prime plus (GE Healthcare, Tokyo) with a HisTrap affinity column (GE Healthcare). Immunoblotting was used to identify the fractions containing the rDAP12 proteins (Fig. (A) and (B)), and SDS-PAGE was used to confirm that the molecular mass of the protein was 12 kDa (Fig. (C)). DAP12 proteins can be present in the hemolymph as a dimer (data not shown), and they were detected as 12 kDa bands at the last purification step. The anti-DAP12 antibody (sc-20783; Santa Cruz Biotechnology, Santa Cruz, CA) reacted with both rDAP12 proteins, and both produced 12-kDa bands on immunoblot (Fig. ). Thus, the antigenicity of anti-DAP12 antibody did not differ between the wild-type and mutant-type rDAP12 proteins. Finally, we purified the two types of rDAP12 protein: 475 μg of DAP12-N and 201 μg of DAP12-M. The conformation of DAP12-M might have differed from that of DAP12-N, because the elution fraction of DAP12-M suggested the presence of two peaks. DAP12-M is thought to have a different binding affinity for Ni. Thus, the conformation of DAP12-M might have differed from that of DAP12-N. However, no differences were identified between DAP12-N and DAP12-M for any parameter examined in this study. We have not yet determined whether the serine 39 in DAP12-N is phosphorylated.
Fig. 2. Expression of Wild-type and Mutant-type rDAP12 Proteins with BmNPV in B. mori Larva and BmN4 Cells.
Expression levels of the rDAP12 proteins with amino acid-coding sequences derived from a healthy subject (DAP12-N) in BmN4 cells (A) and from silkworm larvae (B), from a patient with Nasu-Hakola disease (DAP12-M) in BmN4 cells (C), and from silkworm larvae (D). Protein samples (5 μg) were separated by a SDS-PAGE, transferred to nitrocellulose membranes by the method of Towbin et al. Citation10) and immunoblotted with rabbit anti-DAP12 antibody (SC-20783; Santa Cruz Biotechnology, Santa Cruz, CA) and goat anti-rabbit IgG-conjugated horseradish peroxidase (HRP) (Santa Cruz Biotechnology). The membranes were developed with a chemiluminescent substrate (Pierce, Rockford, IL).
The graphs show the intensity of the respective rDAP12 protein bands plotted against time after infection. Lower panels are immunoblots of the rDAP12 proteins. Lane 1, 24 hours after infection (hpi); lane 2, 48 hpi; lane 3, 72 hpi; lane 4, 96 hpi.
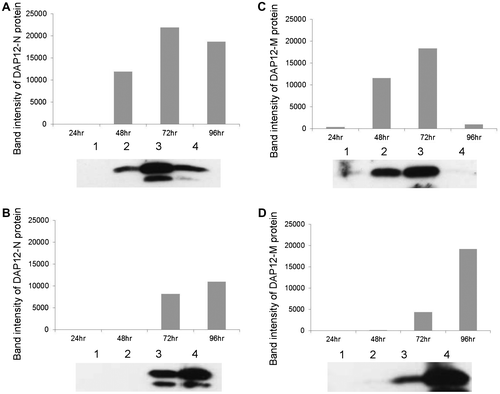
Fig. 3. Purification of rDAP12 Proteins.
Hemolymph collected from virus-infected larvae was diluted with phosphate-buffered saline (PBS; 10 mM phosphate, 150 mM NaCl, pH 6.5 containing 2 mM glutathione), and centrifuged at 10,000 × g for 15 min. The supernatant was collected and dialyzed against binding buffer (20 mM sodium phosphate, 0.5 M NaCl, 20 mM imidazole, pH 7.4), and then applied to a HisTrap affinity column that had been equilibrated with binding buffer. Elution buffer comprising 20 mM sodium phosphate, 0.5 M NaCl, and pH 7.4 diluted with imidazole on a linear gradient (20–500 mM) was supplied at a flow rate of 30 mL/h by AKTA Prime Plus (GE Healthcare). The protein concentration was monitored spectrophotometrically using the ratio of protein absorbance at 280 nm. The purity of each of the rDAP12 proteins was confirmed by 15% SDS-PAGE analysis and immunoblotting. (A) Wild-type protein (DAP12-N), (B) S39N-mutant type protein (DAP12-M). Upper and lower panels show immunoblots of the rDAP12 proteins in the various elution fractions. (C) Lane M, marker, purified rDAP12 protein; lane 1, DAP12-N; lane 2, DAP12-M.
In future studies, we plan to determine whether phosphorylation at serine 39 plays a role in the normal functioning of DAP12 protein and the dysfunction of the S39N mutated form. Further, we plan to determine whether there are conformational changes in DAP12 proteins by NMR analysis. These purified rDAP12 proteins are potentially useful in studies of the molecular mechanisms involved in NHD.
References
- Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, et al. Cell. Mol. Neurobiol. 2004;24:1–24.
- Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, et al. Nat. Genet. 2000;25:357–361.
- Klunemann HH, Ridha BH, Magy L, Wherrett JR, Hemelsoet DM, et al. Neurology. 2005;64:1502–1507.
- Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. J. Exp. Med. 2003;198:669–675.
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Nature. 1998;391:703–707.
- Kaneko M, Sano K, Nakayama J, Amano N. Neuropathology. 2010;30:463–470.
- Call ME, Wucherpfennig KW, Chou JJ. Nat. Immunol. 2010;11:1023–1029.
- Satoh J, Shimamura Y, Tabunoki H. Cell. Mol. Neurobiol. 2012;32:337–343.
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, Saeki Y, Sato Y, Furusawa M. Nature. 1985;315:592–594.
- Towbin H, Staehelin T, Gordon J. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354.
