Abstract
Chimeric enzymes between a cold-adapted isocitrate lyase (ICL) of a psychrophilic bacterium, Colwellia maris, (CmICL) and a mesophilic ICL of a nitrogen-fixing bacterium, Azotobacter vinelandii, (AvICL) were constructed by dividing the ICL genes into four regions of almost equal length and exchanging regions in various combinations. The chimeric ICL, which was replaced C-terminal region 4 of AvICL by the corresponding region of CmICL, showed much lower specific activity and lower optimum temperature and thermostability for activity than wild-type AvICL, indicating that region 4 is involved in its thermal properties. Furthermore, mutual substitution between the Met501 residue in region 4 of CmICL and the corresponding Ile504 residue of AvICL influenced the temperature dependence of their activities, suggesting that these amino acid residues are important to the respective mesophilic and cold-adapted properties of AvICL and CmICL.
Graphical Abstract
Chimeric enzymes of cold-adapted isocitrate lyase of psychrophilic Colwellia maris (CmICL) and mesophilic ICL of Azotobacter vinelandii (AvICL) were constructed by dividing their genes into four regions of almost equal length.
A key enzyme of the glyoxylate cycle, isocitrate lyase (ICL, EC 4.1.3.1), catalyzes the cleavage of isocitrate to glyoxylate and succinate and plays an important role in the acetate and fatty acid metabolism of micro-organisms and plants. Since isocitrate is also the substrate of isocitrate dehydrogenase which catalyzes the oxidative decarboxylation of isocitrate to 2-oxoglutarate and CO2 in the TCA cycle, ICL is also involved in the control of the flux of isocitrate between the two metabolic pathways.Citation1)
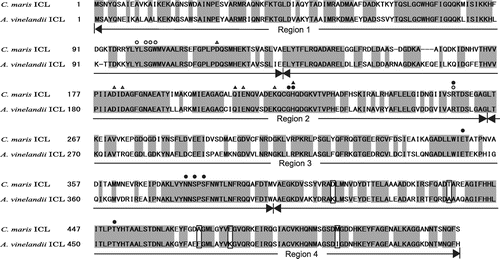
The ICL of a mesophilic nitrogen-fixing bacterium, Azotobacter vinelandii, (AvICL) exhibits maximum activity at 40 °C and retains more than 80% of its activity after incubation for 10 min at 45 °C,Citation2) indicating that it is mesophilic. On the other hand, the optimum temperature for the activity of the ICL of a psychrophilic marine bacterium, Colwellia maris, (CmICL) is 20 °C.Citation3) This enzyme is remarkably thermolabile, and about 80% of its activity is lost under incubation for 2 min at 30 °C. Thus, the CmICL is a cold-adapted enzyme. The two ICLs, then, show thermal properties different from each other, while the homology between their amino acid sequences is high (71% identity) (Fig. ). Bacterial ICLs are categorized into two subfamilies (1 and 3), and AvICL and CmICL are categorized into subfamily 3.Citation4) Studies of subfamily 3 ICLs are limited, but several properties of ICLs from C. maris,Citation3) Colwellia psychrerythraea,Citation5,6) Acinetobacter calcoaceticus,Citation7) Capriavidus necator,Citation8) and A. vinelandiiCitation2) have been reported.
Fig. 1. Amino acid sequences of ICLs from A. vinelandii and C. maris.
Note: Letters in gray boxes indicate conserved amino acid residues between the two ICLs, and boxed letters indicate amino acid residues exchanged by site-directed mutagenesis in this study. The amino acid residues involved in binding with substrates, glyoxylate (○) and succinate (●), and the metal ion (Δ), and the tetrameric assembly of the enzyme protein (▲) in the E. coli ICL are indicated.
In preliminary studies, substitutions of amino acid residues were done in CmICL, AvICL, and the cold-adapted ICL of C. psychrerythraea (CpICL), and the thermal properties, such as optimum temperature and thermostability for activity, of the mutated enzymes were examined.Citation2,4,6) From these studies, several amino acid residues (the Gln207 and Gln217 residues of CmICL, the Ile504 residue of AvICL, and the Ala214 residue of CpICL) were suggested to be involved in their thermal properties, but the possibility that other amino acid residues of ICLs contribute to their thermal properties remain to be tested. Furthermore, structural factors involved in the psychrophilic properties of various cold-adapted enzymes, such as clustering of Gly residues and reduction in Arg residues, have been reported.Citation9) As a first step to understand further the adaptation mechanisms of cold-adapted ICLs, such as CmICL and CpICL, to low temperatures, identification of the region of the enzyme proteins responsible for the differences in thermal properties between AvICL and CmICL was attempted in this study. However, since no crystal structures of subfamily 3 ICL proteins have been resolved, the structural units of the two enzymes, including the domain, remain unclear. Hence, the two ICL genes were divided into four regions of almost equal length (Fig. ) and chimeric ICL genes were constructed by exchanging the regions of the two ICLs in various combinations and the properties of the chimeric ICLs were investigated. In addition, since C-terminal region 4 of AvICL was suggested to be involved in these thermal properties, several mutated CmICL and AvICL genes, in which the corresponding amino acid residues in this region of the two ICLs were replaced mutually, were constructed by site-directed mutagenesis, and the thermal properties of the mutated enzymes were examined.
Materials and methods
Bacteria, plasmids, and culture condition
Escherichia coli strain TOP10 (Invitrogen, Carlsbad, CA) was used for the amplification of plasmids and the production of His-tagged ICL proteins. Plasmid pTrcHisB (Invitrogen) was used as a vector in conferring the N-terminal (His)6-tag on the expressed proteins. Plasmids pHis-CmWTCitation4) and pHis-AvWTCitation2), containing the CmICL and AvICL genes, respectively, in the BamHI-SacI site of pTrcHisB, were used as template DNAs in the construction of chimeric ICL genes and in site-directed mutagenesis. Unless otherwise noted, E. coli strain TOP10 was cultured at 37 °C in Luria–Bertani medium.Citation10)
Construction of chimeric ICL genes
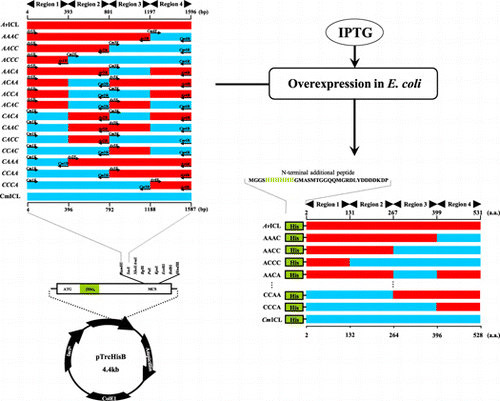
Chimeric genes between cold-adapted CmICL and mesophilic AvICL were constructed as reported previously.Citation11) Four DNA fragments of almost equal length of the two ICL genes (containing about 130 amino acid residues, regions 1–4 in Fig. ) were amplified by PCR. These chimeric ICL genes were termed CmICL (C) and AvICL (A) based on regions 1–4 of the genes. For example, chimeric gene consisting of region 1 of CmICL and regions 2–4 of AvICL was termed ACCC. The reaction mixture (50 μL) contained 50 ng of template DNA, 15 pmol each of the forward and reverse primers, and 1 U KOD-Plus-DNA polymerase (Toyobo, Osaka, Japan) in a buffer system prepared by the manufacturer. Amplification was done through 30 cycles in a DNA thermal cycler (GeneAmp PCR System 2400; Perkin–Elmer, Foster City, CA). The cycling conditions were as follows: denaturation at 94 °C for 15 s, annealing for 30 s, and extension at 68 °C for 24 s per region of the ICL genes, through each of the 30 cycles. The template DNA, primers, annealing temperature, and extension time used for the construction of each of the chimeric ICL genes are shown in Supplemental Tables 1 and 2 (see Biosci. Biotechnol. Biochem. http://dx.doi.org/10.1080/09168451.2014.882744). By these rounds of PCR, cleavage sites for the restriction enzymes, BamHI and SacI, were introduced at the 5′- and 3′-terminals of the two ICL genes, respectively. The purified PCR products were digested with BamHI or SacI and ligated to the BamHI-SacI site of pTrcHisB. To confirm precise production of the chimeric ICL genes, the coding region of each gene was sequenced with a BigDye Terminator V 1.1 Cycle Sequencing Reaction kit (Applied Biosystems, Foster City, CA) with an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems).
Site-directed mutagenesis
Site-directed mutagenesis was carried out by triple PCR.Citation4) Primers to introduce the mutations into the CmICL and AvICL genes were designed as shown in Supplemental Table 3. In the first PCR, Cm1F and Av1F (Supplemental Table 1) as forward primer and reverse primer to introduce substitutions of amino acid residues (D410K-R, T436A-R, A470E-R, E477K-R, and M501I-R for the mutagenesis of the CmICL gene and K413D-R, A439T-R, E473A-R, and K480E-R for that of the AvICL gene (Supplemental Table 3) and pHis-CmWT and pHis-AvWT as template were used to obtain 5’-terminal fragments of the mutated ICL genes. In the second PCR, Cm4R and Av4R (Supplemental Table 1) as reverse primer and forward primer for the substitution of amino acid residues (D410K-F, T436A-F, A470E-F, E477K-F, and M501I-F for mutagenesis of the CmICL gene, and K413D-F, A439T-F, E473A-F, and K480E-F for that of the AvICL gene) and template DNA same as the first PCR were used to obtain 3′-terminal fragments of the mutated ICL genes. In the final PCR, the first and second PCR products as template DNA and Cm1F and Cm4R for the CmICL mutants and Av1F and Av4R for the AvICL mutants as forward and reverse primers, respectively, were used to obtain the full lengths of the mutated genes. Reaction mixture of each PCR (50 μL) contained 50 ng of template DNA, 15 pmol each of the forward and reverse primers, and 1 U KOD-Plus-Neo DNA polymerase in a buffer system prepared by the manufacturer. Amplification was done over 30 cycles in a DNA thermal cycler (Verti 96 well Thermal cycler, Applied Biosystems). The cycling conditions were as follows: denaturation at 98 °C for 10 s, annealing for 30 s, and extension at 68 °C for each of the 30 cycles. The annealing temperatures and extension times are summarized in Supplemental Table 4. The final PCR products were digested with BamHI and SacI, and then ligated to the BamHI-SacI site of pTrcHisB. To certify the introduced mutations, the relevant regions of the plasmids were sequenced in both directions, as described above.
Overexpression and purification of His-tagged ICLs
According to a previous report,Citation4) E. coli strain TOP10 transformed with pTrcHisB carrying wild-type or mutated ICL genes was grown and the expression of His-tagged ICL proteins was induced with isopropyl-β-d-thiogalactopyranoside (IPTG), except for further incubation of the culture for 18–22 h at 10 °C after the addition of IPTG. Cells were harvested by centrifugation and resuspended in 30 mL of a sonication buffer (50 mM sodium phosphate buffer (pH 6.85) containing 2 mM MgCl2, 10 mM imidazole, 0.5 M NaCl, 10% glycerol, and 10 mM 2-mercaptoethanol) per liter of culture. A final concentration of 2 mg/mL of lysozyme was added to the suspension, and the mixture was shaken for 1.5–2 h at 4 °C. A serine protease inhibitor, phenylmethylsulfonyl fluoride (PMSF, final concentration 1 mM), was added to the mixture, and then the cells were disrupted by ultrasonic oscillation. After centrifugation of the cell lysate at 17,741 × g for 20 min at 0 °C to remove cell debris, the same concentration of PMSF was added to the supernatant and this was centrifuged 59,309 × g for 1 h at 4 °C. After further addition of 1 mM PMSF, the resultant supernatant was loaded on a Ni-NTA column (25 mL; Qiagen, Hilden, Germany) equilibrated with the sonication buffer. After a thorough washing with 200 mL of the sonication buffer, the column was further washed with 50 mL of buffer A (the sonication buffer containing 20 mM imidazole instead of 10 mM imidazole), and next with 50 mL of buffer B (buffer A containing 50 mM imidazole instead of 20 mM imidazole), and the enzyme was finally eluted with 50 mL of an elution buffer (buffer B containing 250 mM imidazole instead of 50 mM imidazole). The elutant was concentrated with polyethylene glycol #6000 and dialyzed against 20 mM sodium phosphate buffer (pH 6.85) containing 2 mM MgCl2, 0.25 M NaCl, 5 mM trisodium citrate, 1 mM dithiothreitol (DTT), and 50% glycerol. All purified enzymes were stored at −30 °C until use.
Enzyme assay
ICL activity was assayed at pH 6.85, as described previously.Citation6) The reaction was started by the addition of substrate to the reaction mixture. One unit of ICL activity was defined as the amount of enzyme that forms 1 μmol of glyoxyl-phenylhydrazone per min. The protein concentration was measured by the method of Lowry et al.Citation12) with bovine serum albumin as a standard. To investigate the thermostability of the ICL activity, the purified enzymes were diluted to a protein concentration of 0.2 mg/mL with 20 mM sodium phosphate buffer (pH 6.85) containing 2 mM MgCl2, 0.25 M NaCl, 5 mM trisodium citrate, and 1 mM DTT. They were then dialyzed overnight at 4 °C against 20 mM potassium phosphate buffer (pH 6.85) containing 2 mM MgCl2 and 1 mM DTT. The dialyzed samples were stored on ice and used within 24 h. The kinetic parameters, Vmax, Km, and kcat, of ICL were calculated by Lineweaver-Burk analysis with various concentrations of isocitrate. The molecular masses of the wild-type and the mutant ICLs (AvWT, CmWT, AvI504M, and CmM501I) with the additional N-terminal His-tag were calculated to be 250,436, 246,633, 250,508, and 246,561 Da, respectively, from the amino acid sequences. All data reported for activity and kinetic parameters are mean values for at least two independent experiments.
Western blot analysis
After induction by IPTG as described above, 1 mL of growth culture of E. coli TOP10 transformed with pTrcHisB harboring the chimeric ICL genes was withdrawn, and the cells were harvested by centrifugation. Then, to obtain cell lysate, the cell pellets were resuspended with 50 μL of SDS-PAGE sample buffer containing 50 mM Tris–HCl (pH 6.8), 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 0.71 M of 2-mercaptoethanol, and boiled. After SDS-PAGE of the purified ICLs and the cell lysate with 10% polyacrylamide gel at 120 V,Citation13) the proteins on the gels were transferred to a nitrocellulose membrane (Immobilon-P; Millipore, Bedford, MA) following the manufacturer’s instructions. Western blot analysis of this membrane was carried out at room temperature, as follows: The blotted membrane was washed for 1 min with TBS-T solution (20 mM Tris–HCl (pH 7.6) containing 137 mM NaCl) and soaked overnight in a blocking solution (TBS-T solution containing 5% Irish Cream). Then the membrane was washed twice for 10 min each time with TBS-T solution and incubated for 50 min with rabbit antibody against purified CmICL (20 mg/mL)Citation14) diluted to 1:50,000 in TBS-T solution. After two washings for 10 min with TBS-T solution, the membrane was incubated for 50 min with donkey antibody against rabbit IgG (GE Healthcare, Buckinghamshire, UK) diluted to 1:50,000 in TBS-T solution, and then washed twice for 10 min each time with TBS-T solution. The target protein was detected with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA).
Results
Construction and overexpression of chimeric ICLs in E. coli
Fourteen chimeric enzymes (termed in the same manner as their gene names) were constructed as described in “Materials and Methods,” and the wild-type and chimeric enzyme proteins with His-tags at the N-terminals were overexpressed in E. coli TOP10 cells and purified. However, no ICL activity was detected in many purified chimeric enzymes, containing AACA, ACAA, ACCA, CAAA, CCAA, and CCCA. Western blot analysis of cell lysate of E. coli overexpressing each of the chimeric ICL proteins by IPTG revealed that the chimeric ICLs with no activities (ACCA, AACA, CCAA, and CCCA) had smaller molecular masses than the wild-type CmICL, and that cross-reactive band(s) were hardly present in CACC, CAAC, CACA, and CAAA (data not shown). These results suggest that these eight chimeric enzyme proteins were digested in the E. coli cells during the expression of them. Furthermore, the purified ACAC and ACAA showed very low and no activity, respectively, and only a faint band was detected on SDS-PAGE and Western blot analysis.
The other chimeric ICLs (AAAC, AACC, ACCC, and CCAC) and the wild-type ICLs (AvWT and CmWT) were almost homogeneously purified. However, in spite of the same induction conditions, the yields of purified chimeric ICLs (AACC, ACCC, and CCAC) and CmWT were less than that of AvWT, estimated at 4.80, 2.09, 2.24, 3.50, and 11.82 mg protein per liter of culture, respectively (mean values of two or three independent experiments). On the other hand, the yield of purified AAAC (12.54 mg protein per liter of culture) was similar to that of AvWT.
Thermal properties of chimeric ICLs
Wild-type and chimeric ICL activities were assayed at various temperatures (Fig. ). Based on a comparison of purified native CmICL and its purified recombinant enzyme with His-tag, it was reported that the addition of a His-tag at the N-terminal of CmICL did not affect specific activity, optimal pH for activity, and thermal properties, such as optimum temperature and thermostability for activity.Citation4) On the other hand, since the optimal pH for the activity of His-tagged CmICL and AvICL was found to be 6.85 and 6.7, respectively,Citation2,4) an enzyme assay of all the ICLs was carried out at pH 6.85. In accord with previous reports,Citation2,4) the optimum temperatures for the activities of AvWT and CmWT were 40 °C and 20–25 °C, respectively. However, the specific activity of AvICL purified in this study was about six-fold higher than that in the previous study. This discrepancy may be attributable to the improved purification procedure used in this study, including the addition of PMSF, and the different assay pH for ICL activity (pH 7.8 in the previous reportCitation2)). All the chimeric ICLs showed much lower specific activities than AvWT and optimum temperatures for activities similar to that of CmWT. On the other hand, only AAAC showed slightly higher specific activity than CmWT.
Fig. 2. Effects of temperature on the activity of wild-type and chimeric ICLs.
Note: ICL activity was assayed at the indicated temperatures. Symbols: AvWT (○), CmWT (●), AAAC (Δ), AACC (▲), ACCC (□), and CCAC (■).
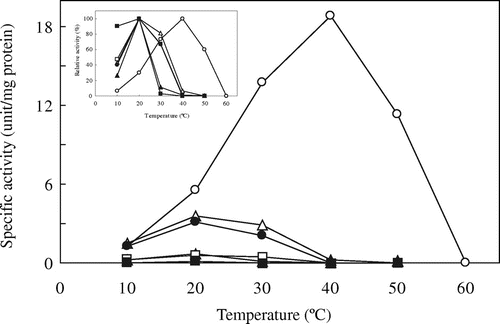
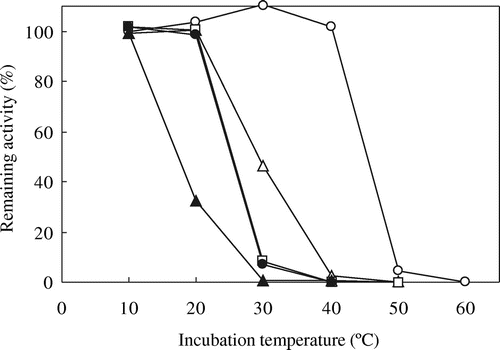
Fig. shows thermostability for the activities of the wild-type and chimeric ICLs. AvWT maintained its activity perfectly under incubation at 40 °C, while CmWT lost more than 90% of activity after incubation at 30 °C for 10 min. Among the chimeric ICLs, only AAAC showed higher thermostability than CmWT at 30 °C, but AACC showed lower thermostability than CmWT at 20 °C. Furthermore, ACCC showed almost the same thermostability as CmWT, at all temperatures tested. On the other hand, all the chimeric ICLs showed considerably lower thermostabilities than AvWT. The thermostability of CCAC could not be determined because of its very low specific activity and the poor yield of the purified protein.
Fig. 3. Thermostability of wild-type and chimeric ICLs.
Note: The thermostabilities of ICL activities were assayed at 20 °C after incubation at the indicated temperatures for 10 min and further incubation on ice for 10 min, and the remaining activities were expressed as percentages of ICL activity without incubation. Symbols are the same as in Fig. .
Overexpression and purification of mutated ICLs
The results presented in Figs. and suggest that C-terminal region 4 of AvICL is involved in its thermal properties. Several replacements of amino acid residues by ones with different properties, (such as charge, hydrophobicity, and so on) were found in this region of AvICL and CmICL (Fig. ), suggesting that such substitutions are responsible for the different thermal properties between the two ICLs. Among them, five amino acid residues were selected (Fig. boxed residues), and the mutated genes of AvICL and CmICL, which were replaced with the corresponding amino acid residues different from each other, were constructed by site-directed mutagenesis, as described in “Materials and Methods.” The mutated ICLs were then overexpressed in E. coli TOP10 cells and purified almost to homogeneity. Unlike the chimeric ICLs, the mutated ICLs were overexpressed and purified without difficulty.
Thermal properties of the mutated ICLs
The temperature dependences of the mutated AvICL and CmICL are shown in Figs. and , respectively. Among the mutated AvICLs (Fig. inset figure), two mutants, AvA439T and AvK480E, in which Ala439 and Lys480 of AvICL were replaced by Thr and Glu residues, respectively, maintained higher relative activities at 50 °C, higher than their optimum temperatures for activity, than AvWT. In contrast, AvK413D and AvI504M, in which Lys413 and Ile504 of AvICL were replaced by Asp and Met residues, respectively, showed slightly higher relative activities at 30 °C than AvWT. However, the specific activities of all the mutated AvICLs were much lower than that of AvWT. On the other hand, among the mutated CmICLs (Fig. inset figure), the relative activity of CmM501I, in which Met501 of CmICL was replaced by Ile residue (corresponding to the mutated AvICL, AvI504M), at 30 °C, higher than the optimum temperature for activity, was higher than that of CmWT. Furthermore, all the mutated CmICLs showed lower specific activity than CmWT.
Fig. 4. Effects of temperature on the activity of the wild-type and mutated AvICLs.
Note: ICL activity was assayed at indicated temperatures. Symbols: AvWT (○), AvK413D (●), AvA439T (Δ), AvE473A (▲), AvK480E (□), and AvI504M (■).
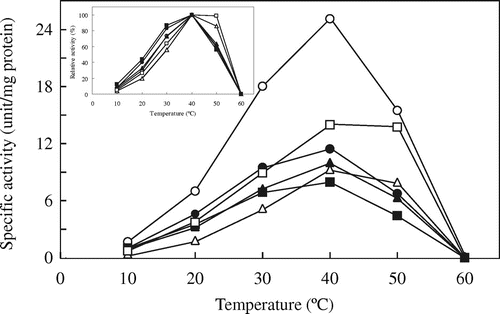
Fig. 5. Effects of temperature on the activity of the wild-type and mutated CmICLs.
Note: ICL activity was assayed at the indicated temperatures. Symbols: CmWT (○), CmD410K (●), CmT436A (Δ), CmA470E (▲), CmE477K (□), and CmM501I (■).
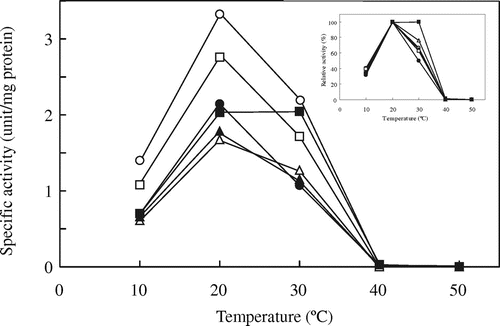
The residual activities of mutated AvICL and CmICL were assayed at 20 °C after incubation at various temperatures for 10 min (Figs. and , respectively). Among the mutated AvICLs, the thermostability for the activities of AvI504M and AvE473A, in which Glu473 was replaced by Ala residue, were slightly decreased at 40 °C as compared with that of AvWT, and the other mutated AvICLs showed almost the same thermostability as AvWT. On the other hand, the thermostabilities for activities of the two mutated CmICLs, CmD410K and CmA470E, in which Asp410 and Ala470 of CmICL were replaced by Lys and Glu residues, respectively, were lower at 20 °C than that of CmWT. In particular, CmD410K lost about 30% of its activity after incubation at 20 °C. No increase in thermostability was observed in the mutated AvICLs or CmICLs.
Fig. 6. Thermostability of wild-type and mutated AvICLs.
Note: The thermostabilities of ICL activities were assayed at 20 °C after incubation at the indicated temperatures for 10 min and further incubated on ice for 10 min. The remaining activities are expressed as percentages of ICL activity without incubation. Symbols are the same as in Fig. .
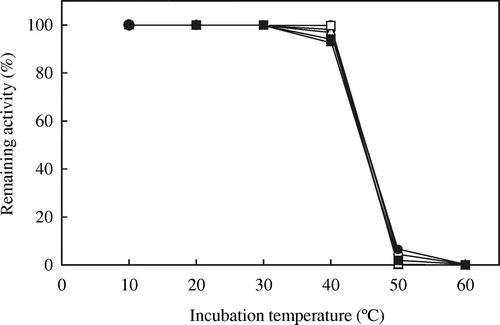
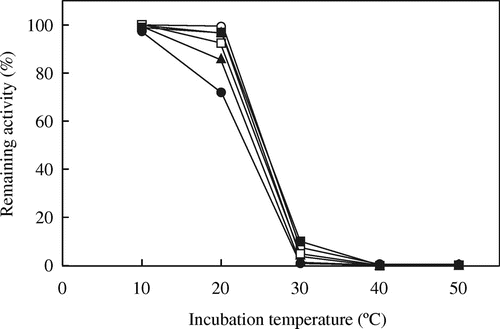
Fig. 7. Thermostability of wild-type and mutated CmICLs.
Note: The thermostabilities of ICL activities were assayed at 20 °C after incubation at the indicated temperatures for 10 min and further incubated on ice for 10 min. The remaining activities are expressed as percentages of ICL activity without incubation. Symbols are the same as in Fig. .
Since among the mutations of AvICL and CmICL, the AvI504M and CmM501I mutations appeared to affect their thermal properties, the kinetic parameters of the two mutated ICLs were compared with the respective wild-type ones (Table ). The values of Km for isocitrate and kcat of CmWT were lower than that of AvWT, but difference for the latter value was larger than that for the former one. Hence, the kcat/Km value of AvWT was higher than that of CmWT. The kinetic parameters of AvI504M and CmM501I were almost the same as those of the respective wild-type ICLs, except that the kcat/Km value of AvI504M was lower than that of AvWT.
Table 1. Kinetic parameters of the wild-type and mutated ICLs at 20 °C.
Discussion
In this study, the activity of many purified chimeric ICLs was not detected in spite of improvements in the conditions for IPTG induction of ICL gene expression. Perhaps these chimeric proteins were partially digested during gene expression in the E. coli cells. Since the chimeric ICLs were constructed by divided ICL genes without consideration of the structures of the AvICL and CmICL proteins, this might have led to structural instabilization and might have caused partial digestion in the E. coli cells. On the other hand, Watanabe and TakadaCitation4) reported that the expression levels of the genes for CmWT and its substitutional mutants of the amino acid residue introduced by site-directed mutagenesis in the E. coli cells were much lower than that of the E. coli ICL (EcICL), and that the wild-type and mutated CmICLs were more sensitive to tryptic digestion than EcICL. From these results, they suggested that the low expression of the CmICLs was due to post-translational proteolysis in the E. coli cells. Since the yields of CmWT and most of the chimeric ICLs expressed in the E. coli cells were also lower than that of AvWT in this study, these ICLs might have been post-translationally proteolyzed in the E. coli cells. Similar to this study, chimeric enzymes between isocitrate dehydrogenases (IDHs) of C. maris and A. vinelandii have been constructed by dividing the two IDH genes into four regions of almost equal length and the exchange of regions in various combinations.Citation11) In addition, based on the crystal structure of the A. vinelandii IDH, chimeric enzymes in which three regions of the two IDHs were replaced in various combinations were constructed.Citation15) In these two cases, all the chimeric IDHs retained their catalytic activities. The two IDHs were monomeric proteins with molecular masses of about 80 kDa, whereas the C. maris ICL is known to be homotetrameric.Citation3) Therefore, the more complex structure of ICL proteins as compared with the IDH proteins may make it difficult to acquire active chimeric ICLs.
Although chimeric ICL, ACCC, showed lower specific activity than CmWT (CCCC), they showed almost the same thermostability and optimum temperature for activity (Figs. and ). This suggests that region 1, located at the N-terminal, of CmICL does not contribute to the thermostability but is involved in its catalytic activity. On the other hand, AAAC showed markedly lower specific activity, optimum temperature, and thermostability for activity than AvWT (AAAA), indicating that C-terminal region 4 of the AvICL contributes considerably to its thermal properties. When AACC was compared to ACCC, no noticeable difference was observed in their temperature dependence for activity, but ACCC showed higher thermostability than AACC, suggesting that not only the specified region but also appropriate combinations of regions are important for their thermal properties.
No remarkable change in thermostability for activity was observed between AvWT and the mutated AvICLs, except that AvE473A and AvI504M were slightly less thermostable at 40 °C than AvWT (Fig. ). In contrast, among the mutated CmICLs, CmD410K and CmA470E showed slightly decreased thermostability at 20 °C as compared to CmWT (Fig. ). Thus, CmA470E and its corresponding AvICL mutant, AvE473A, showed decreased thermostability. These results suggest that Glu473 of AvICL and the corresponding Ala470 of CmICL are involved in their thermostability. On the other hand, AvK413D, AvI504M, and CmM501I showed temperature dependence for activity different from the respective wild-type ICLs (Figs. and inset figures). AvI504M and CmM501I are mutants of AvICL and CmICL, respectively, in which the corresponding position of amino acid residues (Ile504 and Met501) in the two ICLs replaced each other, and the two amino acid residues may be involved in their thermal properties. Tanaka et al.Citation2) have reported that the Ile504 residue of AvICL is conserved in ICLs of mesophilic bacteria and is replaced by Met in the cold-adapted ICLs of the psychrophilic bacteria C. maris and C. psychrerythraea, and that the I504M mutation resulted in a decreased optimum temperature and thermostability for activity. Thus, these amino acid residues may be important to the mesophilic properties of AvICL. On the other hand, the present study elucidated that the corresponding amino acid residue, Met501, of CmICL is involved in its psychrophilic properties. Since the values of the hydropathy index for Ile and Met are 4.5 and 1.9, respectively, the former is more hydrophobic than the latter. Furthermore, the overall hydrophobicity of cold-adapted enzyme proteins have been reported to be lower than the mesophilic counterparts.Citation9) These might have been responsible for the altered thermal properties found for AvI504M and CmM501I.
In this study, single mutations were merely introduced in AvICL and CmICL, but appropriate combinations of amino acid residues may be involved in their thermal properties. Therefore, further studies of multiple mutants are planned to confirm this possibility.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.882744.
Supplementary Fig. 1
Download MS Power Point (195.5 KB)Supplemental Table 4. The Condition of PCR for Site-Directed Mutagenesis
Download PDF (420.3 KB)Supplemental Table 3. Oligonucleotides Used for Site-Directed Mutagenesis
Download PDF (459.6 KB)Supplemental Table 2. The Condition of PCR for the Construction
Download PDF (591.4 KB)Supplemental Table 1. Oligonucleotides Used for the Construction of Chimeric ICL Genes
Download PDF (436.9 KB)Notes
Abbreviations: PCR, polymerase chain reaction; PAGE, polyacrylamide gel electrophoresis.
References
- Cozzone AJ. Annu. Rev. Microbiol. 1998;52:127–164.10.1146/annurev.micro.52.1.127
- Tanaka Y, Hayashi T, Yamaoka N, Takada Y. Biosci. Biotechnol. Biochem. 2012;76:78–83.10.1271/bbb.110518
- Watanabe S, Takada Y, Fukunaga N. Biosci. Biotechnol. Biochem. 2001;65:1095–1103.10.1271/bbb.65.1095
- Watanabe S, Takada Y. Microbiology. 2004;150:3393–3403.10.1099/mic.0.27201-0
- Watanabe S, Yamaoka N, Fukunaga N, Takada Y. Extremophiles. 2002b;6:397–405.10.1007/s00792-002-0271-x
- Sato Y, Watanabe S, Yamaoka N, Takada Y. Extremophiles. 2008;12:107–117.10.1007/s00792-007-0115-9
- Hoyt JC, Johnson KE, Reeves HC. J. Bacteriol. 1991;173:6844–6848.
- Wang ZX, Brämer CO, Steinbüchel A. FEMS Microbiol. Lett. 2012;228:63–71.
- Georlette D, Blaise V, Collins T, D’Amico S, Gratia E, Hoyoux A, Marx J-C, Sonan G, Feller G, Gerday C. FEMS Microbiol. Rev. 2004;28:25–42.10.1016/j.femsre.2003.07.003
- Sambrook J, Russell D. Molecular cloning: a laboratory manual. 3rd ed. Cold spring Harbor, NY: Cold spring Harbor Laboratory; 2001.
- Yoneta M, Sahara T, Nitta K, Takada Y. Curr. Microbiol. 2004;48:383–388.10.1007/s00284-003-4203-5
- Lowry HO, Rosebrough JN, Farr LA, Randall JR. J. Biol. Chem. 1951;193:265–275.
- Laemmli UK. Nature. 1970;227:680–685.10.1038/227680a0
- Watanabe S, Yamaoka N, Takada Y, Fukunaga N. Microbiology. 2002a;148:2579–2589.
- Watanabe S, Yasutake Y, Tanaka I, Takada Y. Microbiology. 2005;151:1083–1094.10.1099/mic.0.27667-0
