Abstract
We quantified trans-resveratrol in each part of fresh Polygonum cuspidatum harvested in Kochi Prefecture. A small amount of trans-resveratrol was detected in the edible stem parts, the content varying with seasonal, geographical, and environmental factors. We also examined the antioxidative activity of each part, suggesting that P. cuspidatum contained many more contributing antioxidants other than resveratrol.
Polygonum cuspidatum, also called Japanese knotweed, is an invasive plant originating in East Asia and has spread to Europe and North America.Citation1–3) The rhizome of P. cuspidatum is commonly used in Chinese medicine to treat inflammatory disease, hepatitis, tumors, and diarrhea.Citation4) In a few places in Japan including Kochi Prefecture, the shoots of P. cuspidatum, known as Itadori, are harvested in spring and eaten as a food. P. cuspidatum has recently been drawing attention from researchers as a notable source of trans-resveratrol (trans-3,5,4′-trihydroxy-stilbene), a kind of polyphenol. Resveratrol has been found to promote human health due to its anti-cancer, antioxidative, anti-fungal, and anti-inflammatory effects.Citation5,6) Many researchers have studied the resveratrol content of dried rhizomes of P. cuspidatum. There have been, however, few studies on the upper ground parts, especially the edible stem part, and there is no available data on the amount of resveratrol and its derivatives in Itadori as a dietary component. We quantified in the present study the resveratrol contents in the edible and inedible parts of fresh P. cuspidatum. Seasonal changes and regional differences in content were apparent, and the antioxidative function of resveratrol in the samples was evaluated.
The samples were harvested in the west (Shimanto), central (Kagami), and east (Muroto) regions of Kochi Prefecture in each season of 2012. The results of quantifying free resveratrol and resveratrol released from the glycosidic forms of P. cuspidatum are shown in Table . The reproducibility of all procedures including extraction and analysis was tested, and the relative standard deviation was within 12%. Resveratrol exists in nature in the trans form, but its cis-isomer, cis-resveratrol, can also be found in plants. Although we discuss here resveratrol in the trans form, we also identified cis-resveratrol and found the content of this compound to be very small and seeming to depend on the trans-resveratrol content (data are not shown). In its overall distribution, resveratrol in the above-ground parts was less concentrated than in the subterranean parts. The edible stem parts contained a trace amount to 0.06 μg/g f.w. of free resveratrol and a trace amount to 0.40 μg/g f.w. of resveratrol from glycosides. There was no major difference apparent in the resveratrol content among three portions of the edible stem. In these stem samples, the amount of resveratrol from glycosides was greater than that of free resveratrol. In the leaves, the content of free resveratrol and resveratrol glycoside was found to be about 1.5–200 times higher than that in the stems. The subterranean parts, however, contained substantially larger amounts than the above-ground parts. Rhizomes, which are often used for medicinal purposes, had relatively more resveratrol than tubers, especially during the growing seasons. It has been mentioned in previous studies that free resveratrolCitation3) and its glycoside formsCitation7) were undetectable in the stems and leaves of P. cuspidatum. However, Vrchotova et al. have reported the presence of free resveratrol, piceid, and resveratroloside in these parts. Although previous studies have reported contradictory results, we found that there were small amounts of free resveratrol and resveratrol glycoside in the above-ground parts of the plant, including the edible portions. Since fresh samples were used in this study, it is important to keep in mind that the shoots have high water content, so resveratrol may be less concentrated in these parts. With our findings, the above-ground parts, which are often discarded in industrial use, may possibly be used as a new resveratrol source. Itadori, the edible stem part of P. cuspidatum, can also be recognized as a food containing resveratrol.
Table 1. The content of free trans-resveratrol and trans-resveratrol glycoside in each part of P. cuspidatum.
Our study has clarified that the resveratrol content in P. cuspidatum varied depending on the harvesting season, growing location, and cultivating condition.Citation1,5,8) The seasonal peaks of free resveratrol content were different between the above-ground and subterranean parts. The free resveratrol content of the subterranean parts peaked in spring, and the above-ground samples had the highest free resveratrol content in fall. From spring to summer, however, we observed less resveratrol glycoside in both the above-ground and subterranean parts. In summer, there are a number of stressful factors such as intense sunlight and high temperatures which could be related to the rapid consumption of stored forms of resveratrol. However, in the three geographical regions, these observed differences in resveratrol content were not always consistent. In particular, samples from Muroto had different seasonal changes in resveratrol glycoside content from the other regions. For example, the resveratrol glycoside contents in the leaves and rhizomes harvested in Kagami and Shimanto were relatively low in summer, but the samples from Muroto showed the highest content in this season. Environmental factors such as hours of sunlight and the presence of harmful insects and/or fungus can affect the production of resveratrol in plants.Citation9) In fact, the weather in Muroto was distinctly different from that in the other regions. According to the meteorological data for Kochi Prefecture, the amount of rainfall in Sakihama-cho, Muroto-shi, was markedly higher than in the other places, including the Shimanto and Kochi regions.Citation10) The hours of sunlight could have been relatively fewer, so that the plants did not need to expend their stored resveratrol. Other possible factors are insects and fungal infections. Kovarova et al. have reported that physical damage to the above-ground parts caused by fungal and insect attacks resulted in increased resveratrol, piceid and other polyphenols in the subterranean parts of P. cuspidatum.Citation6) Some of the Muroto samples were damaged due to insect attacks when compared with the samples from the other places. Further studies are needed to confirm the factors contributing to the uniqueness of the samples from Muroto.
The antioxidative properties of the samples evaluated are shown in Table . Since the reaction mechanisms of the DPPH (1,1-diphenyl-2-picrylhydrazyl) and WST-1 (water-soluble tetrazolium salt-1) methods are different, it was inevitable to see some inconsistent results.Citation11) Overall, the results from both methods showed high antioxidative capacity in the subterranean parts and leaves. Even in the stem parts, the antioxidative capacity may be much higher if the water content is considered. Although antioxidative effects were apparent throughout the plant, free resveratrol contributed only a small part in the samples, accounting for less than 5%. This contribution did not include other resveratrol derivatives including resveratrol glycoside. However, even piceatannol, a hydroxylated analog of resveratrol present in these samples, contributed less than 0.05% of the samples’ antioxidative activity (data not shown). A variety of such antioxidants as anthraquinones, flavanols, and cinnametes have been found in P. cuspidatum.Citation4) Although P. cuspidatum has been reported as a substantial source of resveratrol, we also agree with Yi et al. who mentioned that the quality of this plant cannot be determined only by the amount of resveratrol.Citation8)
Table 2. Antioxidative capacity of each part of P. cuspidautm harvested in shimanto in spring.
We found in this study that there was a small amount of resveratrol present in fresh edible stems of P. cuspidatum which are traditionally consumed by local people in Kochi Prefecture. Resveratrol is distributed throughout all other parts of the plant, but a number of factors can be related to the differences in the resveratrol content. Although resveratrol is a well-known compound found in P. cuspidatum, its antioxidative contribution was found to be little. Further research on the antioxidants contained in this plant may result in more medicinal compounds and health-promoting mechanisms.
Experimental
Preparing the crude extracts
P. cuspidatum was harvested in three different locations in Kochi Prefecture, Japan. Shimanto (Kinokawa, Shimanto-cho, Takaoka-gun), Kagami (Chozu, Kagamiyoshihara, Kochi-shi), and Muroto (Sakihama-cho, Muroto-shi) are, respectively, located in the west, center, and east of Kochi Prefecture. Fresh plants were separated into four different parts: stem, leaf, tuber, and rhizome. The edible plant shoots harvested in spring were further divided into four different sections: the upper, middle, and lower parts of the edible portion, and the inedible skin. The stem was categorized as one whole sample in summer and fall. Before extraction, the stems and leaves were ground, and the subterranean parts were cut into 5-mm-thick pieces. The stem and leaf samples were extracted for 24 h in 80% methanol in water, and the subterranean samples were extracted for 72 h. The methanol extraction was conducted twice. The obtained crude extracts were passed through a 0.45-μm membrane filter.
Pretreatment for quantifying free resveratrol
The crude extracts were fractioned according to the work of Ban et al. with some modifications.Citation12) Each sample extract that was equivalent to 10 g f.w. of the part of P. cuspidatum was concentrated and suspended in 70% methanol in water. The dissolved sample was extracted three times with hexane, and water was added to the aqueous layer to double the volume. The aqueous layer was further extracted three times with ethyl acetate. The ethyl acetate layer was evaporated and dissolved in 10 mL of 30% acetonitrile in water. The dissolved sample was then applied to a C18 Sep-Pak® cartridge (Waters, Milford, MA, USA), the cartridge being eluted with 10 mL of 100% acetonitrile. Both the flow through and the eluate were combined, and a total of 20 mL of the sample solution was evaporated to dryness. The dried material was dissolved in 30% acetonitrile in water to yield 1 g f.w. equiv/mL of solution for an analysis of free resveratrol.
Pretreatment for quantifying total resveratrol
The hydrolysis procedure was conducted according to Ortuno et al.Citation13) Crude extracts equivalent to 1 g f.w. for the stems and leaves and 0.1 g f.w. for the subterranean parts were used. β-Glucosidase purchased from Toyobo Co. (Osaka, Japan) was used for the hydrolysis process. After incubation, each sample was extracted three times with ethyl acetate. The subsequent procedures were the same as the pretreatment just described for quantifying free resveratrol.
Determination of the resveratrol content in P. cuspidatum by HPLC
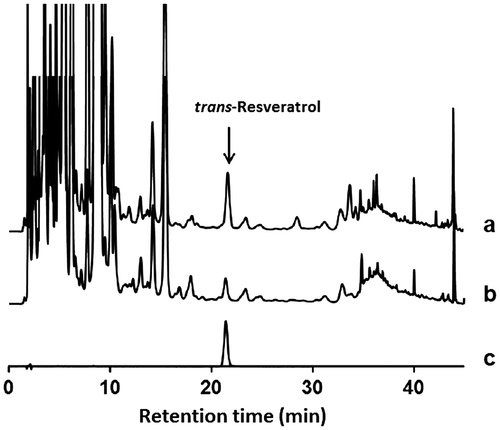
The resveratrol content of each sample was determined by using an HPLC system equipped with a Cosmosil 5C18AR-II column (4.6 i.d. × 150 mm, Nacalai Tesque, Kyoto, Japan). The HPLC system consisted of an ERC-3215α degasser (ERC, Saitama, Japan), and an LC-7100 pump, L-2300 column oven, and L-2420 UV VIS detector (Hitachi, Tokyo, Japan). D-2000 Elite Chromatography Data Station software (Hitachi, Tokyo, Japan) was used for analysis. The HPLC conditions and retention time for resveratrol are shown in Fig. . The resveratrol peak was identified by comparing it with an authentic standard, and the calibration curve of resveratrol was drawn for each experiment. The resveratrol glycoside content, described as the amount of resveratrol released from the glycoside, was calculated by subtracting free resveratrol from total resveratrol.
Fig. 1. HPLC data for the extracts of P. cuspidatum and trans-resveratrol.
Notes: The mobile phase contained 0.4% formic acid (solvent A) and acetonitrile (solvent B) with a flow rate of 1 mL/min. The gradient conditions for the eluent were as follows: 0–30 min, 15–20% (solvent B concentration); 30–40 min, 20–100%; and 40–45 min, 100%. The separation temperature was set at 40 °C, and the wavelength for UV detection was 303 nm. Chromatograms for the leaf samples with hydrolysis (a) and without hydrolysis (b) and the standard of resveratrol (c) are shown. The trans-resveratrol peak appeared at 21.4 min.
Determination of the antioxidative capacity
The antioxidative capacity of each different part of P. cuspidatum was determined by using the DPPH radical scavenging activity method and WST-1 method, as described by Shimamura et al. with some modifications.Citation11) The crude extract of the plant harvested in Shimanto in spring was used here. The radical scavenging rate was plotted and used to measure IC50. The Trolox equivalent antioxidative capacity (TEAC) was calculated for each sample by using following equation:
SOD equivalent activity, however, was redefined according to the following equation:
Acknowledgments
We thank Kenichi Tanabe from Ecology Shimanto Co. and Soichiro Ueta from the NPO Sakihama Genki project for providing the samples used in our study.
Notes
Abbreviations: f.w., fresh weight; DPPH, 1,1-diphenyl-2-picrylhydrazyl; WST-1, water-soluble tetrazolium salt 1; HPLC, high-performance liquid chromatography; UV, ultraviolet; TEAC, trolox-equivalent antioxidative capacity; SOD, superoxide dismutase.
References
- Vrchotová N, Šerá B, Dadáková E. HPLC and CE analysis of catechins, stilbens and quercetin in flowers and stems of Polygonum cuspidatum, P. sachalinense and P. x bohemicum. J. Indian Chem. Soc. 2010;87:1267–1272.
- Grevstad F, Shaw R, Bourchier R, Sanguankeo P, Cortat G, Reardon RC. Efficacy and host specificity compared between two populations of the psyllid Aphalara itadori, candidates for biological control of invasive knotweeds in North America. Biol. Control. 2013;65:53–62.10.1016/j.biocontrol.2013.01.001
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50:3337–3340.10.1021/jf0112973
- Fan P, Hay AE, Marston A, Lou H, Hostettmann K. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem. Syst. Ecol. 2009;37:24–34.10.1016/j.bse.2008.11.018
- Qian G, Leung SY, Lu G, Leung KS. Optimization and validation of a chromatographic method for the simultaneous quantification of six bioactive compounds in Rhizoma et Radix Polygoni Cuspidati. J. Pharm. Pharmacol. 2008;60:107–113.10.1211/jpp.60.1.0014
- Kovářová M, Frantík T, Koblihová H, Bartůňková K, Nývltová Z, Vosátka M. Effect of clone selection, nitrogen supply, leaf damage and mycorrhizal fungi on stilbene and emodin production in knotweed. BMC Plant Biol. 2011;11:98–112.10.1186/1471-2229-11-98
- Kirino A, Takasuka Y, Nishi A, Kawabe S, Yamashita H, Kimoto M, Ito H, Tsuji H. Analysis and functionality of major polyphenolic components of Polygonum cuspidatum (itadori). J. Nutr. Sci. Vitaminol. 2012;58:278–286.10.3177/jnsv.58.278
- Yi T, Zhang H, Cai Z. Analysis of Rhizoma Polygoni Cuspidati by HPLC and HPLC-ESI/MS. Phytochem. Anal. 2007;18:387–392.10.1002/(ISSN)1099-1565
- Giovinazzo G, Ingrosso I, Paradiso A, Gara LD, Santino A. Resveratrol Biosynthesis: Plant Metabolic Engineering for Nutritional Improvement of Food. Plant Foods Hum. Nutr. 2012;67:191–199.10.1007/s11130-012-0299-8
- Japan Meteorological Agency. http://www.jma.go.jp/jma/menu/report.html; 2012–2013. Japanese.
- Shimamura T, Matsuura R, Tokuda T, Sugimoto N, Yamazaki T, Matsufuji H, Matsui T, Matsumoto K, Ukeda H. Comparison of conventional antioxidants assays for evaluating potencies of natural antioxidants as food additives by collaborative study. Nippon Shokuhin Kagaku Kogaku Kaishi. 2007;54:482–487. Japanese.10.3136/nskkk.54.482
- Ban SH, Kwon YR, Pandit S, Lee YS, Yi HK, Jeon JG. Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci. Fitoterapia. 2010;81:30–34.10.1016/j.fitote.2009.06.019
- Ortuño J, Covas MI, Farre M, Pujadas M, Fito M, Khymenets O, Andres-Lacueva C, Roset P, Joglar J, Lamuela-Raventós RM, Torre Rdl. Matrix effects on the bioavailability of resveratrol in humans. Food Chem. 2010;120:1123–1130.10.1016/j.foodchem.2009.11.032
