Abstract
To investigate the combinatorial effects using Salmonella and γ-radiation, the Salmonella typhimurium infection in combination with γ-radiation was investigated on melanoma. We showed that ROS expression and H2AX phosphorylation increased during stress by γ-radiation irrespective of Salmonella infection, inducing apoptosis by caspase-3 and bcl2 in tumor cells. In addition, tumor growth was suppressed by this combinatory therapy suggesting candidates for radiation therapy against melanoma.
Facultative intracellular bacteria Salmonella inhibit the growth of tumor cells using specific invasion strategies including rapid multiplication in hypoxic regions.Citation1,2) However, the mechanisms by which Salmonella exert their anti-cancer properties are still unknown.Citation3,4) It has been reported that genetically engineered Salmonella exhibit many of the advantageous characteristics of an anti-tumor vector, including selective delivery to tumors, replication within tumors, tumor growth suppression, and the capability to express effector genes, such as tumor necrosis factor (TNF)-α or herpes simplex thymidine kinase.Citation5–7)
Moreover, recent progress in the field of Salmonella vaccine delivery systems proved to genetically modify the harboring hyper invasive or attenuated Salmonella-induced apoptosis phenotypes.Citation8) Salmonella is utilized as a vector system for the systemic administration of cytokine therapies such as oral cytokine gene therapy in melanoma cells.Citation9) In addition, Salmonella expressing TNF-α suppressed melanoma tumor cell growth.Citation5)
Melanoma is radioresistant but located in a radiosensitive organ. A combination of therapies consisting of radiation and chemotherapy are typically used to treat melanoma.Citation10) Malignant melanoma patients who have been treated with radio-chemotherapy exhibit favorable effects, including a satisfactory quality of life.Citation11) Although most melanomas are treated with radiotherapy, previous reports have suggested that melanomas are relatively radioresistant compared with other tumor types. However, the molecular mechanisms that evoke the intrinsic resistance to radiation in melanoma are unknown.Citation12) Therefore, a radio-sensitizing agent has been utilized for the treatment of melanoma.Citation13)
In the present study, we investigated whether the combination of Salmonella and γ-radiation is effective in sensitizing melanoma cells to radiotherapy and inhibiting the growth of melanoma tumors. It has previously been reported that the combination of genetically engineered Salmonella and radiotherapy resulted in the suppression of tumor growth.Citation14) However, the underlying mechanism of how this poly-therapy induces tumor suppression is not known. In this regard, the aim of our study was to examine the anti-tumor effect of apoptosis induced by Salmonella in combination with γ-radiation.
Salmonella infection with γ-radiation poly-therapy would be unfavorable to melanoma cells if cytotoxic effects were produced. To investigate the extent of melanoma cell survival after microbial tumor therapy with γ-radiation, we analyzed images of tumor cells by microscopy (100×) (Fig. (A)). The results confirmed that poly-therapy led to the suppression of tumor growth (Fig. ). In cytotoxicity assays, the lactate dehydrogenase in combination therapy-treated melanoma cells steadily increased during from 1 to 72 h (Fig. (B)). This result suggests that Salmonella infection with γ-radiation directs melanoma cell death in vitro. Therefore, the combinatorial treatment of Salmonella and γ-radiation leads to a more potent cytotoxic response in melanoma cells.
Fig. 1. Combinatorial treatment of Salmonella and γ-radiation induced a strong cytotoxic response in melanoma cells.
Note: Murine melanoma cells were infected with Salmonella BRD509 strains at a MOI of 100 for 6 h. The Salmonella infected cells were exposed to 8 Gy of γ-radiation at room temperature using a 137Cs source. (A) Viability of B16F10 melanoma cells after combinatorial treatments. Tumor cells were imaged at 100× magnification with a microscope. Mouse melanoma B16F10 cells were treated with PBS, S. typhimurium alone, γ-radiation, and S. typhimurium with γ-radiation as shown in the methods. (B) Cytotoxicity of B16F10 melanoma cells after the combinatorial treatment was determined at the indicated times. (C) ROS production was measured by FACS analysis in B16F10 cells (no treatment; control), 509 Salmonella infection, γ-radiation, and the combination of Salmonella and γ-radiation using 10 μM of H2DCFDA, which converts to a fluorescent derivative only in the presence of ROS. Results shown are representative of at least three independent experiments. The data presented are the average of three independent experiments that were performed in duplicate. The error bars indicate standard deviation.
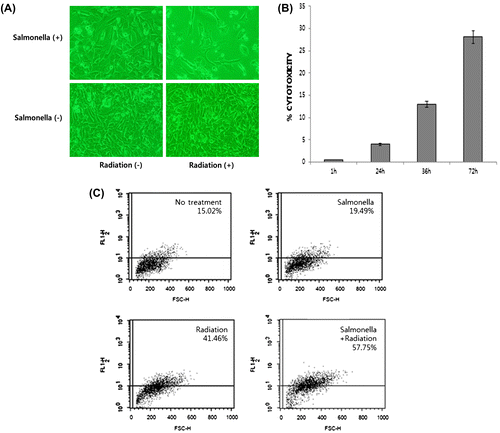
Reactive oxygen species (ROS) are also essential to cellular signal transduction events and may not only require cell functions in relation to ROS burst caused by radiation but also enhance the pathways that induce cell destruction. Next, we evaluated the measurement of cellular ROS production in B16F10 cells with no treatment, Salmonella treatment, radiation treatment, and combined treatment of Salmonella and radiation by DCFDA staining. As shown in Fig. (C), 41.16% of IR–treated B16F10 murine melanoma cells produced ROS compared with 19.49% of Salmonella-treated cells. Next, we analyzed cellular ROS measurements in B16F10 cells treated with Salmonella and radiation combination therapy to determine if ROS production increased in cancer cells. As expected, 57.75% of the combined Salmonella and radiation-treated cells produced ROS compared with radiation only-treated cells. Therefore, this study shows that the sensitizing effect of poly-therapy using Salmonella and radiation leads to increased apoptotic cell death.
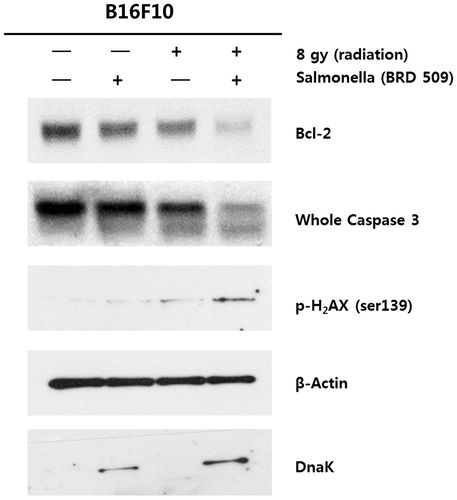
The induction of apoptosis in cancer cells is an important mechanism for most anti-tumor therapies, including chemotherapy, γ-radiation, immunotherapy, and cytokine therapy. Indeed, apoptosis is weakly induced by the decreased expression of anti-apoptotic protein Bcl2 and whole Caspase-3. In addition, H2AX phosphorylation slightly increases inducing apoptosis in response to DNA damage in B16F10 cells after the treatment of γ-radiation (Fig. ). However, apoptosis is not enhanced in Salmonella-infected tumor cells by immunotherapy. We investigated the combined treatment with γ-radiation and Salmonella BRD 509 to determine whether cell apoptosis pathways are decreased in B16F10 cells. Indeed, the level of Bcl and whole Caspase 3 expression in B16F10 cells was significantly decreased by the combined treatment with γ-radiation and Salmonella BRD 509 (Fig. ). Additionally, our results confirmed that H2AX expression was increased by the combined treatment of γ-radiation and Salmonella BRD 509 compared with individual treatments of γ-radiation (8 Gy) and Salmonella BRD 509 (Fig. ). Taken together, the combined treatment with ionizing radiation and Salmonella enhances apoptotic cell death in B16F10 cells.
Fig. 2. Induced apoptosis by combinatorial effects of Salmonella infection and exposure of ionizing radiation in B16F10 cells.
Note: B16F10 cells were infected with BRD509 Salmonella at a multiplicity of infection (MOI) of 100:1 for 6 h, treated with γ-radiation (8 Gy) and incubated for 17 h. Whole cell lysates were subjected to SDS-PAGE followed by western blot analysis using antibodies against Bcl2, phosphorylated H2AX, DnaK (for the detection of Salmonella), and actin (as a loading control). Results shown are representative of at least three independent experiments.
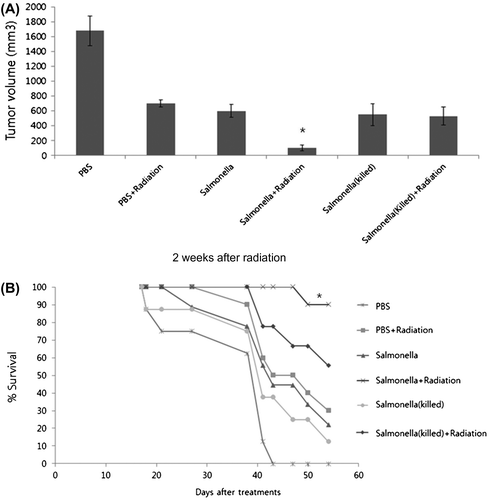
To confirm the activation of apoptotic cell death using combination therapy, we performed growth and survival tests in tumor-bearing C57BL/6 mice (Fig. ). As expected, B16F10 tumor volume was significantly decreased using the combinatorial therapy with Salmonella and γ-radiation compared with PBS treatment, Salmonella infection only, and γ-radiation only (Fig. (A)). Furthermore, poly-therapy with Salmonella and radiation led to a prolonged overall survival rate of 90% in tumor-bearing mice over 55 days (Fig. (B)). However, treatments with PBS, Salmonella infection only, and γ-radiation only resulted in a survival rate of below 40% in tumor-bearing mice. We also confirmed that heat-killed Salmonella, which induces an innate immune response, such as LPS, lacked the ability to extend survival in tumor-bearing mice. Taken together, increased apoptosis due to the poly-therapeutic effect of Salmonella and γ-radiation treatment is responsible for the sensitivity of melanoma cells, and this treatment further extends the survival of tumor-bearing mice.
Fig. 3. Effects of poly-therapy with S. typhimurium-infection and γ-radiation in melanoma-bearing mice.
Note: (A) Growth inhibition of B16F10 tumors by subcutaneous inoculation of Salmonella with γ-radiation treatment. B16F10 melanoma cells were implanted in the skin of mice and allowed to grow for 20 d. Tumor-bearing mice were inoculated with 1 × 108 Salmonella and treated with γ-radiation, and the size of the tumor after 2 weeks was measured with microcalipers in two dimensions. *p < 0.05 was considered statistically significant compared with all other groups. (B) Survival of tumor-bearing mice after poly-therapy treatments. B16F10 cells were implanted by hypodermic inoculation. After 20 d, tumor-bearing mice were orally inoculated with PBS, 1 × 108 Salmonella and heat killed Salmonella with or without γ-radiation treatment. The survival of the animals was examined everyday after inoculation. *p < 0.05 was considered statistically significant compared with all other groups.
Our data demonstrate that Salmonella in combination with γ-radiation can be utilized to suppress tumor growth. We have provided a mechanistic explanation for how melanoma cells are induced by apoptosis and are more sensitive to combinational effects using Salmonella typhimurium and radiation (Figs. and ). Using FACS analysis, this finding has been further confirmed by demonstrating that ROS expression was increased during stress by γ-radiation irrespective of Salmonella infection (Fig. ). However, this striking phenotype could not be killed by γ-radiation (Fig. (A)) or induction of apoptosis due to unknown reasons (Fig. ). The development of resistance to apoptosis in cancer cells during sequential radiotherapy is a known cause of therapeutic failure. Therefore, Salmonella infection in cancer cells is required for induction of apoptosis as a radiosensitizer in combinatorial cancer therapy. Furthermore, we confirmed in vivo that the effect of poly-therapy is enhanced by the induction of apoptosis in tumor-bearing mice (Fig. (A) and (B)). These results suggest that the combination therapy using Salmonella with γ-radiation confers radiosensitization of cancer cells by inducing apoptotic cell death. Overall, our data demonstrated that cancer radiation therapy is significantly improved with the use of bacteria. For this reason, our findings indicate that bacteria could be utilized as an effective cancer radiation therapy in the future.
Acknowledgment
Thank you for the technical assistance by Nim Hana.
Funding
This study was supported by a grant of the Korea University. This study was also supported by NRF Fund [grant number 2012R1A1A2038549].
Notes
Abbreviations: H2AX, H2A histone family member X; ROS, Reactive oxygen species; TNF, tumor necrosis factor.
References
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. J. Cell Biochem. 2009;106:992–998.10.1002/jcb.v106:6
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10170–10174.
- Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, Longhi R, Colombo MP, Dougan G, Rescigno M. Cancer Res. 2005;65:3920–3927.10.1158/0008-5472.CAN-04-3002
- Leschner S, Weiss S. J. Mol. Med. (Berl). 2010;88:763–773.10.1007/s00109-010-0636-z
- Yoon WS, Chae YS, Hong J, Park YK. Appl. Microbiol. Biotechnol. 2011;89:1807–1819.10.1007/s00253-010-3006-4
- Pawelek JM, Low KB, Bermudes D. Cancer Res. 1997;57:4537–4544.
- Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin SL, Luo X, Miller SI, Zheng L, King I, Pawelek JM, Bermudes D. Nat. Biotechnol. 1999;17:37–41.
- Kong W, Brovold M, Koeneman BA, Clark-Curtiss J, Curtiss 3rd R. Proc. Natl. Acad. Sci. U.S.A. 2012;109:19414–19419.
- Agorio C, Schreiber F, Sheppard M, Mastroeni P, Fernandez M, Martinez MA, Chabalgoity JA. J. Gene Med. 2007;9:416–423.10.1002/(ISSN)1521-2254
- Jenrette JM. Semin. Oncol. 1996;23:759–762.
- Patonay P, Naszaly A, Mayer A, Pocza K, Kovacs L. Magy. Onkol. 2004;48:303–308.
- Pak BJ, Lee J, Thai BL, Fuchs SY, Shaked Y, Ronai Z, Kerbel RS, Ben-David Y. Oncogene. 2004;23:30–38.10.1038/sj.onc.1207007
- Jori G, Soncin M, Friso E, Vicente MG, Hao E, Miotto G, Colautti P, Moro D, Esposito J, Rosi G, Nava E, Sotti G, Fabris C. Appl. Radiat. Isot. 2009;67:S321–S324.10.1016/j.apradiso.2009.03.071
- Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low KB, Pawelek J. Eur. J. Cancer. 2000;36:2397–2402.10.1016/S0959-8049(00)00336-1
