Abstract
Meiotic chromosomes are of basic interest to the geneticist and cell biologist who study their behavior. A rapid and highly repeatable method for visualization of meiotic chromosomes is useful. Here we describe a fast staining protocol for Arabidopsis male meiotic chromosomes. Meiocytes were squashed into a labeling buffer, the chromosome morphology could be analyzed using fluorescence without any additional treatment.
With the advances in genetics and in molecular biology, Arabidopsis has become an important model for the analysis of meiosis in plants. Through mutagenesis and homologous study, a number of meiotic genes were identified.Citation1−3) For many experimental purposes, a rapid and easy method for visualization of meiotic chromosomes is desired.
Chromosome spreading,Citation4) which is usually done in conjunction with 4′,6-diamidino-2-phenylindole (DAPI) staining, is the most widely used technique for visualization of meiotic chromosomes, and a basic preparative technique for fluorescence in situ hybridization analysis. Typical procedures for this technique involve the fixation of flower buds, enzymatic digestion and dissection of buds, chromosome spreading and staining with DAPI. This method is a tedious and time-consuming task not only in slides preparation, but also in microscopic investigation, since only a small portion of cells in the microscopic preparations are meiocytes, thus, it does not allow for acquisition of data in a timely manner. In some cases, researchers are discouraged from chromosome losing during long experiment operation or due to slide contamination. In addition, this protocol uses toxic (Carnoy’s solution) and strong-smelling substances (acetic acid). The method using ferrous acetocarmine provides a fast procedure for chromosome observation,Citation5) which includes the preparation of a saturated solution of carmine in acetic acid, the fixation of the material, the crushing, smearing, and the subsequent coloring of the material at elevated temperature. While the preparation of the acetocarmine is a lengthy work, the beginners have difficulties frequently in making the preparation by crushing, smearing, and in the coloring of the preparation. Here, a simple, quick, and easily reproducible protocol is described for labeling of male meiotic chromosomes in Arabidopsis thaliana.
This protocol uses Molecular Probes SYTOX® Green Nucleic Acid Stain (Life Technologies, Carlsbad, CA, USA) to label the meiotic chromosomes in unfixed tissues. SYTOX Green is a high-affinity nucleic acid stain that easily penetrates damaged membranes, and exhibits bright fluorescence when bound to DNA. It was commonly used as an indicator of dead cells in published studies.Citation6)
A glass slide was placed on the dissecting microscope stage, 2–3 drops of labeling buffer was applied (around 2.5 μl/each drop) onto the slide at different positions. We used a labeling buffer containing 5 μM SYTOX Green diluted by phosphate-buffered saline. On the glass slide, anthers were dissected from the flower buds (Fig. (A)) with disposable syringe, 4–6 anthers looked transparent (Fig. (B)) were collected into a drop of labeling buffer. A cover slip was placed over the sample, a gentle press was applied to break the anthers and release meiocytes. The anthers in different buffer drops could release meiocytes in different position of the slide. In bright field light, meiocytes could be easily distinguished from somatic cells by the form of cell clusters in early stage (Fig. (A)) and a transparent callose-containing wall in later stages (Fig. (C)). The chromosomes were analyzed with a fluorescence microscope. Meiocytes incubated with the labeling buffer will result in bright green fluorescence (Figs. (B), (D) and ). The meiosis stages could be determined at the individual cell level (Fig. ). Higher concentration of SYTOX Green might bring more background, while lower concentration of SYTOX Green could result in quick fluorescence quenching (Supplemental Fig. 1 (A)–(C); see http://dx.doi.org/10.1080/09168451.2014.905191). Another nuclei acid stain, DAPI (Life Technologies), was tested with the same cell preparations; however, the chromosomes were barely clear enough (Supplemental Fig. 1(D)).
Fig. 1. An illustration of the flowers and anthers used for visualizing of Arabidopsis male meiotic chromosomes.
Notes: (A) Young healthy Arabidopsis inflorescence. In our long-day growing conditions,Citation7) the average number of flower buds in a wild-type (Col-0) inflorescence undergoing meiosis is 5.1. (B) Anthers dissected from the floral buds in the framed part show in (A), those transparent ones (left and middle) are at meiosis stage. Bar = 1 mm in (A), 0.1 mm in (B).
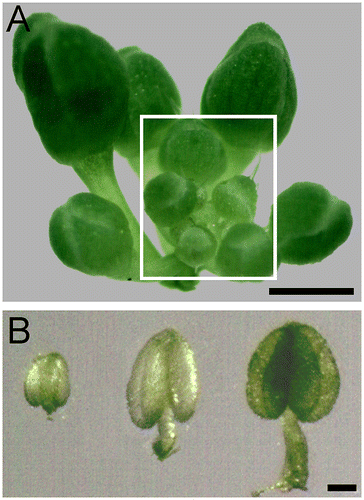
Fig. 2. Released two types of Arabidopsis meiocytes.
Notes: (A) Released meiocytes like “worms”. (C) Released separated meiocytes. (B) and (D) are corresponding fluorescent images of (A) and (C), respectively. Images were taken using a Zeiss Axioskop 40 microscope (Carl Zeiss, Jena, Germany). The green fluorescence was detected with a FITC filter. Star refers the meiocytes;
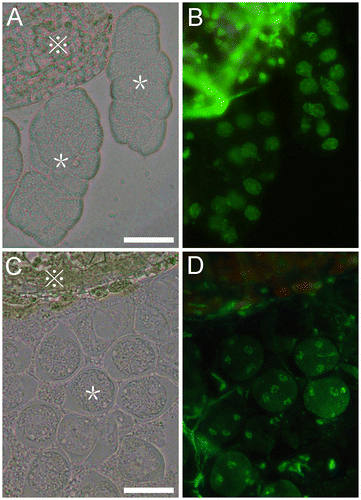
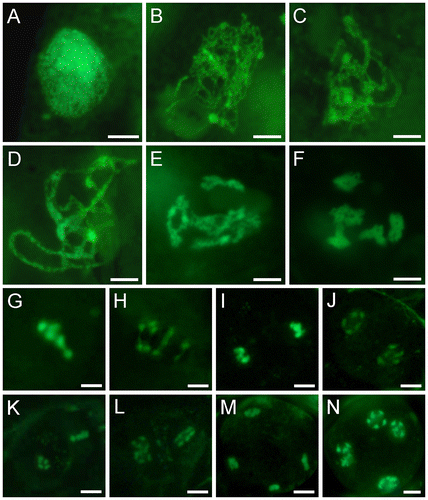
Fig. 3. Meiotic Stages of SYTOX Green-stained male meiocytes of wild-type Arabidopsis.
Notes: (A) Preleptotene. (B) Leptotene. (C) Zygotene. (D) Pachytene. (E) Late diplotene. (F) Diakinesis. (G) Metaphase I. (H) Anaphase I. (I) Telophase I. (J) Dyad. (K) Metaphase II. (L) Anaphase II. (M) Telophase II. (N) Tetrad. Bars = 5 μm.
Pollen mother cells develop more or less synchronously through meiosis,Citation4) thus, our protocol allowing rapid identification of the meiotic stage of a flower by determining only one anther dissected from a bud, with other anthers were left intact. This modification could be a substantial aid for enriching of meiocytes in particular stage.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.905191.
Supplemental Fig. 1
Download PDF (305.3 KB)Acknowledgments
We thank Life Technologies for providing sample of Molecular Probes SYTOX® Green Nucleic Acid Stain. This work was supported by the Scientific Research Starting Foundation of Henan Normal University (qd12127), the Plan for Scientific Innovation Talent of Henan Province (114200510013), and the Innovation Scientists and Technicians Troop Construction Projects of Henan Province.
Notes
Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
References
- Osman K, Higgins JD, Sanchez-Moran E, Armstrong SJ, Franklin FC. Pathways to meiotic recombination in Arabidopsis thaliana. New Phytol. 2011;190:523–544.10.1111/nph.2011.190.issue-3
- Ma H. A molecular portrait of Arabidopsis meiosis. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis book. Rockville (MD): American Society of Plant Biologists; 2006. p. 1–39.
- Mercier R, Grelon M. Meiosis in plants: ten years of gene discovery. Cytogenet. Genome Res. 2008;120:281–290.10.1159/000121077
- Ross K, Fransz P, Jones G. A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res. 1996;4:507–516.10.1007/BF02261778
- Belling J. The iron-acetocarmine method of fixing and staining chromosomes. Biol. Bull. 1926;50:160–162.10.2307/1536680
- Truernit E, Haseloff J. A simple way to identify non-viable cells within living plant tissue using confocal microscopy. Plant methods. 2008;4:15.10.1186/1746-4811-4-15
- Li J, Han Y, Zhao Q, Li C, Xie Q, Chong K, Xu Y. The E3 ligase AtRDUF1 positively regulates salt stress responses in Arabidopsis thaliana. PLoS One. 2013;8:e71078.10.1371/journal.pone.0071078
