Abstract
Basic amino acids (lysine, histidine and arginine) accumulated in Saccharomyces cerevisiae vacuoles should be mobilized to cytosolic nitrogen metabolism under starvation. We found that the decrease of vacuolar basic amino acids in response to nitrogen starvation was impaired by the deletion of AVT4 gene encoding a vacuolar transporter. In addition, overexpression of AVT4 reduced the accumulation of basic amino acids in vacuoles under nutrient-rich condition. In contrast to AVT4, the deletion and overexpression of AVT3, which encodes the closest homologue of Avt4p, did not affect the contents of vacuolar basic amino acids. Consistent with these, arginine uptake into vacuolar membrane vesicles was decreased by Avt4p-, but not by Avt3p-overproduction, whereas various neutral amino acids were excreted from vacuolar membrane vesicles in a manner dependent on either Avt4p or Avt3p. These results suggest that Avt4p is a vacuolar amino acid exporter involving in the recycling of basic amino acids.
Graphical Abstract
In the budding yeast Saccharomyces cerevisiae, basic amino acids that are highly accumulated in vacuoles are extruded by vacuolar amino acid transporter Avt4.
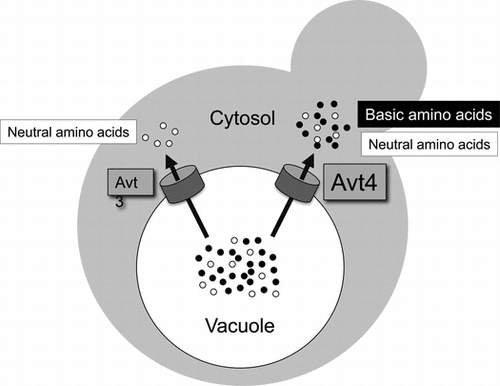
Vacuoles of the budding yeast Saccharomyces cerevisiae serve as a storage compartment for a variety of amino acids.Citation1,2) Basic amino acids are highly accumulated in this organelle, whereas glutamate, which is the most abundant amino acid in cytosol, is almost excluded.Citation1,2) While other amino acids are also compartmentalized in vacuoles, their concentrations are lower than those of the basic amino acids. These differences in concentration of free amino acids between vacuolar and cytosolic pools imply the existence of active amino acid transport systems on the vacuolar membrane. ATP-dependent amino acid uptake, driven by the proton electrochemical gradient generated by the action of the proton-pumping vacuolar ATPase (V-ATPase), has been observed in purified vacuolar membrane vesicles.Citation3,4) Subsequently, several vacuolar transporters in two gene families, AVT and VBA, were reported to be involved in the uptake of amino acids.Citation5,6) Avt1p in the AVT family is involved in vacuolar uptake of glutamine, isoleucine, and tyrosine.Citation5) Vba1p, Vba2p, and Vba3p in the VBA family mediate vacuolar uptake of basic amino acids: arginine, histidine, and lysine.Citation6) These are likely proton/amino acid antiporters. For extrusion of vacuolar amino acids, involvement of three transporters in the AVT family, Avt3p, Avt4p, and Avt6p, was reported.Citation5) Avt3p and its closest homologue Avt4p are both involved in efflux of glutamine, isoleucine, and tyrosine from vacuoles. Avt6p mediates vacuolar efflux of aspartate and glutamate. An interplay among these and possibly other as yet unidentified transporters should be important for vacuolar compartmentalization of amino acids.
Vacuolar compartmentalization of amino acids dynamically changes depending upon a variety of growth conditions. Under nutrient starvation, amino acids accumulated in vacuoles should be supplied to the cytosolic amino acid pool for nitrogen metabolism and for protein synthesis.Citation2,7) Vacuolar basic amino acids occupying more than 70% of total amino acids are supposed to be especially important as nitrogen source.Citation2,8) It was reported that vacuolar arginine was completely lost within a few hours by exposing cells to nitrogen starvation.Citation2) However, the molecular mechanism underlying arginine efflux from the vacuoles has not been determined. To elucidate the physiological role of vacuolar amino acid pool in more detail, identification and further characterization of transporter(s) mediating extrusion of vacuolar amino acids is indispensable. Here, we found that both Avt4p and Avt3p are transporters for extrusion of a variety of amino acids from vacuoles. In particular, Avt4p was involved in transport of basic amino acids. This is the first report on the component of a transport system involved in efflux of basic amino acids from vacuoles.
Materials and methods
Strains and media
S. cerevisiae X2180-1B (MATa SUC2 mal mel gal2 CUP1) and the derivatives used in this study are listed in Table . Yeast strains were grown aerobically at 30 °C in SD+CA medium (0.17% yeast nitrogen base w/o amino acids and ammonium sulfate, 0.5% ammonium sulfate, 0.5% casamino acids, 20 mg/l of tryptophan, and 2% glucose). Nitrogen starvation medium was SD-N (0.17% yeast nitrogen base w/o amino acids and ammonium sulfate and 2% glucose).
Table 1. Yeast strains used in this study.
Plasmid construction
AVT3 and AVT4 open reading frames with the flanking sequences were cloned into pRS316.Citation9) SalI and BglII sites were created after the initiation codon of AVT3 and AVT4, respectively, by use of a QuikChange mutagenesis kit (Stratagene). The GFP cassette (XhoI or BamHI) was inserted into the created restriction site to construct pGFP-AVT3 and pGFP-AVT4, respectively. GFP-AVT3 and GFP-AVT4 were amplified using pGFP-AVT3 and pGFP-AVT4, respectively, as a template, and then cloned into p416GPDCitation10) to construct pGPD-AVT3 and pGPD-AVT4. A single point mutant, AVT4E496A, was constructed by use of the QuikChange kit.
Amino acid analysis
To prepare a vacuolar fraction, the cupric ion treatment method was used.Citation11) Amino acid contents in the fractions were measured by an automatic amino acid analyzer (Hitachi L-8800).
Fluorescence microscopy
Vacuolar membranes were stained with the fluorescent dye FM4-64 as described.Citation12) Fluorescence signals were visualized with the use of an IX71 fluorescence microscope (Olympus).
Immunoblot analysis
Vacuolar membrane vesicles (10 μg protein) were analyzed by immunoblotting using anti-GFP polyclonal antibody and anti-VPH1 monoclonal antibody (10D7) (Molecular Probes). Immunodetection utilized an enhanced chemiluminescence system (GE Healthcare).
Amino acid transport assays
Vacuolar membrane vesicles were prepared as described.Citation3) To measure the export activity for amino acids, vesicles (200 μg of protein) were preloaded with amino acids by incubation for 5 min at 25 °C in the assay mixture (500 μl) consisting of 25 mm 2-(N-morpholino)ethanesulfonic acid (MES)-Tris (pH 7.0), 4 mm MgCl2, 25 mm KCl, and 14C-labeled amino acid (final 0.1 mm; 4.8–11.1 GBq/mmol). Under this condition, equilibrium of amino acid inside and outside the vesicles was attained. After preloading, the reaction was started by adding 2 mm ATP and then stopped by diluting a 100 μl sample with 5 ml of ice-cold wash buffer consisting of 25 mm MES-Tris (pH 7.0), 5 mm MgCl2, and 25 mm KCl at indicated time points. Vesicles were recovered by vacuum filtration onto 0.45-μm cellulose acetate filter membrane (Advantec). Filters were washed once with 5 ml of ice-cold wash buffer. Radioactivity remained on the filters was determined by a xylene scintillator in a liquid scintillation counter. Uptake of [U-14C]arginine (0.1 mm; 12.9 GBq/mmol) and 45CaCl2 (2.5 mm; 18.5 GBq/mmol) by vesicles was assayed as described.Citation3,6) Radioactive materials were obtained either from Perkin Elmer ([U-14C]alanine, [U-14C]proline, [U-14C]arginine and 45CaCl2) or GE Healthcare ([U-14C]valine).
Results
Avt4p is involved in the reduction of vacuolar basic amino acids under nitrogen starvation
It has been reported that the amount of vacuolar arginine in S. cerevisiae rapidly decreased upon nitrogen starvation.Citation2) However, the underlying mechanism is poorly understood. By the experiments using vacuolar membrane vesicles, Avt3p, Avt4p, and Avt6p have been suggested to be involved in excretion of several amino acids from vacuoles.Citation5) These transporters are good candidates for the system mediating vacuolar arginine excretion. A limited number of amino acid species, however, has been examined as their transport substrates. In addition, in vivo function of these transporters such as the effect on vacuolar amino acid contents has not been addressed so far. Since microarray analysis suggested that expression of these transporters is induced under nitrogen starvation,Citation13) we expected a possible involvement of these transporters in the starvation-induced excretion of arginine from vacuoles.Citation2) In the case of Avt6p, however, we found that the vacuolar levels of aspartate and glutamate but not of basic amino acids including arginine increased in avt6∆ cells under nitrogen starvation.Citation8) Here, we examined the vacuolar levels of basic amino acids in avt3∆ and/or avt4∆ cells (Table ). At 6 h after shifting from SD+CA to SD-N, the amounts of lysine, histidine, and arginine of the vacuolar fraction of the wild-type strain decreased to less than 10% of the values in SD+CA medium. This tendency was also observed in avt3∆ cells. However, about 30% of the vacuolar amounts of lysine, histidine, and arginine remained in avt4∆ cells. The amounts of these amino acids did not further increase in avt3∆avt4∆ cells. On the other hand, the vacuolar amount of glutamate was little affected in these mutant cells. These results suggest that Avt4p is particularly important for export of basic amino acids from vacuoles under nitrogen starvation.
Table 2. Change in the amount of amino acids in vacuoles by nitrogen starvation.
Effect of overproduction of Avt3p or Avt4p on vacuolar levels of amino acids
We next examined the effect of overproduction of Avt4p or Avt3p on the amino acid compositions of the vacuolar fractions in cells cultured in SD+CA medium (Fig. (A)). Here either GFP-AVT3 or GFP-AVT4 gene was expressed under the control of GPD promoter in the avt3∆avt4∆ cells by plasmids. GFP-Avt3p and GFP-Avt4p exclusively localized to the vacuolar membrane (Fig. (B)) and densitometric analysis of proteins on Western blot confirmed that 6- and 11-fold increase of GFP-Avt3p and GFP-Avt4p, respectively, when compared with those expressed from each native promoter (data not shown). In comparison with the amino acid composition of the vacuolar fraction of the wild-type strain (Fig. (A), white bars), various neutral amino acids remarkably increased in avt3∆avt4∆ vacuoles (Fig. (A), black bars), whereas moderate or no obvious increase was observed in avt3∆ and avt4∆ single mutant vacuoles (data not shown), showing a redundant function in the excretion of vacuolar neutral amino acids between Avt3p and Avt4p. The amounts of aspartate, glutamate, lysine, histidine, and arginine were little changed in the mutants. When either GFP-Avt3p (Fig. (A), light gray bars) or GFP-Avt4p (Fig. (A), dark gray bars) was overproduced in the avt3∆avt4∆ cells, the vacuolar amounts of neutral amino acids were highly reduced. Furthermore, it was noteworthy that the amounts of basic amino acids (lysine, histidine, and arginine) highly accumulating in vacuolesCitation1,2) were drastically reduced upon overproduction of GFP-Avt4p, but not GFP-Avt3p. The vacuolar amounts of aspartate and glutamate were little affected by overproduction of GFP-Avt3p but somewhat increased by overproduction of GFP-Avt4p. This observation was discussed below. These results suggest that Avt3p and Avt4p are involved in excretion of various neutral amino acids, but not aspartate and glutamate, from vacuoles in a redundant manner, and that Avt4p is particularly involved in excretion of basic amino acids.
Fig. 1. Vacuolar amino acid amounts in wild-type and mutants with altered expression of AVT3 and/or AVT4.
Notes: (A) Cells grown in SD+CA medium were treated with CuCl2 as described in “Materials and methods,” and their pools were analyzed with an amino acid analyzer. Levels of basic amino acids are indicated in the right panel. The results are means±SD of three independent experiments. Symbols: wild-type/empty vector (white bar), avt3∆avt4∆/empty vector (black bar), avt3∆avt4∆/pGPD-AVT3 (light gray bar), avt3∆avt4∆/pGPD-AVT4 (dark gray bar). (B) Subcellular localization of overproduced GFP-Avt3p and GFP-Avt4p was visualized using a fluorescence microscope. Images demonstrating colocalization of GFP fluorescence with the vacuolar membrane stained with FM4-64 are shown in merged images. Bar, 5 μm.
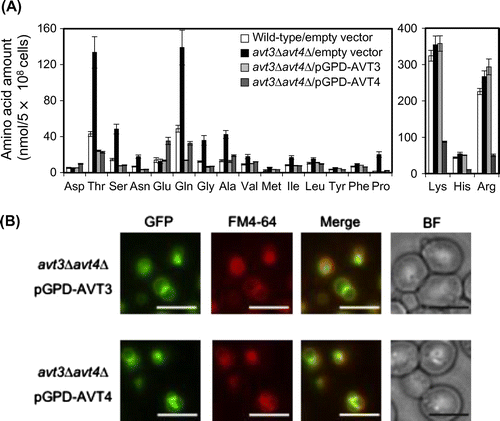
Avt3p- or Avt4p-dependent net efflux of alanine, valine, and proline by vacuolar membrane vesicles
Both Avt3p and Avt4p have been suggested to export isoleucine, glutamine, and tyrosine from vacuoles, since ATP-dependent uptake of these amino acids was accelerated in the vacuolar membrane vesicles of avt3∆ and/or avt4∆ cells.Citation5) Very recently, we found ATP-dependent efflux activities for neutral amino acids, such as alanine, valine, and proline across the vesicles of the wild-type strain.Citation14) Avt3p- or Avt4p-dependent efflux of amino acids by the vesicles was here examined (Fig. ). The vesicles were prepared from avt3∆avt4∆ cells overproducing either GFP-Avt3p or GFP-Avt4p employed for the experiment of Fig. . After preloading 14C-labeled amino acids into vesicles in the absence of ATP, the reaction was started by addition of ATP. Alanine, valine, or proline were further taken up into the avt3∆avt4∆ vesicles upon addition of ATP (Fig. , open circles) when compared with those in the absence of ATP (Fig. , closed circles). Upon overproduction of GFP-Avt3p or GFP-Avt4p, these amino acids in vesicles rapidly decreased in the presence of ATP (Fig. , open triangles and squares), whereas such rapid decrease was not observed in the absence of ATP (Fig. , closed triangles and squares). These results suggest that both Avt3p and Avt4p mediate export of neutral amino acids from vacuoles in a redundant manner. Avt3p- (Fig. (A)) or Avt4p- (Fig. (B)) dependent alanine efflux as well as influx by avt3∆avt4∆ vesicles (Fig. (C)) was inhibited by a V-ATPase inhibitor: concanamycin A and by a protonophore: CCCP. Avt3p and Avt4p are the transporters depending on the proton electrochemical gradient across the vacuolar membrane, likely proton/amino acid symporters.
Fig. 2. Amino acid efflux by Avt3p or Avt4p-overproduction.
Notes: 14C-labeled amino acids were preloaded into vacuolar membrane vesicles isolated from avt3∆avt4∆ cells carrying an empty vector (circles), pGPD-AVT3 (triangles), or pGPD-AVT4 (squares). Assay was performed in the presence (open symbols) or absence (closed symbols) of 2 mm ATP. The amount of preloaded 14C-labeled amino acids (0 min) was taken as 100%. The relative amounts trapped on the filters were plotted. The results are means±SD of three independent experiments.
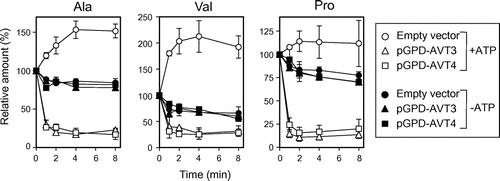
Fig. 3. Effect of concanamycin A and CCCP on efflux/influx of alanine.
Notes: Vacuolar membrane vesicles isolated from avt3∆avt4∆ cells carrying either pGPD-AVT3 (A), pGPD-AVT4 (B) or empty vector (C) were preincubated with either 1 μm concanamycin A (triangles) or 10 μm CCCP (squares), at 25 °C for 5 min before preloading 14C-labeled alanine. As a control, vesicles were preincubated with solvent (1% ethanol, circles). The data represents the efflux or influx of alanine in the presence of 2 mm ATP and are means ± SD of three independent experiments.
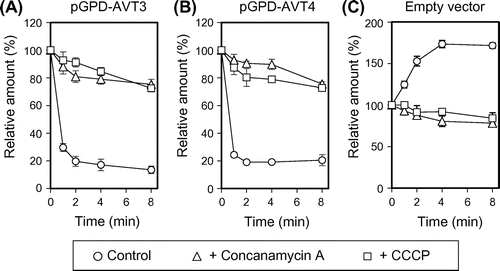
Involvement of Avt4p in ATP-dependent transport of arginine by vacuolar membrane vesicles
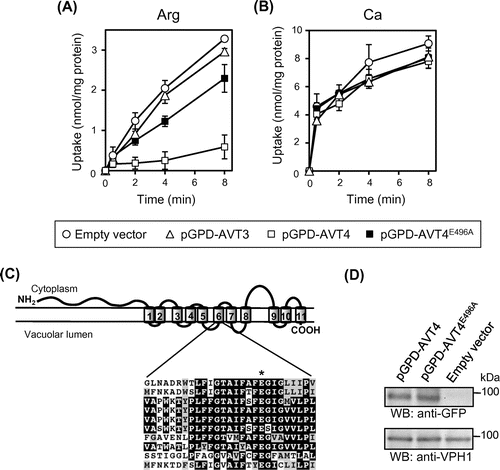
From the results on Avt4p-dependence of the vacuolar levels of basic amino acids (Table and Fig. ), it is likely that Avt4p, not Avt3p, exports basic amino acids as well as other neutral amino acids from vacuoles. We, however, could not observe ATP-dependent efflux of basic amino acids by vacuolar membrane vesicles due to the robust uptake activity by other transporters (data not shown). Instead, we examined the effect of Avt4p overproduction on net uptake of arginine by vacuolar membrane vesicles without the preloading. Vacuolar membrane vesicles were prepared from the same strains employed for Figs. . When compared with avt3∆avt4∆ vesicles (Fig. (A), open circles), ATP-dependent arginine uptake was largely decreased by overproduction of GFP-Avt4p (Fig. (A), open squares) but not GFP-Avt3p (Fig. (A), open triangles). Unlike arginine uptake, calcium uptake by vesicles, which is primarily mediated by a calcium/proton antiporter,Citation15) was not largely affected by GFP-Avt4p overproduction (Fig. (B)). As the members of AAAP transporter family,Citation16) both Avt3p and Avt4p share a number of highly conserved amino acid residues. Glu-496 is one such residue embedded in the sixth transmembrane helix of the putative 11 transmembrane helices of Avt4p (Fig. (C)). Vacuolar membrane vesicles were isolated from cells overproducing GFP-fusion of mutated Avt4p (Avt4pE496A), in which the Glu-496 was substituted with alanine, and the arginine uptake was examined. Although the GFP-Avt4pE496A was equally expressed at the vacuolar membrane as GFP-Avt4p (wild-type) (Fig. (D)), arginine uptake was only partially decreased (Fig. (A), closed squares). In addition, the vacuolar amounts of arginine, lysine, histidine, as well as various neutral amino acids in avt3∆avt4∆ cells were not fully decreased by Avt4pE496A-overproduction as compared to Avt4p (wild-type)-overproduction (data not shown). These results suggest that Avt4p is involved in vacuolar transport of various amino acids, mediating export of basic amino acids as well as other neutral ones.
Fig. 4. Arginine uptake by vacuolar membrane vesicles overproducing GFP-Avt3p or GFP-Avt4p.
Notes: (A) and (B) Vacuolar membrane vesicles were isolated from avt3∆avt4∆ cells carrying an empty vector (open circles), pGPD-AVT3 (open triangles), pGPD-AVT4 (open squares), or pGPD-AVT4E496A (closed squares) and were used for uptake assay of arginine (A) or calcium (B). The reactions were initiated by addition of 14C-labeled arginine or 45CaCl2 at 0 min. The results are means±SD of three independent experiments. (C) Top, predicted secondary structure of Avt4p showing the location of TM6 (amino acids 480–502). Bottom, sequence alignments of Avt4p TM6 and analogous regions in Avt3p, human (hPAT1 and hPAT2), mouse (mPAT1), rat (rPAT1), Caenorhabditis elegans (ceT27A1.5), fly (dmCG13384-PA), Arabidopsis thaliana (ANT1), and fission yeast Schizosaccharomyces pombe (spAC3H1.09C) homologues by ClustalW. Amino acid residues that are identical are shown against a black background, whereas residues that are similar are against a gray background. The conserved glutamate (E496) is indicated with an asterisk. (D) Western blot analysis for overproduced GFP-Avt4 and GFP-Avt4E496A proteins. Vacuolar membrane vesicles isolated from avt3∆avt4∆ cells carrying a vector as indicated were subjected to Western blot analysis using anti-GFP antibody. As a loading control, Vph1p was detected by anti-Vph1 antibody.
Discussion
In nutrient-rich condition, S. cerevisiae cells accumulate about 70–90% of cellular basic amino acids in vacuoles.Citation1,2) Vacuolar accumulation of basic amino acids has been reported in several fungal species,Citation17Citation–Citation19) implicating that it is a common feature among fungi. Under nitrogen starvation, basic amino acids (Table ) as well as other amino acids (data not shown) rapidly disappeared from vacuoles, suggesting the existence of export system(s). Avt3p and Avt4p were suggested to be involved in excretion of a limited number of amino acid species.Citation5) In this study, we examined the change in vacuolar amino acid levels in cells with altered expression of AVT3 and/or AVT4. Our amino acid composition analysis implicated the involvement of Avt3p and Avt4p for excretion of various amino acids from vacuoles. Broad substrate specificity of these transporters was further supported by amino acid transport assay using vacuolar membrane vesicles. It is noteworthy that Avt4p is especially involved in excretion of basic amino acids (Table and Figs. and ). The reduction of vacuolar basic amino acids under nitrogen-starved condition was obviously suppressed in avt4∆ cells (Table ). Recently, Ypq1p, Ypq2p, and Ypq3p, of which mammalian homologue, PQLC2, was shown to export basic amino acids from lysosomes, were suggested to be involved in homeostasis of basic amino acids.Citation20) Our preliminary results indicated that, disruption of YPQ genes in avt4∆ cells further increased the amount of vacuolar basic amino acids under nitrogen starvation, whereas disruption of YPQ genes in the AVT4 wild-type background did not largely affect the vacuolar amino acid pool. Thus, Avt4p seems to act as the main system for excretion of basic amino acids from vacuoles.
There are multiple transport systems for uptake and export of a variety of amino acids at the vacuolar membrane.Citation5,6,21,22) ATP-dependent efflux of alanine, valine, and proline was clearly observed (Fig. ). These amino acids were taken up into vesicles from the avt3∆avt4∆ mutant in an ATP-dependent manner. However, these activities were not observed by vesicles from avt1∆avt3∆avt4∆ mutant (data not shown), suggesting interplay among Avt1p, Avt3p, and Avt4p transporters for balancing amino acid flux across the vacuolar membrane.
It was reported that ATP-dependent efflux of glutamate or aspartate was deficient in the vesicles of avt6∆ cells,Citation5) and we observed that glutamate efflux was normal in the vesicles from avt3∆, avt4∆, and avt3∆avt4∆ cells (data not shown). From the data in this report (Figs. and ) and in the previous paper,Citation5) we suggest that Avt3p and Avt4p are the vacuolar amino acid exporters for a variety of amino acids, except for glutamate and aspartate. Both are likely proton/amino acid symporters, since the ATP-dependent activities were inhibited by concanamycin A or CCCP (Fig. ). Avt4p but not Avt3p is likely involved in transport of basic amino acids from vacuoles (Table , Figs. and ). Discrimination of basic amino acids as the substrate between Avt4p and Avt3p is an important feature for elucidating the substrate binding site at the molecular level. The identity and similarity in amino acid sequences of the stretches for the putative 11 transmembrane helices between Avt3p and Avt4p are 47 and 77%, respectively. There are a number of highly conserved portions in the sequences as shown in Fig. (C). Recent analyses of structure–function relationship of transporters have revealed the functional importance of the conserved charged amino acid residues in transmembrane domains. Our results suggest that the conserved glutamate residue in the predicted sixth transmembrane helices is important for the excretion of amino acids from vacuoles (Fig. ). In any case, the details in kinetic features of amino acid transport by Avt3p and Avt4p should be examined.
Although AVT4 disruption obviously suppressed the reduction of vacuolar basic amino acids under nitrogen-starved condition, it did not affect the amounts of these amino acids in nutrient-rich condition (Table ). Robust uptake activity for basic amino acids operating in nutrient-rich condition may overwhelm the change in the vacuolar extrusion activity resulted from AVT4 disruption. In addition, it is very likely that the Avt4p activity is regulated depending on nutritional conditions. It has been suggested that transcription of AVT4 gene is induced upon nitrogen starvation.Citation13) In addition, predicted topology of Avt4p suggests that this protein possesses a long N-terminal hydrophilic region (Fig. (C)), which is possibly involved in the regulation. Avt3p also has a hydrophilic region at the N-terminal, but the conservation to that of Avt4p is limited. It is peculiar that the vacuolar amounts of aspartate and glutamate increased by overproduction of Avt4p (Fig. ). We suppose that overproduction of Avt4p in nutrient-rich condition may alter the nutritional status in cells, affecting expression or activity of other transporter(s) for acidic amino acids. Comprehensive identification of vacuolar amino acid transporters and investigation of those substrate specificity and regulation are important for understanding of the significance of vacuolar amino acid compartmentalization in eukaryotic cells.
Acknowledgments
We thank Kana Hondo for technical help and Venture Business Laboratory, Ehime University, for assistance in amino acid analysis. This work was supported in part by a grant-in-aid from Elizabeth Arnold Fuji Foundation (to Y.K. and T.S.), from the Noda Science Foundation (to Y.K. and T.S.), and from JSPS Postdoctoral Fellowship (to S.C.).
Notes
Abbreviations: V-ATPase, vacuolar H+-ATPase; GFP, green fluorescent protein; CCCP, carbonylcyanide m-chlorophenylhydrazone; MES, 2-(N-morpholino)ethanesulfonic acid.
References
- Wiemken A, Durr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch. Microbiol. 1974;101:45–57.10.1007/BF00455924
- Kitamoto K, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J. Bacteriol. 1988;170:2683–2686.
- Ohsumi Y, Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1981;256:2079–2082.
- Sato T, Ohsumi Y, Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J. Biol. Chem. 1984;259:11505–11508.
- Russnak R, Konczal D, McIntire SL. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 2001;276:23849–23857.10.1074/jbc.M008028200
- Shimazu M, Sekito T, Akiyama K, Ohsumi Y, Kakinuma Y. A family of basic amino acid transporters of the vacuolar membrane from Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:4851–4857.
- Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 2005;280:31582–31586.10.1074/jbc.M506736200
- Chahomchuen T, Hondo K, Ohsaki M, Sekito T, Kakinuma Y. Evidence for Avt6 as a vacuolar exporter of acidic amino acids in Saccharomyces cerevisiae cells. J. Gen. Appl. Microbiol. 2009;55:409–417.10.2323/jgam.55.409
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27.
- Mumberg D, Müller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122.10.1016/0378-1119(95)00037-7
- Ohsumi Y, Kitamoto K, Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 1988;170:2676–2682.
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995;128:779–792.10.1083/jcb.128.5.779
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257.10.1091/mbc.11.12.4241
- Ishimoto M, Sugimoto N, Sekito T, Kawano-Kawada M, Kakinuma Y. ATP-dependent export of neutral amino acids by the vacuolar membrane vesicles of Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2012;76:1802–1804.10.1271/bbb.120372
- Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1983;258:5614–5617.
- Paulsen IT, Sliwinski MK, Nelissen B, Goffeau A, Saier MH Jr. Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 1998;430:116–125.10.1016/S0014-5793(98)00629-2
- Vaughn LE, Davis RH. Purification of vacuoles from Neurospora crassa. Mol. Cell. Biol. 1981;1:797–806.
- Förster C, Marienfeld S, Wilhelm R, Krämer R. Organelle purification and selective permeabilisation of the plasma membrane: two different approaches to study vacuoles of the filamentous fungus Ashbya gossypii. FEMS Microbiol. Lett. 1998;167:209–214.10.1111/fml.1998.167.issue-2
- Chardwiriyapreecha S, Hondo K, Inada H, Chahomchuen T, Sekito T, Iwaki T, Kakinuma Y. A simple and specific procedure to permeabilize the plasma membrane of Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2009;73:2090–2095.10.1271/bbb.90319
- Jezegou A, Llinares E, Anne C, Kieffer-Jaquinod S, O’Regan S, Aupetit J, Chabli A, Sagne C, Debacker C, Chadefaux-Vekemans B, Journet A, Andre B, Gasnier B. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. USA. 2012;109:E3434–E3443.10.1073/pnas.1211198109
- Yang Z, Huang J, Geng J, Nair U, Klionsky DJ. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol. Biol. Cell. 2006;17:5094–5104.10.1091/mbc.E06-06-0479
- Sekito T, Fujiki Y, Ohsumi Y, Kakinuma Y. Novel families of vacuolar amino acid transporters. IUBMB Life. 2008;60:519–525.10.1002/iub.v60:8
