Abstract
Endo-α-mannosidase, a GH99-family glycoside hydrolase, cleaves α-mannoside linkages with glucose residues. This enzyme is proposed to play a critical role in N-glycan processing for deglucosylation. To measure endo-α-mannosidase activity, we synthesized a fluorescently labeled tetrasaccharide derivative (Glcα1-3Manα1-2Manα1-2Manα1-O–C3H6–NH-Dansyl) in a stereocontrolled manner. The tetrasaccharide skeleton was prepared by step-wise coupling using mannose donors 4 and 7. The 1,2-cis α-glycosidic linkage on the non-reducing end of the glucose residue was constructed by inversion of the stereochemistry of the C-2 hydroxyl group in the α-mannose residue. Finally, the dansyl group was introduced at the reducing end via an aminopropyl linker. This probe successfully measured endo-α-mannosidase activity.
Graphical Abstract
A fluorescently labeled tetrasaccharide 1 (Glcα1-3Manα1-2Manα1-2Manα1-O-C3H6-NH-Dansyl) was chemically synthesized. Using this probe, we successfully measured endo-α-mannosidase activity.
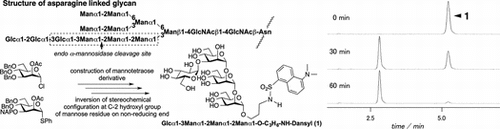
N-linked glycans on proteins are processed in the endoplasmic reticulum (ER) through a system that is highly conserved in eukaryotes. In the early processing of N-linked glycans, glucosidase I, glucosidase II, and α-mannosidase I sequentially trim the glucosylated high mannose-type tetradecasaccharide (Glc3Man9GlcNAc2) structure to yield ER-type N-glycansCitation1) These glycans play important roles in glycoprotein quality control in the ER.Citation2) Endo-α-mannosidase, a member of the glycoside hydrolase (GH) 99 family, is localized in the Golgi apparatus and in the intermediate compartment.Citation3) The enzyme cleaves the glucose-substituted α-mannoside linkage within Glc3Man9GlcNAc2, Glc2Man9GlcNAc2, or Glc1Man9GlcNAc2 to give Man8GlcNAc2, which lacks the outermost α-1,2-linked mannose residue in the A-arm (Fig. (A)). This alternative deglucosylation process allows glycoproteins to bypass the ER-localized quality control machinery. Therefore, this enzyme has been proposed to play an important role in the post-ER quality control pathway.Citation4,Citation5) Recently, crystal structures of GH99 from two types of enteric bacteria have been determined.Citation6) The kinetics and catalytic mechanisms of mammalian endo-α-mannosidase remain incompletely understood.
Fig. 1. Structure of glucosylated high mannose-type N-Glycan and Dansyl-labeled tetrasaccharide 1 and disaccharide derivative 2.
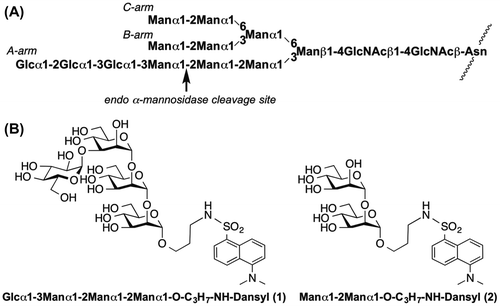
Fig. 2. 150 MHz 13C NMR spectra of monosaccharide 9 measured at different temperatures (A: 22 °C; B: 70 °C).
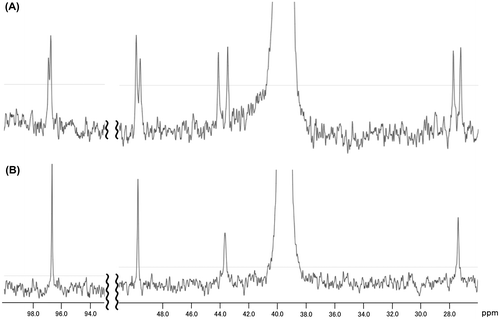
Fig. 3. 1H NMR spectra of tetrasaccharide 1 (A) and disaccharide 2 (B).
Note: The anomeric signals of α-glucose and α-mannose residues are indicated by white and black arrow, respectively.
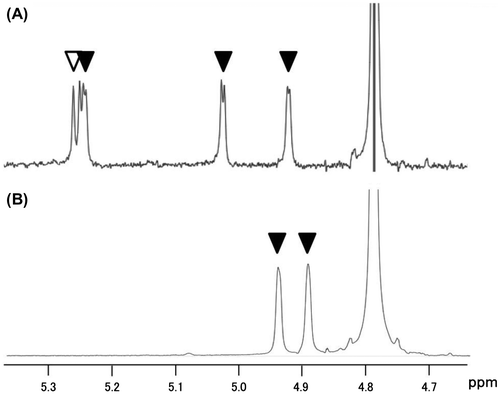
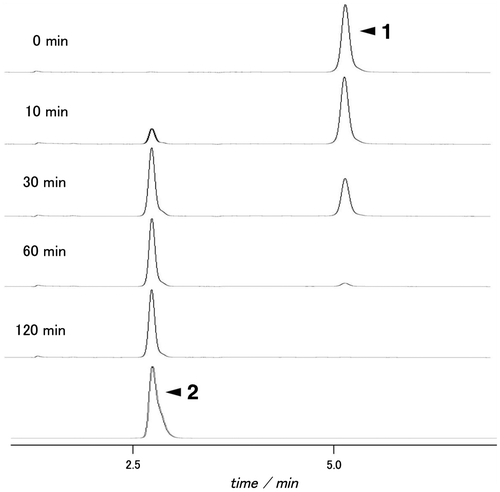
Fig. 4. HPLC analysis of the hydrolysates of compound 1 by endo-α-mannosidase. dansyl-oligosaccharides were monitored via fluorescent signals (excitation wavelength, 340 nm; emission wavelength, 515 nm).
Fluorescently labeled glycans are powerful tools for investigating the function of glycosidases and glycosyltransferases because they deem the enzymatic reactions easily detectable by spectrofluorometer combined with high performance liquid chromatography (HPLC).Citation7,Citation8) For example, the activity of endo-α-mannosidase was measured using a labeled pyridyl-amino or 2-anthranilic acid group in the high mannose-type glycan substrate.Citation9,Citation10) However, preparation of an adequate amount of glucosylated high mannose glycan from natural sources is difficult. Substrates with less complex structures are required to create a simple assay system for measuring endo-α-mannosidase activity. In this study, we synthesized the partial structure of a high mannose-type glycan containing an aminopropyl linker at the reducing end and introduced a fluorescent group for the detection of enzymatic activity. Using this probe, we succeeded in measuring endo-α-mannosidase activity.
Results and discussion
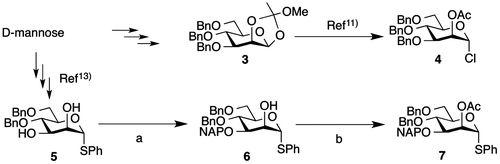
We designed the tetrasaccharide derivative shown in Fig. . based on the mechanism of endo-α-mannosidase activity.Citation6) To construct this tetrasaccharide, we prepared 2 mannosyl donors (compounds 4 and 7 as shown in Scheme ). Preparation of mannosyl chloride donor 4 proceeded in six steps starting from D-mannose as previously described.Citation11) Thiophenyl donor 7 was synthesized from compound 5. Regioselective introduction of 3-naphthylmethyl (NAP) ether via stanylene acetal to compound 5 gave C-3 NAP ether derivative 6 (yield, 87%). Acetylation of the C-2 position of compound 6 yielded thiophenyl donor 7 (yield, 98%).
Scheme 1. Reagents and conditions.
Note: (a) (i) dibutyltin oxide, toluene, 110 °C, (ii) 2-(bromomethyl)naphthalene, tetrabutylammonium bromide, toluene, 80 °C, 87%; (b) Ac2O, pyridine, 40 °C, 98%.
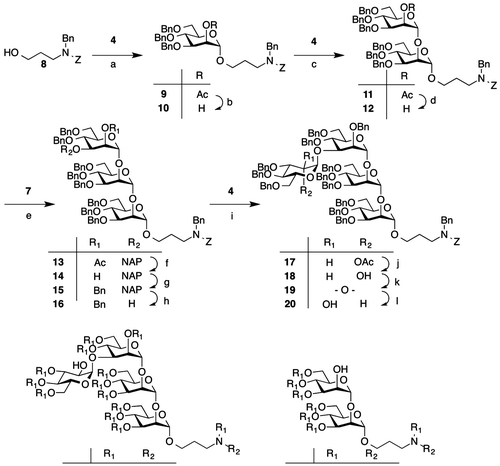
With these two donors in hand, we constructed a tetrasaccharide skeleton as shown in Scheme . Coupling of N-protected aminopropyl alcohol 8 with mannosyl chloride 4 using silver trifluoromethane sulfonate (AgOTf) as a promoter gave compound 9. The 13C spectrum of compound 9 shows two anomeric carbons caused by the configuration of the benzyloxy carbonyl group (Z) used for amine protection. In fact, these two anomeric 13C NMR signals of compound 9 (97.0 ppm and 96.8 ppm in DMSO) at 22 °C were changed into one signal (96.7 ppm in DMSO) at 70 °C (Fig. ). Furthermore, removal of the acetyl, benzyl, and Z protecting groups from compound 9 gave a single product. Removal of the acetyl group from compound 9 gave compound 10. Coupling of compound 10 with compound 4 gave mannobiose derivative 11, which was converted into the glycosyl acceptor 12. Glycosylation of compound 12 with thioglycoside 7 was performed using N-iodosuccinimide (NIS) and AgOTf as a promoter to give mannotriose derivative 13 (yield, 86%). Removal of acetyl group of compound 13 gave compound 14. The liberated C-2 hydroxyl group of compound 14 was protected with a benzyl group to give compound 15. The C-3 NAP ether of compound 15 was chemoselectively removed with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in two steps to give compound 16 (yield, 46%). The resultant OH-3 of compound 16 was mannosylated with compound 4 and AgOTf to give mannotetraose derivative 17. Removal of the acetyl group at the C-2 position of compound 17 gave compound 18 (yield, 90%). Having obtained the mannotetraose derivative 18 possessing a C-2 free hydroxyl group at the non-reducing end of the mannose residue, we converted the stereochemistry at the C-2 hydroxyl group to the gluco configuration using an oxidation–reduction method.Citation12,Citation13) The treatment of compound 18 with DMSO and Ac2O gave ulosyl derivative 19. The product was reduced by sodium triacetoxyborohydride (NaBH(OAc)3) to give gluco-derivative 20 (yield, 63%) as a major product. The epimeric gluco- and manno-derivatives were separated by column chromatography to yield the pure compound. The structure of compound 20 was confirmed by 1H, 13C, and 2D NMR spectroscopy. The coupling constant of J1H–2H (3.7 Hz) corresponding to the reducing end of glucosyl residue supported the assigned structure. Removal of the benzyl ether and Z groups on tetrasaccharide 20 was performed in the presence of Pd(OH)2 to give compound 21. The free amino group on the aglycon of compound 21 was dansylated using dansyl chloride to give the target compound 1 (Fig. ). Dansyl-labeled mannobiose derivative 2 was then synthesized for monitoring the enzymatic reaction using HPLC.
Scheme 2. Reagents and conditions.
Note: (a) AgOTf, MS 4A, toluene, −20 °C, 93%; (b) NaOMe, MeOH, r.t., 83%; (c) AgOTf, MS 4A, toluene, −20 °C, 87%; (d) NaOMe, THF, MeOH, r.t., 96%; (e) NIS, AgOTf, MS 4A, toluene, −20 °C, 86%; (f) NaOMe, MeOH, THF, r.t., 90%; (g) BnBr, NaH, DMF, r.t., 94%; (h) DDQ, ClCH2CH2Cl, MeOH, r.t., 49%; (i) AgOTf, MS 4A, toluene, −20 °C, 90%; (j) NaOMe, MeOH, THF, 40 °C, 90%; (k) DMSO, Ac2O, r.t.; (l) NaBH(OAc)3, THF, 0 °C, 63%; (m) Pd(OH)2/C, H2, THF, H2O, r.t., 44%; (n) Dansyl-Cl, NaHCO3, H2O, acetone, r.t., 98%; (o) Pd(OH)2/C, H2, THF, H2O, r.t., 89%; (p) Dansyl-Cl, NaHCO3, H2O, acetone, r.t., 71%.
To confirm whether enzyme activity could be detected using Dansyl-labeled tetrasacccharide derivative 1, we incubated compound 1 with a recombinant human endo-α-mannosidase. The reaction was monitored by HPLC. The HPLC elution profile showed a new peak at 2.8 min (Fig. ). We identified the peak as hydrolysis product 2 by comparison with HPLC and MALDI-TOF-MS analysis. These results clearly show that probe 1 serves as a substrate for endo-α-mannosidase.
Conclusion
Fluorescence-labeled tetrasaccharide 1 was successfully synthesized. We then used this probe to measure endo-α-mannosidase activity. Based on this result, we have designed a more sensitive fluorescent probe using rhodamine derivatives and a BODIPY-labeled tetrasaccharide. A detailed investigation of endo-α-mannosidase activity is currently underway, and results will be reported in due course.
Experimental
Starting materials and reagents were purchased from standard vendors and used. All reactions sensitive to moisture were carried out under argon atmosphere with anhydrous solvents. Analytical thin layer chromatography was developed on silica gel 60 F plate (Merck). Silica gel column chromatography was performed on Silica gel 60 N (40–100 mesh or 100–210 mesh, Kanto Kagaku Co., Ltd, Japan). NMR spectra were obtained on a JEOL ECX 400 or 600 spectrometers at ambient temperature unless otherwise noted. MALDI-TOF MS was recorded in the high resolution mode with positive ion mode on an AXIMA-Performance (Shimadzu, Kyoto, Japan). HPLC was performed on Prominence HPLC system equipped with a fluorescence detector RF-20Axs (Shimadzu, Kyoto, Japan).
Phenyl 4,6-di-O-benzyl-3-O-naphthylmethyl-1-thio-α-D-mannopyranoside (6)
To a solution of 5 (1.1 g, 2.4 mmol) and dibutylthin oxide (0.76 g, 3.1 mmol) in toluene (30 mL) was stirred for 3 h at 110 °C and concentrated in vacuo. The residue was dissolved in toluene (20 mL) and 2-(bromomethyl)naphthalene (0.98 g, 4.5 mmol) and tetrabutylammonium bromide (1.5 g, 4.8 mmol) were added into the mixture, which was stirred at 80 °C for 1 h. The reaction was quenched by the addition of sat. NaHCO3 aq. The mixture was diluted with EtOAc and filtered through Celite. The organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography using gradient solvent system (toluene/EtOAc = 100:0–90:10) to give the title compound 6 (1.1 g, 87%). Rf 0.51 (toluene/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3): δH 7.85–7.19 (m, 22H, Ar), 5.61 (d, 1H, J = 1.4 Hz, H-1), 4.90–4.84 (m, 3H, –O–CH2–Ph, –O–CH2–Nap), 4.62 (d, 1H, J = 11.9 Hz, –O–CH2–Ph), 4.55 (d, 1H, J = 10.4 Hz, –O–CH2–Ph), 4.46 (d, 1H, J = 11.9 Hz, –O–CH2–Ph), 4.32–4.30 (m, 2H, H-2, H-5), 3.99–3.94 (m, 2H, H-3, H-4), 3.81 (dd, 1H, J = 10.7, 4.6 Hz, H-6), 3.69 (dd, 1H, J = 1.9 Hz, H-6), 2.71 (bs, 1H, –OH). 13C NMR (150 MHz, CDCl3): δC 138.4, 138.3, 135.2, 134.0, 133.4, 133.3, 131.8, 129.1, 128.6, 128.5, 128.4, 128.1, 128.0, 127.9, 127.7, 127.6, 127.0, 126.4, 126.3, 126.0, 87.5 (C-1), 80.4 (C-3), 75.4 (–O–CH2–Ph), 74.6 (C-4), 73.5 (–O–CH2–Ph), 72.5 (–O–CH2–Nap), 72.4 (C-5), 70.1 (C-2), 69.0 (C-6) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 615.218, Found: 615.227.
Phenyl 2-O-acetyl-4,6-di-O-benzyl-3-O-naphthylmethyl-1-thio-α-D-mannopyranoside (7)
To a solution of 6 (1.1 g, 1.9 mmol) in pyridine (20 mL) was added acetic anhydrate (10 mL) at room temperature. The reaction mixture was stirred at 40 °C for 4 d and quenched with methanol. The reaction mixture was concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc = 75:25) to give the title compound 7 (1.2 g, 98%). Rf 0.51 (hexane/EtOAc = 7:3). 1H NMR (600 MHz, CDCl3): δH 7.83–7.23 (m, 22H, Ar), 5.66 (s, 1H, H-2), 5.55 (s, 1H, H-1), 4.90 (t, 2H, J = 16.2, 17.4 Hz, –O–CH2–Nap), 4.73–4.45 (m, 4H, –O–CH2–Ph), 4.33 (m, 1H, H-5), 4.00–3.99 (m, 2H, H-3, H-4), 3.86 (dd, 1H, J = 6.0, 5.4 Hz, H-6), 3.73 (d, 1H, J = 16.2, H-6), 2.16 (s, 3H, Ac). 13C NMR (150 MHz, CDCl3): δC 170.3 (–CO–CH3), 138.2, 138.1, 135.0, 133.6, 133.2, 133.0, 131.7, 129.0, 128.3, 128.2, 127.9, 127.8, 127.7, 127.5, 127.0, 126.1, 126.0, 86.2 (C-1), 78.5 (C-3), 75.2 (–O–CH2–Ph), 74.5 (C-4), 73.3 (–O–CH2–Nap), 72.5 (C-5), 71.9 (–O–CH2–Ph), 70.3 (C-2), 68.8 (C-6), 21.1 (–OC–CH3) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 657.229, Found: 657.219.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-D-mannopyranoside (9)
A mixture of 3-(N-benzyl-N-benzyloxycarbonyl)aminopropanol (8) (0.72 g, 2.4 mmol), AgOTf (0.90 g, 3.5 mmol), and freshly activated molecular sieve (4Å, 8.0 g) in toluene (25 mL) was stirred under argon. The mixture was cooled to −20 °C and then, a solution of 4 (1.5 g, 2.9 mmol) in toluene (15 mL) was added. The reaction mixture was stirred for 3 h. The reaction was quenched by the addition of Et3 N and diluted with EtOAc. The mixture was filtered through a pad of Celite, the filtrate was washed successively with 1 M HCl, sat. NaHCO3 aq., and brine. The organic layer was dried through Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography using gradient solvent system (hexane/EtOAc = 93:7–85:15) to give the title compound 9 (1.7 g, 93%). Rf 0.27 (hexane/EtOAc = 13:7). 1H NMR (600 MHz, CDCl3): δH 7.34–7.14 (m, 25H, Ar), 5.31 (d, 1H, H-2, J = 7.3 Hz), 5.19 (bs, 1H, –COO–CH2–Ph), 5.15 (bs, 1H, –COO–CH2–Ph), 4.84 (d, 1H, J = 10.6 Hz, –O–CH2–Ph), 4.78 (bs, 0.5H, H-1), 4.75 (bs, 0.5H, H-1), 4.66 (m, 2H, –O–CH2–Ph), 4.53–4.45 (m, 5H, –O–CH2–Ph, –O–CH2–CH2–), 3.92–3.86 (m, 2H, H-3, H-4), 3.77–3.62 (m, 4H, –N–CH2–Ph, H-5, H-6, H-6), 3.40–3.27 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.14 (s, 3H, –CO–CH3), 1.82–1.74 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 170.6 (Ac), 156.8 and 156.3 (–N–CO–CH2Ph), 138.5, 138.3, 138.0, 137.9, 136.9, 136.8, 128.7, 128.6, 128.5, 128.4, 128.2, 128.0, 127.9, 127.7, 127.4, 97.93 (C-1), 97.86 (C-1’) 78.3 (C-3), 75.3 (–O–CH2–Ph), 74.4 (C-4), 73.6 (–O–CH2–Ph), 71.9 (–O–CH2–Ph), 71.6 (C-5), 68.9 (C-2, C-6), 67.3 (–COO–CH2–Ph), 65.7 and 65.5 (–N–CH2–Ph), 50.9 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.3 and 27.8 (–CH2–CH2–CH2–), 21.3 (–OC–CH3). 13C NMR (150 MHz, (CD3)2SO, 22 °C): δC 169.7 (Ac), 155.9 and 155.4, 138.4, 138.3, 138.1, 138.0, 137.0, 136.9, 128.5, 128.4, 128.2, 127.7, 127.6, 127.5, 127.3, 127.2, 97.0 and 96.8 (C-1), 77.5, 74.3, 74.2, 72.3, 71.0, 70.7, 68.8, 68.1, 66.3 (–COO–CH2–Ph), 65.0 and 64.7 (–N–CH2–Ph), 49.9 and 49.6 (–O–CH2–CH2), 44.2 and 43.5 (–CH2–CH2–N–), 27.8 and 27.3 (–CH2–CH2–CH2–), 20.8 (–OC–CH3). 13C NMR (150 MHz, (CD3)2SO, 70 °C): δC 169.2 (Ac), 155.3, 138.2, 137.8, 136.7, 128.0, 127.8, 127.7, 127.3, 127.2, 127.1, 127.0, 126.7, 96.7 (C-1), 77.2, 74.1, 73.8, 72.2, 71.0, 70.6, 68.9, 68.2, 66.0 (–COO–CH2–Ph), 64.7 (–N–CH2–Ph), 49.7 (–O–CH2–CH2), 43.7 (–CH2–CH2–N–), 27.4 (–CH2–CH2–CH2–), 20.3 (–OC–CH3). MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 796.346, Found: 796.350.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl 3,4,6-tri-O-benzyl-α-D-mannopyranoside (10)
To a solution of 9 (0.42 g, 0.55 mmol) in methanol (5 mL) was added 0.28 mL of 1 M NaOMe in methanol at room temperature. The reaction mixture was stirred at the same temperature for 19 h and then neutralized with Amberlyst 15E. The mixture was filtered, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc = 45:55) to afford the title compound 10 as colorless syrup (0.33 g, 83%). Rf 0.28 (hexane/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3): δH 7.31–7.15 (m, 25H, Ar), 5.17 (bs, 1H, –COO–CH2–Ph), 5.15 (bs, 1H, –COO–CH2–Ph), 4.82–4.79 (m, 2H, H-1, –O–CH2–Ph), 4.64–4.61 (m, 3H, –O–CH2–Ph), 4.52–4.47 (m, 4H, –O–CH2–Ph, –O–CH2–CH2–), 3.97–3.66 (m, 7H, H-2, H-3, H-4, H-5, H-6, H-6, –N–CH2–Ph), 3.33–3.28 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.42 (bs, 1H, –OH), 1.74 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 156.6 and 156.1 (–N–CO–CH2Ph), 138.2, 137.9, 137.7, 136.7, 128.5, 128.3, 127.9, 127.8, 99.2 and 99.1 (C-1), 80.1 (C-3), 75.1 (–O–CH2–Ph), 74.2 (C-4), 73.4 (–O–CH2–Ph), 71.9 (–O–CH2–Ph), 71.1 (C-5), 68.8 (C-6), 68.3 (C-2), 67.2 (–COO–CH2–Ph), 65.2 and 65.0 (–N–CH2–Ph), 50.6 and 50.4 (–O–CH2–CH2), 44.5 and 43.6 (–CH2–CH2–N–), 28.1 and 27.6 (–CH2–CH2–CH2–), MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 754.336 Found: 754.332.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (2-O-acetyl-3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (11)
A mixture of 10 (0.36 g, 0.49 mmol), AgOTf (0.18 g, 0.70 mmol), and freshly activated molecular sieve (4Å, 3.0 g) in toluene (6 mL) was stirred under argon. The mixture was cooled to −20 °C, and then solution of 4 (0.36 g, 0.46 mmol) in toluene (4 mL) was added. The reaction mixture was stirred at −20 °C for 6 h and stirred for 11 h at ambient temperature. The reaction was quenched by the addition of Et3 N and diluted with EtOAc. The mixture was filtered through a pad of Celite. The filtrate was washed successively with 1 M HCl, sat. NaHCO3 aq., and brine. The organic layer was dried through Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography using gradient solvent system (toluene/EtOAc = 100:0–90:10) to give 11 (0.52 g, 87%). Rf 0.45 (hexane/EtOAc = 2:1). 1H NMR (600 MHz, CDCl3): δH 7.32–7.14 (m, 40H, Ar), 5.53 (s, 1H, H-2), 5.17 (bs, 1H, –COO–CH2–Ph), 5.13 (bs, 1H, –COO–CH2–Ph), 5.06 (s, 0.5H, H-1), 5.04 (s, 0.5H, H-1), 4.85–4.81 (m, 3H, H-1), 4.66–4.38 (m, 12H), 3.98–3.54 (m, 12H), 3.31–3.17 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.12 (s, 3H, CH3CO–), 1.74–1.67 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 170.3 (Ac), 138.6, 138.5, 138.3, 138.1, 128.7, 128.5, 128.4, 128.3, 128.2, 128.1, 127.9, 127.8, 127.7, 127.6, 127.3, 99.7 (C-1), 98.9 and 98.8 (C-1), 79.8, 78.3, 75.3, 75.2, 75.0, 74.7, 74.5, 73.5, 73.4, 72.2, 72.0, 71.9, 69.3, 69.2, 68.9, 67.3 (–COO–CH2–Ph), 65.4 and 65.3 (–N–CH2–Ph), 50.9 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–), 21.3 (–OC–CH3) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1228.540, Found: 1228.537.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (12)
To a solution of 11 (0.52 g, 0.43 mmol) in methanol (2 mL) and THF (1 mL) was added 0.22 mL of 1 M NaOMe in methanol at room temperature. The reaction mixture was stirred at room temperature for 23 h and then neutralized with Amberlyst 15E. The mixture was filtered and the filtered was concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc = 65:35) to afford the title compound 12 as colorless syrup (0.48 g, 96%). Rf 0.23 (hexane/EtOAc = 3:2). 1H NMR (600 MHz, CDCl3): δH 7.32–7.13 (m, 40H, Ar), 5.17–5.10 (m, 3H, H-1), 4.86–4.80 (m, 3H, H-1), 4.68–4.42 (m, 12H), 4.11 (s, 1H, H-2), 3.96–3.52 (m, 12H), 3.29–3.15 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.41 (s, 1H, –OH), 1.73–1.63 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 156.8, 156.2, 138.7, 138.6, 138.5, 138.4, 138.3, 138.1, 137.9, 137.0, 136.8, 128.7, 128.6, 128.5, 128.4, 128.3, 128.1, 127.8, 127.7, 127.6, 127.5, 127.3, 101.3 (C-1), 99.01 and 98.95 (C-1), 80.1, 79.8, 75.3, 75.2, 74.9, 74.3, 73.5, 73.4, 72.4, 72.3, 72.1, 71.7, 69.4, 68.6, 67.3 (–COO–CH2–Ph), 65.4 and 65.3 (–N–CH2–Ph), 50.8 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1186.529, Found: 1186.529.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (2-O-acetyl-4,6-di-O-benzyl-3-O-naphthylmethyl-α-D-mannopyranosyl) -(1-2)-(3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (13)
A mixture of NIS (29 mg, 0.13 mmol), AgOTf (29 mg, 0.11 mmol), and freshly activated molecular sieve (4Å, 1.0 g) in toluene (2 mL) was stirred under argon at −20 °C. The mixture was added a solution of thioglycoside 7 (55 mg, 0.09 mmol) and 12 (0.93 mg, 0.08 mmol) in toluene (8 mL). The mixture was stirred under argon at −20 °C for 3 h and stirred for 15 h at 0 °C. The reaction was quenched by the addition of Et3 N and diluted with EtOAc. The mixture was filtered through a pad of Celite. The filtrate was washed with sat. Na2S2O3 aq., 1 M HCl aq., sat. NaHCO3 aq., and brine. The organic layer was dried with MgSO4 and concentrated in vacuo. The resultant residue was purified by column chromatography on silica gel (toluene/EtOAc = 100:0–83:17) to afford 13 (0.12 g, 86%). Rf 0.23 (toluene/EtOAc = 9:1). 1H NMR (600 MHz, CDCl3): δH 7.72–7.01 (m, 57H, Ar), 5.55 (d, 1H, J = 3.3 Hz), 5.16–5.10 (m, 3H), 4.90–4.88 (m, 2H), 4.82–4.78 (m, 2H), 4.72–4.26 (m, 19H), 4.02–3.91 (m, 3H), 3.82–3.45 (m, 13H), 3.28–3.05 (m, 3H), 2.04 (s, 3H), 1.71–1.62 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 170.3 (Ac), 138.7, 138.6, 138.4, 138.3, 135.7, 133.4, 133.1, 128.6, 128.4, 128.3, 128.2, 128.0, 127.8, 127.6, 127.5, 100.8 (C-1), 99.5 (C-1), 98.98 and 98.90 (C-1), 80.2, 77.4, 76.0, 75.5, 75.1, 75.0, 74.6, 73.7, 73.2, 71.8, 71.5, 71.4, 71.2, 70.3, 68.9, 68.2, 67.3 (–COO–CH2–Ph), 65.4 and 65.3 (–N–CH2–Ph), 50.8 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–), 21.4 (–OC–CH3). MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1710.749, Found: 1710.747.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (4,6-di-O-benzyl-3-O-naphthylmethyl-α-D-mannopyranosyl) -(1-2)-(3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (14)
To a solution of 13 (0.36 g, 0.21 mmol) in methanol (5 mL) and THF (5 mL) was added 0.11 mL of 1 M NaOMe in methanol at room temperature. The reaction mixture was stirred at room temperature for 15 h and then neutralized with Amberlyst 15E. The mixture was filtered and the filtered was concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc = 65:35) to give 14 (0.32 g, 90%). Rf 0.23 (hexane/EtOAc = 17:3). 1H NMR (600 MHz, CDCl3): δH 7.79–7.12 (m, 57H, Ar), 5.17–5.12 (m, 4H, H-1, COO–CH2–Ph), 4.88–4.70 (m, 6H, H-1), 4.64–4.40 (m, 14H), 4.31 (d, 1H, J = 12.0 Hz, –O–CH2–Ph), 4.16 (s, 1H, H-2), 4.11 (s, 1H, H-2), 3.94–3.88 (m, 6H), 3.80–3.50 (m, 11H), 3.27–3.13 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.40 (bs, 1H, –OH), 1.72–1.64 (m, 2H, –CH2–CH2–CH2– ). 13C NMR (150 MHz, CDCl3): δC 156.7, 156.2, 138.6, 138.4, 138.3, 137.9, 136.8, 135.6, 133.4, 133.1, 130.0, 128.7, 128.6, 128.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 127.5, 127.3, 126.7, 101.1 (C-1), 101.0 (C-1), 98.98 and 98.91 (C-1), 80.0, 79.5, 75.4, 75.2, 75.1, 74.9, 74.4, 73.4, 73.3, 72.5, 72.3, 72.0, 71.8, 69.8, 69.7, 69.4, 68.9, 68.8, 67.3 (–COO–CH2–Ph), 65.4 and 65.3 (–N–CH2–Ph), 50.8 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–). MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1168.739, Found: 1168.716.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (2,4,6-tri-O-benzyl-3-O-naphthylmethyl-α-D-mannopyranosyl) -(1-2)-(3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (15)
To a solution of 14 (83 mg, 0.05 mmol) and benzyl bromide (8 μL, 0.06 mmol) in DMF (2 mL) was added NaH (6 mg, 0.13 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 18 h. The reaction mixture was quenched with TEA. The mixture was diluted with ethyl acetate and washed with sat. Na2S2O3 aq., 1 M HCl aq., sat. NaHCO3 aq., and brine. The organic layer was dried with MgSO4 and concentrated in vacuo. The residue was purified by silica gel chromatography (hexane/EtOAc = 100:0–75:25) to afford 15 (82 mg, 94%). Rf 0.23 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3): δH 7.77–7.10 (m, 62H, Ar), 5.19–5.12 (m, 4H, H-1, –COO–CH2–Ph), 4.91–4.82 (m, 3H, H-1, –O–CH2–Ph), 4.74–4.32 (m, 20H), 4.14 (s, 1H, H-2), 4.07 (t, 1H, J = 9.6 Hz), 3.95–3.48 (m, 17H), 3.27–3.11 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 1.69–1.63 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 156.7, 156.2, 138.8, 138.6, 138.5, 138.4, 137.9, 136.9, 136.8, 136.3, 133.3, 133.0, 128.7, 128.6, 128.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 127.6, 127.5, 127.3, 126.3, 126.1, 125.9, 125.8, 101.0 (C-1), 99.4 (C-1), 98.96 and 98.88 (C-1), 79.8, 79.7, 79.5, 75.2, 75.1, 74.8, 73.4, 73.3, 72.7, 72.4, 72.3, 72.2, 72.1, 72.0, 69.8, 69.7, 69.3, 69.2, 67.3 (–COO–CH2–Ph), 65.4 and 65.2 (–N–CH2–Ph), 50.8 and 50.5 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.8 (–CH2–CH2–CH2–) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1758.786, Found: 1758.780.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (2,4,6-tri-O-benzyl-α-D-mannopyranosyl) -(1-2)-(3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (15)
To a stirred solution of 15 (43 mg, 0.03 mmol) in 1,2-dichloroethane (1.9 mL) and methanol (0.1 mL) was added DDQ (9 mg, 0.04 mmol). The mixture was stirred at room temperature for 6 h. The reaction mixture was diluted with CHCl3 and washed with 1 M HCl, sat. NaHCO3 aq., and brine. The organic layer was dried through Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography using gradient solvent system (toluene/EtOAc = 100:0–90:10) to give 16 (19 mg, 49%). Rf 0.23 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3): δH 7.31–7.12 (m, 55H, Ar), 5.20 (s, 1H, H-1), 5.16–5.12 (m, 3H, H-1, COO–CH2–Ph), 4.88–4.82 (m, 4H), 4.68–4.40 (m, 15H), 4.31 (d, 1H, J = 12.0 Hz, –O–CH2–Ph), 4.23 (d, 1H, J = 11.6 Hz, –O–CH2–Ph), 4.16 (s, 1H, H-2), 4.02 (ddd, 1H, J = 9.6, 3.7 Hz), 3.95–3.88 (m, 3H), 3.82–3.50 (m, 14H), 3.26–3.14 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.26 (d, 1H, J = 9.4 Hz, –OH), 1.73–1.64 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, CDCl3): δC 156.8, 156.2, 138.8, 138.7, 138.5, 138.4, 138.0, 137.9, 137.0, 136.8, 128.7, 128.6, 128.5, 128.4, 128.1, 128.0, 127.9, 127.8, 127.6, 127.5, 127.3, 100.9 (C-1), 99.03 and 98.96 (C-1), 98.7 (C-1), 80.0, 79.7, 78.2, 76.5, 75.3, 75.2, 74.9, 73.4, 72.9, 72.4, 72.2, 72.1, 71.9, 71.6, 69.6, 69.5, 69.4, 69.1, 67.3 (–COO–CH2–Ph), 65.4 and 65.3 (–N–CH2–Ph), 50.9 and 50.6 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–). MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 1618.723, Found: 1618.687.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (2-O-acetyl-3,4,6-tri-O-benzy-α-D-mannopyranosyl)-(1–3)- (2,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- (3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (17)
A mixture of 16 (0.10 g, 0.07 mmol), AgOTf (24 mg, 0.09 mmol), and molecular sieve 4Å (0.5 g) in dichloromethane (3 mL) was stirred under argon. The mixture was cooled to −20 °C and added a solution of 4 (50 mg, 0.09 mmol) in dichloromethane (2 mL). The reaction mixture was stirred at −20 °C for 2 h. The reaction was quenched by the addition of Et3 N and diluted with EtOAc. The mixture was filtered through a pad of Celite. The filtrate was washed successively with 1 M HCl, sat. NaHCO3 aq., and brine. The organic layer was dried through Na2SO4 and concentrated in vacuo. The residue was purified by gel permeation chromatography (toluene/EtOAc = 1:1) to give 17 (0.12 g, 90%). Rf 0.30 (toluene/EtOAc = 5:1). 1H NMR (600 MHz, (CD3)2SO, 70 °C): δH 7.31–7.13 (m, 70H, Ar), 5.38 (s, 1H, H-2), 5.24 (d, 1H, J = 1.3 Hz, H-1), 5.15 (d, 1H, J = 1.8 Hz, H-1), 5.09–5.07 (m, 3H, H-1, –COO–CH2–Ph), 4.87 (d, 4H, J = 1.4 Hz, H-1), 4.75–4.29 (m, 26H), 4.11–4.08 (m, 2H), 3.98–3.45 (m, 21H), 3.22–3.17 (m, 2H, –N–CH2–Ph, –CH2–CH2–N–), 2.02 (s, 3H, Ac), 1.68–1.63 (m, 2H, –CH2–CH2–CH2–). 13C NMR (150 MHz, (CD3)2SO, 70 °C): δC 169.0 (Ac), 138.3, 138.2, 138.1, 138.0, 137.9, 137.8, 137.7, 136.6, 128.0, 127.9, 127.7, 127.4, 127.3, 127.2, 127.1, 127.0, 126.9, 126.8, 126.7, 99.8 (C-1), 98.5(C-1), 98.1 (C-1), 98.0 (C-1), 78.4, 77.0, 74.7, 74.5, 74.2, 73.9, 73.8, 73.7, 73.6, 72.3, 72.2, 72.1, 71.6, 71.4, 71.3, 71.0, 70.9, 70.6, 70.5, 69.2, 69.0, 68.9, 68.5, 68.3 (–COO–CH2–Ph), 66.0, 64.6 (–N–CH2–Ph), 49.7 (–O–CH2–CH2), 43.7 (–CH2–CH2–N–), 27.5 (–CH2–CH2–CH2–), 20.2 (–OC–CH3), MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 2092.927, Found: 2092.942.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (3,4,6-tri-O-benzy-α-D-mannopyranosyl)-(1–3)- (2,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- (3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (18)
Compound 17 (0.12 mg, 0.06 mmol) was dissolved in THF/MeOH (4.0 mL, 1:1). 1 M NaOMe in MeOH (29 μL, 0.03 mmol) was added into the stirred solution, which was stirred at 40 °C for 21 h. The reaction was quenched by the addition of amberlyst 15E. Amberlyst was removed by glass filter and the organic layer was concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc = 100:0–63:27–54:46) to give compound 18 (0.11 g, 90%). Rf 0.25 (hexane/EtOAc = 65:35). 1H NMR (600 MHz, CDCl3): δH 7.31–7.13 (m, 70H, Ar), 5.21–5.12 (m, 5H, H-1, –COO–CH2–Ph), 4.88–4.79 (m, 4H, H-1), 4.67–4.40 (m, 22H), 4.30–4.24 (m, 2H), 4.18–4.16 (m, 2H), 3.99–3.44 (m, 22H), 3.25–3.11 (m, 3H, –N–CH2–Ph, –CH2–CH2–N–), 2.29 (bs, 1H, –OH), 1.71–1.61 (m, 2H, –CH2–CH2–CH2–). 1H NMR (600 MHz, (CD3)2SO, 25 °C): δH 7.25–7.02 (m, 70H, Ar), 5.11–4.92 (m, 5H, H-1, –COO–CH2–Ph), 4.77 (s, 1H), 4.67–4.12 (m, 24H), 4.07–3.87 (m, 3H), 3.74–3.28 (m, 16H), 3.08–2.94 (m, 2H, –N–CH2–Ph, –CH2–CH2–N–), 1.49 (s, 2H), 1H NMR (600 MHz, (CD3)2SO, 70 °C): δH 7.31–7.08 (m, 70H, Ar), 5.19 (s, 1H, H-1), 5.10 (s, 1H, H-1), 5.06 (s, 1H), 5.03 (s, 1H, H-1), 4.84 (m, 2H, H-1), 4.74–4.62 (m, 5H), 4.55–4.26 (m, 22H), 4.07–4.02 (m, 3H), 3.94 (s, 1H), 3.87–3.41 (m, 22H), 3.21–3.08 (m, 1H), 1.64–1.59 (m, 2H), 13C NMR (150 MHz, CDCl3): δC 156.7, 156.2, 138.8, 138.7, 138.5, 138.4, 138.3, 138.1, 137.9, 137.0, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.9, 127.7, 127.5, 127.4, 127.2, 108.8 (C-1), 98.9 (C-1), 79.9, 78.3, 77.5, 77.3, 75.4, 75.2, 75.0, 74.8, 74.7, 74.1, 73.4, 73.3, 72.4, 72.2, 72.0, 71.8, 69.8, 69.7, 69.3, 69.0, 68.8, 67.2 (–COO–CH2–Ph), 66.4, 65.3 and 65.2 (–N–CH2–Ph), 50.8 and 50.5 (–O–CH2–CH2), 44.7 and 43.8 (–CH2–CH2–N–), 28.4 and 27.9 (–CH2–CH2–CH2–), 13C NMR(150 MHz, (CD3)2SO, 25 °C): δC 138.7, 138.6, 138.5, 138.4, 138.2, 138.0, 128.4, 128.3, 128.2, 128.1, 127.9, 127.7, 127.6, 127.4, 127.3, 127.1, 127.0, 100.2 (C-1), 98.3 (C-1), 98.1 (C-1), 79.4, 78.5, 77.1, 74.7, 74.6, 74.2, 74.0, 73.9, 72.4, 72.2, 71.7, 71.3, 71.1, 70.5, 70.1, 69.0, 68.8, 68.7, 67.0 (–COO–CH2–Ph), 66.3, 64.7 and 64.6 (–N–CH2–Ph), 49.9 and 49.7 (–O–CH2–CH2), 44.1 and 43.6 (–CH2–CH2–N–), 27.9 and 27.4 (–CH2–CH2–CH2–), 13C NMR (150 MHz, (CD3)2SO, 70 °C): δC 138.5, 138.4, 138.3, 138.1, 138.0, 137.9, 137.8, 136.6, 128.0, 127.9, 127.7, 127.6, 127.4, 127.3, 127.2, 127.1, 127.0, 126.9, 126.8, 126.7, 101.7 (C-1), 99.9 (C-1), 98.3 (C-1), 97.9 (C-1), 79.2, 78.5, 78.4, 77.3, 74.6, 74.5, 74.2, 74.0, 73.7, 73.4, 72.2, 72.1, 71.6, 71.4, 71.3, 71.1, 70.9, 70.5, 70.0, 69.3, 69.0, 68.9, 68.8, 67.0 (–COO–CH2–Ph), 66.0 (–N–CH2–Ph), 64.6, 49.7 (–O–CH2–CH2), 43.7 (–CH2–CH2–N–), 27.5 (–CH2–CH2–CH2–). MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 2050.917, Found. 2050.905.
3-N-benzyl-3-(N-benzyloxycarbonyl)aminopropyl (3,4,6-tri-O-benzy-α-D-glucopyranosyl)-(1–3)- (2,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)-(3,4,6-tri-O-benzyl-α-D-mannopyranosyl)-(1-2)- 3,4,6-tri-O-benzyl-α-D-mannopyranoside (20)
To a solution of 18 (76 mg, 0.04 mmol) in DMSO (1 mL) was added acetic anhydrate (0.5 mL) at room temperature and stirred for 17 h. The reaction mixture was diluted with ethyl acetate and washed with brine. The organic layer was dried with Na2SO4 and concentrated in vacuo. The residue was dissolved in THF (1.2 mL) and treated with NaBH(OAc)3 (44 mg, 0.21 mmol) at 0 °C. The reaction mixture was stirred at the same temperature for 5 d. The reaction mixture was diluted with ethyl acetate and washed with sat. NaHCO3 aq. and brine. The organic layer was dried with Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (toluene/EtOAc = 100:0–85:15–80:20) and gave compound 20 (48 mg, 63%). Rf 0.26 (toluene/EtOAc = 17:3). 1H NMR (600 MHz, (CD3)2SO, 70 °C): δH 7.37–7.13 (m, 70H, Ar), 5.24 (d, 1H, J = 1.4 Hz, H-1), 5.18 (d, 1H, J = 1.8 Hz, H-1), 5.12–5.09 (m, 5H), 5.07 (d, 1H, J = 3.7 Hz, H-1), 5.03 (m, 1H), 4.94 (d, 1H, J = 11.5 Hz), 4.89 (d, 1H, J = 1.3 Hz, H-1), 4.76–4.73 (m, 4H), 4.60–4.35 (m, 20H), 4.28 (d, 1H, J = 12.2 Hz), 4.13–4.11 (m, 2H), 3.98–3.94 (m, 2H), 3.89–3.82 (m, 6H), 3.77–3.46 (m, 16H), 3.23–3.20 (m, 1H), 1.67–1.65 (m, 2H), 13C NMR (150 MHz, (CD3)2SO, 70 °C): δC 155.3, 138.9, 138.3, 138.1, 138.0, 137.9, 137.8, 136.6, 128.3, 128.0, 127.9, 127.8, 127.7, 127.6, 127.4, 127.3, 127.2, 127.1, 127.0, 126.9, 126.8, 126.5, 100.3 (C-1), 99.9 (C-1), 98.3 (C-1), 97.9 (C-1), 81.8, 78.8, 78.6, 78.4, 77.2, 77.1, 74.5, 74.2, 73.8, 73.7, 73.4, 72.2, 72.1, 71.0, 70.5, 69.2, 69.0, 68.5 (–COO–CH2–Ph), 66.0 (–N–CH2–Ph), 64.6, 49.7 (–O–CH2–CH2), 43.7 (–CH2–CH2–N–), 27.5 (–CH2–CH2–CH2–), MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 2050.917, Found. 2050.905.
Aminopropyl α-D-glucopyranosyl-(1–3)-α-D-mannopyranosyl-(1-2)- α-D-mannopyranosyl-(1-2)-α-D-mannopyranoside (21)
To a solution of 20 (21 mg, 0.01 mmol) in THF/H2O (5:1, 6 mL) and Pd(OH)2/C (20 wt %, 19 mg) was stirred under H2 at room temperature for 120 h . The mixture was filtered through a pad of Celite. The filtrate was evaporated in vacuo. The residue was purified by C-18 Sep-Pak® (H2O) to give 21 (3 mg, 44%). Rf 0.20 (1-butanol/AcOH/H2O = 2:1:1). 1H NMR (600 MHz, D2O): δH 5.28 (s, 1H, H-1), 5.25 (d, 1H, J = 3.5 Hz, H-1), 5.10 (s, 1H, H-1), 5.03 (s, 1H, H-1), 4.23 (s, 1H), 4.11 (s, 1H), 3.95–3.54 (m, 24H), 3.41–3.38 (m, 1H), 3.16- 3.08 (m, 1H), 1.99–1.98 (m, 1H) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 746.270, Found: 746.277.
3-N-Dansylaminopropyl- α-D-glucopyranosyl-(1–3)-α-D-mannopyranosyl-(1-2)-α-D-mannopyranosyl-(1-2)-α-D-mannopyranoside (1)
To a solution of 21 (3 mg, 5 μmol) in 0.5 M NaHCO3 aq. (0.6 mL) was added Dansyl chloride (2 mg, 7 μmol) in acetone (0.2 mL) at room temperature. The reaction mixture was stirred for 1 h. Dansyl chloride (1 mg, 2 μm) and acetone (0.1 mL) were added into the mixture. After stirring for 30 min, ethyl acetate was added into mixture and the organic layer was removed. The water layer was purified by C-18 Sep-Pak® (H2O/MeOH = 100:0–0:100) and HPLC (column; Imtakt, Unison US-C18 250 × 20 mm. H2O/acetonitrile = 80:20–30:70, 5 mL/min. detected by UV absorption at 254 nm) to give compound 1 (4 mg, 98%). Rf 0.30 (acetonitrile/H2O = 4:1). 1H NMR (600 MHz, D2O): δH 8.54 (d, 1H, J = 12.9 Hz, Ar), 8.32 (d, 1H, J = 12.9, Ar), 8.28 (dd, 1H, J = 11.2, 1.6 Hz, Ar), 7.76–7.71 (m, 2H, Ar), 7.46 (d, 1H, J = 11.4 Hz, Ar), 5.25 (d, 1H, J = 6.0 Hz, H-1), 5.24 (d, 1H, J = 2.4 Hz, H-1), 5.03 (d, 1H, J = 2.7 Hz, H-1), 4.92 (d, 1H, J = 1.9 Hz, H-1), 4.23 (dd, 1H, J = 4.9, 3.0 Hz), 4.09 (dd, 1H, J = 5.0, 2.9 Hz), 3.95–3.53 (m, 24H), 3.42–3.36 (m, 3H), 3.02–2.99 (m, 2H), 1.62–1.57 (m, 2H) MALDI-TOF MS m/z (M+Na): Calcd. for C47H51NO9Na: 979.321, Found. 979.634.
Aminopropyl α-D-mannopyranosyl-(1-2)-α-D-mannopyranoside (22).
To a solution of 12 (14 mg, 0.01 mmol) in THF/H2O (1:1, 4 mL) and Pd(OH)2/C (20 wt %, 13 mg) was stirred under H2 at 40 °C for 43 h. The mixture was filtered through a pad of Celite. The filtrate was evaporated in vacuo. The residue was purified by C-18 Sep-Pak® (H2O) to give 22 (4 mg, 89%). Rf 0.20 (1-butanol/AcOH/H2O = 2:1:1). 1H NMR (400 MHz, D2O): δH 5.10 (s, 1H, H-1), 5.00 (s, 1H, H-1), 4.06 (m, 1H), 3.69–3.55 (m, 13H), 3.11 (m, 2H), 1.95 (m, 2H), MALDI-TOF MS m/z (M+Na): Calcd. for C15H29NO11Na: 422.164, Found: 422.163.
3-N-Dansylaminopropyl α-D-mannopyranosyl-(1-2)-α-D-mannopyranoside (2)
To a solution of 22 (2 mg, 6 μmol) in 0.5 M NaHCO3 aq. (0.4 mL) was added Dansyl chloride (4 mg, 14 μmol) in acetone (0.15 mL) at room temperature. The reaction mixture was stirred for 30 min. Dansyl chloride (2 mg, 9 μm) and acetone (0.2 mL) were added into the mixture. After stirring for 1 h, ethyl acetate was added into mixture and the organic layer was removed. The water layer was purified by C-18 Sep-Pak® (H2O/MeOH = 100:0–25:75) and HPLC (column; Imtakt, Unison US-C18 250 × 20 mm. H2O/acetonitrile = 80:20–30:70, 5 mL / min. detected by UV absorption at 254 nm) to give compound 2 (3 mg, 71%). Rf 0.55 (acetonitrile/MeOH = 4:1). 1H NMR (400 MHz, D2O): δH 8.49 (d, 1H, J = 12.9 Hz, Ar), 8.30 (d, 1H, J = 12.5, Ar), 8.23 (d, 1H, J = 11.3, Ar), 7.69 (dd, 1H, J = 11.2, 1.6 Hz, Ar) 7.41 (d, 1H, J = 12.9, Ar), 4.94 (s, 1H, H-1), 4.90 (s, 1H, H-1), 4.04 (m, 1H,), 3.86–3.48 (m, 11H), 3.40 (m, 1H), 3.31 (m, 1H), 2.96 (m, 2H), 1.57 (m, 2H), MALDI-TOF MS m/z (M+Na): Calcd. for C27H40N2O13SNa: 655.215, Found. 655. 225.
Enzyme assay
A reaction mixture (50 μL) containing 1 μg of endo-α-mannosidase, 1 μmol of 1, and 15 mM phosphate buffer (pH 7.4) was incubated at 37 °C. The reaction was stopped by adding of acetonitrile, and analyzed by HPLC (using a Mightysil NH2 column (250 mm × 3.0 mm, Kanto Kagaku Co., Ltd., Japan) with solvent A (97% acetonitrile, 0.3% acetic acid-TEA buffer pH 7.0) and solvent B (30% acetonitrile, 0.3% acetic acid-TEA buffer pH 7.0), and mixed solvent (70:30–40:60, linear gradient for 12 min) at 40 °C, 0.8 mL min−1. Quantification was achieved with fluorescent intensity (excitation 340 nm, emission 515 nm).
Supplementary material
The supplementary material for this paper is available online at http://dx.doi.10.1080/09168451.2014.910101.
Supplemental Fig. 1 caption
Download MS Word (34.5 KB)Supplemental Fig.1
Download MS Power Point (491.5 KB)Acknowledgments
This work was supported in part by the Sasakawa Scientific Research Grant from The Japan Science Society (S.I). We thank Ms. Kyoko Kobayashi for technical assistance.
Notes
Abbreviations: Ac2O, acetic anhydride; BnBr, benzyl bromide; DMF, N,N-dimethylformamide; Dansyl-Cl, 5-(dimethylamino)naphthalene-1-sulfonyl chloride; DMSO, dimethyl sulfoxide; EtOAc, ethyl acetate; NaH, sodium hydride; NaOMe, sodium methoxide; TEA, triethylamine; THF, tetrahydrofuran.
References
- Tomiya N, Narang S, Lee YC, Betenbaugh MJ. Glycoconj. J. 2004;21:343–360.10.1023/B:GLYC.0000046275.28315.87
- Ito Y, Takeda Y. Proc. Jpn. Acad. Ser. B. 2012;88:31–40.10.2183/pjab.88.31
- Zuber C, Spiro JM, Guhi B, Spiro GR, Roth J. Mol. Biol. Cell. 2000;11:4227–4240.10.1091/mbc.11.12.4227
- Lubas WA, Spiro RG. J. Biol. Chem. 1987;262:3775–3781.
- Roth J, Yam HFG, Fan J, Hirano K, Kysala GK, Fourn LV, Guhl B, Santimaria R, Torossi T, Ziak M, Zuber C. Histochem. Cell Biol. 2008;129:163–177.10.1007/s00418-007-0366-7
- Thompson JA, Williams JR, Hakki Z, Alonzi SD, Wennekes T, Gloster TM, Songsrirote K, Thomas-Oates JE, Wrodnigg TM, Spreitz J, Stütz AE, Butters TD, Williams SP, Davies GJ. Proc. Nat. Acad. Sci. USA. 2012;109:781–786.10.1073/pnas.1111482109
- Aikawa J, Matsuo I, Ito Y. Glycoconj. J. 2012;29:35–45.10.1007/s10719-011-9362-1
- Iwamoto S, Isoyama M, Hirano M, Yamaya K, Ito Y, Matsuo I, Totani K. Glycobiology. 2012;23:121–131.
- Matsuda K, Kurakata Y, Miyazaki T, Matsuo I, Ito Y, Nishikawa A, Tonozuka T. Biosci. Biotechnol. Biochem. 2011;75:797–799.10.1271/bbb.100874
- Kukushkin NV, Easthope IS, Alonzi DS, Butters TD. Glycobiology. 2012;22:1282–1288.10.1093/glycob/cws088
- Ogawa T, Katano K, Matsui M. Carbohydr. Res. 1978;64:C3–C9.10.1016/S0008-6215(00)83724-6
- Lemieux RU, James K, Nagabhushan TL. Can. J. Chem. 1973;51:27–32.10.1139/v73-004
- Iino K, Iwamoto S, Kasahara Y, Matsuda K, Tonozuka T, Nishikawa A, Ito Y, Matsuo I. Tetrahedron Lett. 2012;53:4452–4456.10.1016/j.tetlet.2012.06.061
