Abstract
We show here that the transformation efficiency of Saccharomyces cerevisiae is improved by altering carbon sources in media for pre-culturing cells prior to the transformation reactions. The transformation efficiency was increased up to sixfold by combination with existing transformation protocols. This method is widely applicable for yeast research since efficient transformation can be performed easily without changing any of the other procedures in the transformation.
Introducing genes into Saccharomyces cerevisiae by transformation methods is indispensable for genetic and cell biology research. A number of studies have been performed to improve efficiency of S. cerevisiae transformation. In particular, methods in which yeast cells are treated with lithium acetate and polyethylene-glycol (PEG) are commonly used.Citation1) Several modifications of this lithium method aimed at improving efficiency have been reported. The addition of single-stranded carrier DNA is an example.Citation2) However, the effects of altering cell culture conditions before the transformation reactions have not been evaluated carefully thus far. Here, we examine the effects of the concentration of glucose in the culture of S. cerevisiae prior to the transformation reaction on the transformation efficiency.
BY4733 (MATa his3Δ200 trp1Δ63 leu2Δ0 met15Δ0 ura3Δ0) and W303–1A (MATa ade2–1 trp1–1 his3–11,15 ura3–1 leu2–3,112 can1–100) cells grown on YPD (1% yeast extract, 2% bactopeptone, and 2% glucose) solid medium were inoculated into synthetic complete (SC: 0.67% yeast nitrogen base (Difco) supplemented with appropriate amino acids) liquid media containing 2% lactic acid, 3% glycerol, and low- (0–0.05%), mid- (0.5–1%), or high- (2%) glucose (pH 5.5) for preliminary culture. To maintain the growth of cells even in the low glucose concentration conditions, lactic acid and glycerol were added as additional carbon sources. After the cells were grown to the log phase at 30 °C, the preliminary culture was diluted with the same medium for the pre-culture and grown overnight to the log phase (1−2 × 107 cells/ml) at 30 °C. The pre-cultured cells were transformed with the 2-micron plasmid pRS423 or the centromere-containing plasmid pRS316. The transformation of S. cerevisiae cells was performed according to a general protocol,Citation3) with slight modifications. In brief, approximately 1 × 107 cells were collected by centrifuging from the pre-culture. The cells were washed twice with sterile water and suspended in 50 μL of the transformation mix containing 34% (w/v) PEG, 100 mm lithium acetate, and 0.125, 0.25, 0.5, or 1 μg of plasmid DNA. The suspension containing W303-1A or BY4733 cells was incubated for 15, 60, or 90 min at 42 °C. Although the cells were incubated for 15 min at 42 °C in previous protocols,Citation2,4) it has been reported that longer incubation at 42 °C increases the yield of transformants.Citation3) Indeed, when we transformed W303-1A cells pre-cultured in high-glucose conditions with 1 μg of pRS316, the transformation efficiencies were 0.01, 1.10, and 0.23% after 15, 60, and 90 min of incubation at 42 °C, respectively. Therefore, we analyzed the effect of glucose concentration on transformation efficiency after 60 or 90 min of incubation at 42 °C. The incubated cells were spread onto SC solid medium lacking histidine or uracil. A portion of the suspension was diluted in sterile water and spread onto YPD solid medium to count viable cells. In this study, the transformation efficiency is presented as the percentage of the transformants relative to the viable cells to eliminate influence of cell viability (e.g. sensitivity to heat shock) on the transformation efficiency.
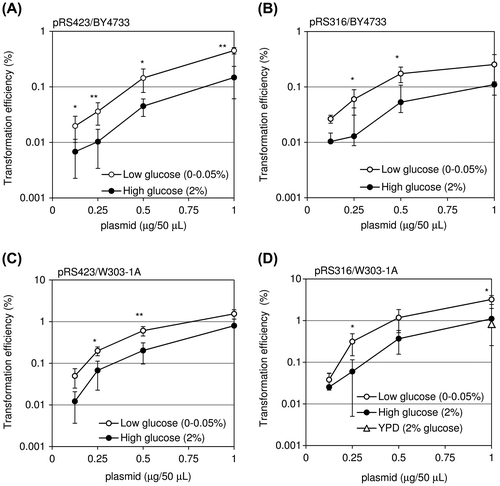
Fig. shows the transformation efficiency under low- and high-glucose culture conditions. BY4733 and W303-1A cells pre-cultured under low- or high-glucose conditions were transformed with different amounts of pRS423 or pRS316 as described above. In all the experiments, it was demonstrated that cells pre-cultured in low-glucose conditions had higher transformation efficiency than those in high-glucose conditions, and that the difference was on average about threefold (Fig. (A–D)). When W303-1A cells pre-cultured in YPD medium, containing 2% glucose, was transformed with 1 μg of pRS316, the efficiency was nearly the same as the case using the medium containing 2% glucose, 2% lactic acid, and 3% glycerol (Fig. (D), open triangle). When the incubation time at 42 °C in the transformation reaction was shortened into 15 min, the significant increase of the transformation efficiency was not observed (data not shown). The maximum difference in the efficiency between the low- and high-glucose conditions was obtained in the transformation of W303-1A cells pre-cultured in low-glucose conditions with 0.25 μg of pRS316, and in this case, the difference was 5.2-fold (Fig. (D)). These results indicate that the transformation efficiency is affected by conditions (e.g. carbon sources) of pre-culturing cells and that higher transformation efficiency is obtained using the medium containing low glucose.
Fig. 1. Transformation efficiency after pre-culturing cells in low- and high-glucose conditions with varying amount of plasmids.
Notes: BY4733 (A and B) and W303-1A (C and D) cells pre-cultured in low- or high- glucose conditions were transformed with different amounts of pRS423 (A and C) or pRS316 (B and D). BY4733 or W303-1A cells were incubated in the presence of 50 μL of the transformation mix (see the text) for 90 min or 60 min, respectively, at 42 °C. The transformation efficiency is represented as percentage of transformants relative to number of cells in the transformation reaction. An open triangle (D) represents the transformation efficiency of cells pre-cultured in YPD medium, containing 2% glucose (standard deviation; 0.8%). Data shown are depicted on a log scale and as the mean ± standard deviation of at least three independent experiments. Single and double asterisks indicate p < 0.05 and p < 0.01, respectively (Student’s t test).
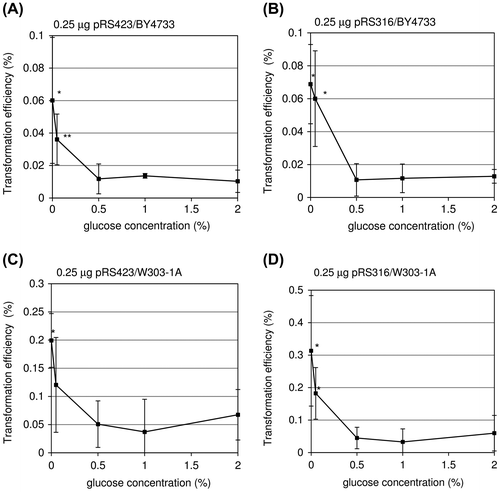
To examine these phenomena in more detail, BY4733 or W303-1A cells pre-cultured in different glucose concentrations were transformed with 0.25 μg of pRS423 or pRS316. In all the experiments, 0% glucose conditions gave the highest transformation efficiency (Fig. (A–D)). In the 0% glucose conditions, the efficiency was 5.8-fold higher than in 2% glucose conditions in the transformation of BY4733 cells with pRS316 (Fig. (B)). Similarly, but less effectively, 0.05% glucose conditions gave an increase in the efficiency. In contrast, the increase in transformation efficiency was not observed on cells pre-cultured in mid- and high-glucose media. These results indicate that the transformation efficiency is increased in a glucose-dependent manner when the concentration of glucose is less than or equal to 0.05%.
Fig. 2. Effect of the glucose concentration of the media on yeast transformation efficiency.
Notes: BY4733 (A and B) or W303-1A (C and D) cells pre-cultured in low-, mid-, or high-glucose media were transformed with 0.25 μg of pRS423 (A and C) and pRS316 (B and D). BY4733 and W303-1A cells were incubated in the presence of 50 μL of the transformation mix for 90 min or 60 min, respectively, at 42 °C. The transformation efficiency is represented as the percentage of transformants relative to number of cells in the transformation reaction. Data shown represent the mean ± standard deviation of at least three independent experiments. Single and double asterisks indicate p < 0.05 and p < 0.01 vs. 2% glucose conditions, respectively (Student’s t test).
Although the molecular mechanisms by which low-glucose conditions produce higher transformation efficiency has not been identified, it has been reported that the cell wall plays an important role in the transformation of S. cerevisiae. It is known that the capacity of the cell wall to absorb DNA is one of the determinants of the transformation efficiency.Citation5) Spf1 is an endoplasmic reticulum-located P-type ATPase,Citation6) Pmr1 is a Golgi-located Mn2+/Ca2+ P-type ATPase,Citation7) and Pde2 is a high affinity cyclic adenosine monophosphate (cAMP) phosphodiesterase.Citation8) The deletion of SPF1 and PMR1 alters the ER-Golgi functions resulting in an altered cell wall structure,Citation7,9) and the disruption of PDE1 also modulates cell wall structure.Citation8) It is reported that that spf1, pmr1, and pde2 cells adsorb more DNA than wild-type cellsCitation5) and demonstrate high-transformation efficiency.Citation10) These findings suggest that the alteration of the cell wall structure can result in a high-transformation phenotype.
It has also been suggested that the structure of the yeast cell wall is affected by culture conditions. Slowly growing cells in limited nutrient conditions show resistance to digestion of their cell walls by Zymolyase, which is a complex mixture of proteases and β-1,3-glucanases, due to increased cross-linking, increased mass of the cell wall, or both.Citation11,12) In this study, W303-1A cells cultured in the medium without glucose grew after a longer lag phase (approximately 1.8-fold as compared to the case in the medium containing 2% glucose), and showed a slight decrease (approximately 26%) in growth rate during log phase. It has been also reported that cells in media containing solely non-fermentable carbon sources grow after a long lag phase.Citation13) This alteration of the cell wall structure according to the slow growth is expected to result in resistance to heat shock. Indeed, W303-1A cells pre-cultured in the low-glucose conditions were more resistant to heat shock than those cultured in the high-glucose conditions (data not shown). Therefore, it is most likely that low-glucose culture conditions change the yeast cell wall such that it absorbs more DNA and this causes high transformation efficiency.
We showed here that high transformation efficiency can be induced by pre-culturing cells in low-glucose conditions. This method is useful for a wide range of yeast research because it is easy to modify various existing protocols for high transformation efficiency. In particular, this method may be suitable for generating the large numbers of transformants required for screening of complex plasmid libraries. In yeast two-hybrid analysis, for instance, a high-efficiency transformation protocol is essential for effectively covering the library complexity. Since this low-glucose pre-culture method gives high-efficiency transformation a simple modification of the culture method would result in a more effective screen. In addition, since W303-1A cells pre-cultured in the low-glucose conditions were resistant to heat shock as mentioned above, this modification would be useful for temperature-sensitive mutant strains. While the transformation of S.cerevisiae cells was performed following a general protocol in this study, the low-glucose method is also effective for transformation when using a kit. When the S. cerevisiae Direct Transformation Kit (Wako) was used, a tendency for an increase in the transformation efficiency was observed when using the low-glucose pre-culture method for the transformation of W303-1A cells with 1 μg of pRS316 (data not shown).
This study raises the possibility that highly efficient methods for the transformation of S. cerevisiae could be developed not only by improving transformation reactions but also by manipulating culture conditions before the reactions. It is expected that previous knowledge about the relationship between culture conditions and cellular phenotypes maybe applied to further increase transformation efficiency.
Acknowledgments
We thank Dr Chihiro Horigome for the culture method; Dr Takahiro Shintani for pRS423 plasmid.
References
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168.
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 1989;16:339–346.10.1007/BF00340712
- Gietz RD. Woods RA. Yeast Transformation by the LiAc/SS Carrier DNA/PEG Method. In: Wei X, editor. Yeast protocols. 2nd ed. vol. 313. New Jersey: Humana Press; 2006. p. 107–120.
- Gietz RD, St. Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425.10.1093/nar/20.6.1425
- Pham TA, Kawai S, Kono E, Murata K. The role of cell wall revealed by the visualization of Saccharomyces cerevisiae transformation. Curr. Microbiol. 2011;62:956–961.10.1007/s00284-010-9807-y
- Cronin SR, Rao R, Hampton RY. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 2002;157:1017–1028.10.1083/jcb.200203052
- Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell. 1998;9:1149–1162.10.1091/mbc.9.5.1149
- Tomlin GC, Hamilton GE, Gardner DC, Walmsley RM, Stateva Li, Oliver SG. Suppression of sorbitol dependence in a strain bearing a mutation in the SRB1/PSA1/VIG9 gene encoding GDP-mannose pyrophosphorylase by PDE2 overexpression suggests a role for the Ras/cAMP signal-transduction pathway in the control of yeast cell-wall biogenesis. Microbiology. 2000;146:2133–2146.
- Suzuki C, Shimma YI. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 1999;32:813–823.10.1046/j.1365-2958.1999.01400.x
- Kawai S, Pham TA, Nguyen HT, Nankai H, Utsumi T, Fukuda Y, Murata K. Molecular insights on DNA delivery into Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2004;317:100–107.10.1016/j.bbrc.2004.03.011
- Elliott B, Futcher B. Stress resistance of yeast cells is largely independent of cell cycle phase. Yeast. 1993;9:33–42.10.1002/(ISSN)1097-0061
- Klosinska MM, Crutchfield CA, Bradley PH, Rabinowitz JD, Broach JR. Yeast cells can access distinct quiescent states. Genes. Dev. 2011;25:336–349.10.1101/gad.2011311
- Swinnen S, Klein M, Carrillo M, McInnes J, Nguyen HT, Nevoigt E. Re-evaluation of glycerol utilization in Saccharomyces cerevisiae: characterization of an isolate that grows on glycerol without supporting supplements. Biotechnol. Biofuels. 2013;6:157.10.1186/1754-6834-6-157
