Abstract
Human macrophage dectin-1, a type II transmembrane β-glucan receptor, was expressed as a fusion protein with an N-terminal hexahistidine tag in a baculovirus–silkworm expression system and assayed for binding activity. Recombinant dectin-1 specifically bound to some β-glucans, and the neck domain and N-linked oligosaccharide chains of human dectin-1 did not affect the ligand binding activity and specificity of the receptor.
β-Glucans are well known immunocyte activators, although the molecular mechanisms of their activities are not fully understood in humans.Citation1) It has been found that curdlan, a linear β-1,3-glucan, exhibits significant activity to stimulate nuclear factor-κB (NF-κB) in mouse macrophages,Citation2) resulting in the production of reactive oxygen species and cytokines, such as tumor necrosis factor-α. This suggests that macrophages respond to linear β-1,3-glucans, possibly released from fungal cell walls, via lectin-like receptors. Dectin-1, a C-type lectin-like receptor, is widely expressed in mouse tissues, particularly on monocytes/macrophages. The human homolog of dectin-1 is thought to be functionally similar to the mouse molecule, but human dectin-1 differs from the mouse molecule in that the neck domain linking the extracellular C-type lectin-like domain to the transmembrane domain is glycosylated.Citation3,4) Human dectin-1 mRNA is alternatively spliced and generates two major isoforms (isoforms A and B). Isoform A of human dectin-1 (hDectin-1A) possesses a cytoplasmic domain, transmembrane domain, neck domain, and C-type lectin-like domain, while isoform B of human dectin-1 (hDectin-1B) lacks a neck domain. It has been found that N-linked glycosylation of dectin-1 affects the cell surface expression of the receptor.Citation4) Thus N-linked oligosaccharides of dectin-1 play an important role in vivo because the expression levels on the cell surface influence the ligand binding. However, it remains unknown whether the neck domain and N-linked oligosaccharide chains of human dectin-1 directly affect the ligand binding activity and specificity. Here we report the β-glucan binding activity of two major splice variants of human dectin-1. This study is the first to report functional expression of recombinant human dectin-1 using baculovirus–silkworm expression system.
To produce hexahistidine (His6)-tagged human dectin-1 fusion proteins in a baculovirus–silkworm expression system, transfer vectors that encode human dectin-1 extracellular domain were constructed. The cDNA encoding human dectin-1A extracellular domain (the neck domain and C-type lectin-like domain, hDectin-1A-ECD) or human dectin-1B extracellular domain (the C-type lectin-like domain alone, hDectin-1B-ECD) was prepared by polymerase chain reaction (PCR) using human leukocyte cDNA (BD Biosciences, San Jose, USA) as a template based on the published nucleotide sequence.Citation5,6) For expression of recombinant hDectin-1A-ECD, the sense and antisense primers were 5′-GGAGATCTGCTATTTGGAGATCCAATTCAGGAAGC and 5′-GGCTCGAGTTACATTGAAAACTTCTTCTCACAAATAC (The BglII and XhoI sites are underlined). The PCR product encoding amino acid residues 68–247 of human dectin-1A was digested with BglII and XhoI, and then cloned in the BglII and XhoI sites of pMSNHT06 (transfer vector for expression of secretory proteins, Sysmex Corporation, Kobe, Japan), yielding pMSNHT06/hDectin-1A-ECD. For expression of recombinant hDectin-1B-ECD, the sense and antisense primers were 5′-GGAGATCTGGGGTTCTTTCCAGCCCTTGTCCTCC and 5′-GGCTCGAGTTACATTGAAAACTTCTTCTCACAAATAC (The BglII and XhoI sites are underlined). The PCR product encoding amino acid residues 114–247 of human dectin-1A was digested with BglII and XhoI, and then cloned in the BglII and XhoI sites of pMSNHT06, yielding pMSNHT06/hDectin-1B-ECD. Recombinant pMSNHT06/hDectin-1A-ECD or pMSNHT06/hDectin-1B-ECD was introduced into competent Escherichia coli DH5α cells. The nucleotide sequence of the construct was analyzed by dideoxy nucleotide sequencing to ensure absence of errors. The recombinant human dectin-1 with an N-terminal His6 tag (His-hDectin-1A-ECD or His-hDectin-1B-ECD) was expressed in a baculovirus–silkworm expression system (Sysmex Corporation) and purified by HPLC with a metal chelating column as described in Ref.Citation7). The purified recombinant proteins were analyzed by 12% SDS-PAGE and immunoblotting.Citation7) Proteins in the gel were transferred to a nitrocellulose membrane, and in some cases, the membrane transferred with proteins was treated with 25 mM H2SO4 at 80 °C for 1 h to remove sialic acids from glycoproteins.Citation7) The membrane was then stained with horseradish peroxidase (HRP)-conjugated anti-His6 antibody (Roche) to detect His6-tagged proteins or lectins to characterize partially oligosaccharide chains on glycoproteins.Citation7) Positive bands were visualized using the enhanced chemiluminescence detection system (GE Healthcare).
To determine the β-glucan binding activity of His-hDectin-1A-ECD or His-hDectin-1B-ECD, paramylon (β-1,3-glucan from Euglena gracilis, Sigma) was washed and suspended with Tris-buffered saline (TBS, 150 mM NaCl, 10 mM Tris-HCl, pH 7.5) containing 0.05% (v/v) Triton X-100 and 10 mM CaCl2. The purified recombinant protein solution (50 μL) was added to the paramylon suspension (50 μL), and the mixture was incubated for 30 min at room temperature with continuous end-over-end mixing.Citation8) Following incubation, the insoluble paramylon was pelleted by centrifugation, washed thrice with the same buffer, and eluted with Laemmli buffer with 130 mM dithiothreitol (DTT). The bound and unbound proteins were analyzed by SDS-PAGE and immunoblotting as described above. The binding specificity of human dectin-1 was determined by incubating the purified recombinant proteins with the following insoluble polysaccharides and gels: chitin beads (New England Biolabs), dextran (GE Healthcare), lichenan, cellulose, mannan-agarose, heparin-agarose, deaminated heparin-agarose, xylan, maltoheptaose-agarose, β-glucose-agarose, N-acetylglucosamine (GlcNAc)-agarose, mannose-agarose, N-acetylgalactosamine (GalNAc)-agarose (all from Sigma), maltose-agarose, chitooligo-agarose, lactose-agarose, melibiose-agarose, and fucose-agarose (all from Seikagaku, Tokyo, Japan).Citation8) The binding assay was done as described above. In some cases, competing carbohydrates (0.5 or 1 mg/mL polysaccharides and 50 or 100 mM monosaccharide) were added to the reactions.
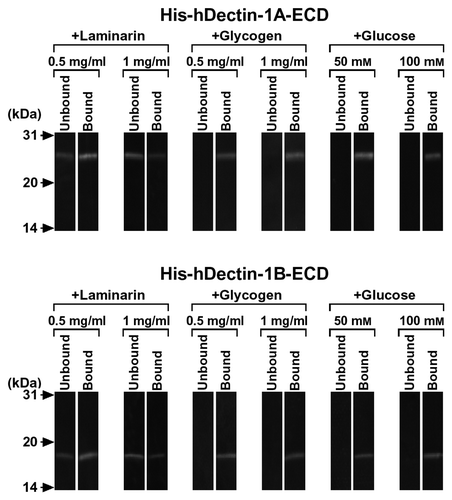
His-hDectin-1A-ECD or His-hDectin-1B-ECD (Fig. (A)) was purified and used to test for binding activity. SDS-PAGE and immunoblot analysis showed that His-hDectin-1A-ECD and His-hDectin-1B-ECD had the expected molecular sizes of 23 and 18 kDa, respectively, and was detectable with anti-His6 antibody (Fig. (B)). Furthermore, structures of oligosaccharide chains of these purified recombinant proteins were estimated by lectin blot analysis. Concanavalin A (Con A) reacted with His-hDectin-1A-ECD, but not with His-hDectin-1B-ECD (Fig. (C), lanes 1 and 8). Phytohemagglutinin (PHA)-E, peanut agglutinin (PNA), Ricinus communis agglutinin (RCA)-I, soybean agglutinin (SBA), Ulex europaeus agglutinin (UEA)-I, and wheat germ agglutinin (WGA) did not react with these recombinant proteins (Fig. (C), lanes 2–7 and 9–14). Treatment with H2SO4 did not affect the reactivity of PNA, RCA-I, and WGA (data not shown), indicating that these recombinant proteins barely contain sialic acids. These results suggest that the neck domain of His-hDectin-1A-ECD is glycosylated and contains Con A-positive oligosaccharides (high mannose-type, hybrid-type, or biantennary complex-type N-linked oligosaccharides).
Fig. 1. Expression, Purification, and Lectin Blot Analysis of His-hDectin-1 Fusion Proteins.
Note: (A) Schematic representation of structural domains of human dectin-1 and His-hDectin-1 fusion proteins. The domain organization is indicated by boxes, as follows: cytoplasmic domain (Cyto), transmembrane domain (TM), neck domain (Neck), and C-type lectin-like domain (CTLD).Citation3,6) SS and His represent signal sequence and His6, respectively. (B) The purified His-hDectin-1A-ECD and His-hDectin-1B-ECD were electrophoresed on a 12% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane. The membrane was stained with anti-His6 antibody. Molecular mass markers are shown to the left. (C) SDS-PAGE and blotting were done as described in B. The membrane was stained with lectins, as indicated. Lanes: 1 and 8, Con A; 2 and 9, PHA-E; 3 and 10, PNA; 4 and 11, RCA-I; 5 and 12, SBA; 6 and 13, UEA-I; 7 and 14, WGA.
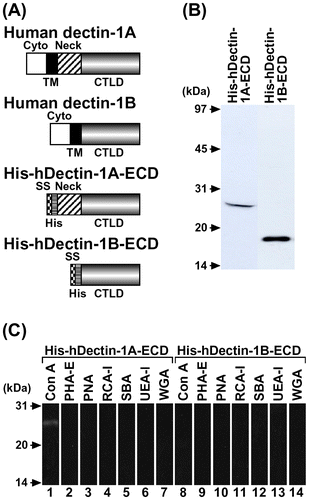
Fig. 2. Carbohydrate Binding Specificity of His-hDectin-1 Fusion Proteins.
Note: The purified His6-tagged human dectin-1 fusion proteins (His-hDectin-1A-ECD and His-hDectin-1B-ECD) were incubated with the indicated insoluble gels. Following incubation, the insoluble gels were pelleted by centrifugation, washed three times, and eluted with Laemmli buffer with DTT. The bound and unbound proteins were analyzed by SDS-PAGE and immunoblotting as described in the legend to Fig. (B).
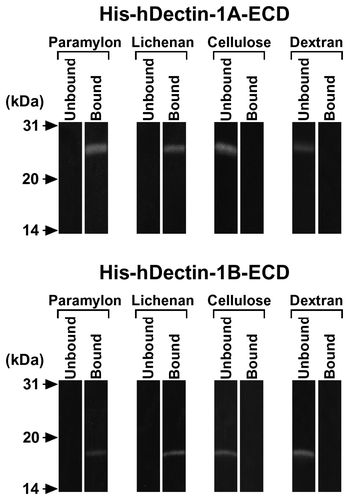
Fig. 3. β-Glucan Binding Specificity of His-hDectin-1 Fusion Proteins.
Note: To determine the binding specificity of human dectin-1, the ability of various carbohydrates to block the β-glucan binding of His-hDectin-1A-ECD or His-hDectin-1B-ECD was examined. Binding assay was done as described in the legend to Fig. 2, except for the addition of competing carbohydrates. Competing polysaccharides (laminarin and glycogen) were present at a concentration of 0.5 or 1 mg/mL in the reaction mixtures, as indicated, to inhibit the binding of His-hDectin-1A-ECD or His-hDectin-1B-ECD to lichenan. Glucose was present at a concentration of 50 or 100 mM in the reactions, as indicated, to inhibit lichenan binding of these recombinant proteins.
We first examined the ability of soluble His-hDectin-1A-ECD or His-hDectin-1B-ECD to recognize paramylon (β-1,3-glucan) and lichenan (β-1,3- and β-1,4-glucan), insoluble β-glucans, as a model of fungal β-glucans. His-hDectin-1A-ECD and His-hDectin-1B-ECD bound to paramylon and lichenan, but did not bind cellulose (β-1,4-glucan) or dextran (α-1,6-glucan with α-1,3-branches) (Fig. ). These recombinant proteins did not bind to the other polysaccharides, polysaccharide-agaroses, oligosaccharide-agaroses, or monosaccharide-agaroses tested in this study (data not shown). In addition, the binding of His-hDectin-1A-ECD or His-hDectin-1B-ECD to lichenan was dose-dependently inhibited by the addition of laminarin, a soluble β-1,3-glucan with β-1,6-branches, but not by glucose and glycogen, a soluble α-1,4-glucan with α-1,6-branches (Fig. ). These results indicate that His-hDectin-1A-ECD and His-hDectin-1B-ECD specifically recognize β-1,3-glucan and β-1,3/1,4-glucan among the carbohydrates tested. These results also show that there are no differences in the β-glucan binding activity and specificity between His-hDectin-1A-ECD and His-hDectin-1B-ECD in this assay. Furthermore, these data suggest that the neck domain and N-linked oligosaccharide chains are not essential to the β-glucan binding of this receptor. It is interesting to note that recombinant hDectin-1A expressed in an E. coli cell-free translation system also bound specifically to paramylon and lichenan.Citation8) In this binding assay, the buffer containing Ca2+ was used as described in Ref.Citation8) to compare the binding activities of hDectin-1A expressed in a baculovirus–silkworm expression system and hDectin-1A expressed in a cell-free translation system. Baculovirus–insect expression systems have been widely utilized in the synthesis of recombinant secretory glycoproteins including pharmaceutical proteins because of their advantages, such as high yield and glycosylation.Citation7) His-hDectin-1A-ECD and His-hDectin-1B-ECD can be used as a useful tool in elucidating the physiological functions of human dectin-1. Our study might lead to a better understanding of antitumor effects exerted by some fungal β-glucans.
Funding
This work was supported in part by a Grant-in-Aid [grant number 25450186, to M.U.] from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Health Research Center for Medicinal and Food Sciences of Meijo University.
Notes
Abbreviations: Con A, concanavalin A; ECD, extracellular domain; His6, hexahistidine; HRP, horseradish peroxidase; PCR, polymerase chain reaction; PHA, phytohemagglutinin; PNA, peanut agglutinin; RCA, Ricinus communis agglutinin; SBA, soybean agglutinin; TBS, Tris-buffered saline; UEA, Ulex europaeus agglutinin; WGA, wheat germ agglutinin.
References
- Herre J, Gordon S, Brown GD. Mol. Immunol. 2004;40:869–876.10.1016/j.molimm.2003.10.007
- Kataoka K, Muta T, Yamazaki S, Takeshige K. J. Biol. Chem. 2002;277:36825–36831.10.1074/jbc.M206756200
- Willment JA, Gordon S, Brown GD. J. Biol. Chem. 2001;276:43818–43823.10.1074/jbc.M107715200
- Kato Y, Adachi Y, Ohno N. Biol. Pharm. Bull. 2006;29:1580–1586.10.1248/bpb.29.1580
- Hermanz-Falcón P, Arce I, Roda-Navarro P, Fernández-Ruiz E. Immunogenetics. 2001;53:288–295.10.1007/s002510100326
- Yokota K, Takashima A, Bergstresser PR, Ariizumi K. Gene. 2001;272:51–60.10.1016/S0378-1119(01)00528-5
- Ujita M, Yamanaka M, Maeno Y, Yoshida K, Ohshio W, Ueno Y, Banno Y, Fujii H, Okumura H. J. Biosci. Bioeng. 2011;111:22–25.10.1016/j.jbiosc.2010.08.020
- Ujita M, Nagayama H, Kanie S, Koike S, Ikeyama Y, Ozaki T, Okumura H. Biosci. Biotechnol. Biochem. 2009;73:237–240.10.1271/bbb.80503
