Abstract
Hyphal fusion is involved in the formation of an interconnected colony in filamentous fungi, and it is the first process in sexual/parasexual reproduction. However, it was difficult to evaluate hyphal fusion efficiency due to the low frequency in Aspergillus oryzae in spite of its industrial significance. Here, we established a method to quantitatively evaluate the hyphal fusion ability of A. oryzae with mixed culture of two different auxotrophic strains, where the ratio of heterokaryotic conidia growing without the auxotrophic requirements reflects the hyphal fusion efficiency. By employing this method, it was demonstrated that AoSO and AoFus3 are required for hyphal fusion, and that hyphal fusion efficiency of A. oryzae was increased by depleting nitrogen source, including large amounts of carbon source, and adjusting pH to 7.0.
Graphical Abstract
We established a new method to quantitatively evaluate hyphal fusion efficiency in the industrial filamentous fungus Aspergillus oryzae, and demonstrated that the fusion efficiency was varied by media composition.
Key words:
Aspergillus oryzae is an important micro-organism in Japanese food fermentation industry.Citation1) A. oryzae is also used for heterologous protein productionCitation2) owing to its prominent ability of protein secretion and guaranteed safety. For breeding of A. oryzae strains with more favored industrial properties, mutation breeding has been mainly performed.Citation3) In contrast, crossbreeding strategy, in which favored properties from different strains can be combined into a single strain, has not been extensively employed in A. oryzae except for protoplast fusion method.Citation4) Although sexual cycle has not yet been discovered in A. oryzae, the finding of two mating types suggests its potential sexuality.Citation5) Hyphal fusion is the first step for sexual and parasexual reproduction, and thus elucidation of its mechanism is important for crossbreeding. In traditional sake-making, conidia from apparently different strain sources are mixed and inoculated onto steamed rice.Citation6) It can be hypothesized that hyphal fusion may occur between the strains during the koji-making, of which industrial significance has not been focused yet. However, there has been poor knowledge about hyphal fusion in A. oryzae except for an old report by Ishitani and Sakaguchi.Citation7) Very recently, we reported the evidence that hyphal fusion occurs in A. oryzae in paired culture, in which two auxotrophic strains are separately inoculated on the agar medium and heterokaryotic hyphae appear by hyphal fusion on the border of the two colonies.Citation8) However, this method cannot be used to quantitatively evaluate hyphal fusion efficiency.
It is generally regarded in filamentous fungi that hyphal fusion is an important process for the formation of an interconnected colony, which provides cytoplasmic transport and nuclear mixing.Citation9) Physiological effects of hyphal fusion and detailed morphological features of heterokaryotic hyphae have been clarified.Citation10,11) Moreover, there are several reports that media composition affects the hyphal fusion efficiency.Citation12−16) Taking advantages of very high fusion frequency in Neurospora crassa, its molecular mechanism has been extensively characterized.Citation17) The most studied proteins are MAK-2Citation18) and SOCitation19); MAK-2 is a homolog of Sacccharomyces cerevisiae Fus3p MAP kinase, and SO is a Pezizomycotina-specific protein with WW domain. Hyphal fusion between germling conidia is associated with specialized structures, conidial anastomosis tubes (CATs),Citation9) in which MAK-2 and SO alternately appear at CAT tip.Citation20) MAK-2 and SO are also essential for sexual reproduction.Citation19−21) In Aspergillus nidulans, AnFus3/MpkB homologous to N. crassa MAK-2 is required for hyphal fusion and sexual development.Citation22−24) AoSO protein homologous to N. crassa SO accumulates at the septal pore in response to various stresses,Citation25) and it colocalizes with stress granules.Citation26) However, functional involvement of AoSO in hyphal fusion has not been investigated since an effective assay of hyphal fusion efficiency has been lacking in A. oryzae.
In this study, we established a method to quantitatively analyze the hyphal fusion efficiency of A. oryzae in mixed culture, where conidia from two auxotrophic strains were mixed and inoculated on the agar medium. By employing this method, the roles of AoSO and AoFus3 in hyphal fusion were examined. The effects of media composition on the hyphal fusion efficiency were investigated, and then the fusion efficiency was improved by optimizing the nitrogen/carbon sources and pH.
Materials and methods
Strains, media, and transformation
The strains used in this study are listed in Table . A. oryzae wild-type strain RIB40Citation27) was used as a DNA donor. The uridine/uracil auxotrophic strain PlD-HR1-c (ΔpyrG) and adenine auxotrophic strain AblD-HG1-c (ΔadeB)Citation8) were used in the assay of hyphal fusion. Nuclei of the strains PlD-HR1-c and AblD-HG1-c were fluoresced in red and green, respectively.Citation8) CD medium (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, and 2% glucose, pH 5.5) and M medium (0.2% NH4Cl, 0.1% (NH4)2SO4, 0.05% KCl, 0.05% NaCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, and 2% glucose, pH 5.5) with required supplements (20 mM uridine/0.2% uracil, and 0.01% adenine) were used for mixed culture of A. oryzae strains. To investigate the effect of nitrogen source, NaNO3 in the CD medium was replaced with 10 mM amino acids or 0.2% NH4Cl. In the CD medium without NaNO3, 0.2% NaCl was added to supply sodium. DPY medium (2% dextrin, 1% polypeptone, 0.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4·7H2O, pH 5.5) and PD (potato dextrose) medium (Nissui Pharmaceutical, Tokyo, Japan) were used as rich media. A. oryzae was transformed according to a method described in Maruyama and Kitamoto.Citation28)
Table 1. Strains used in this study.
Molecular techniques
The BP and LR recombination reactions with the MultiSite Gateway system (Invitrogen, Carlsbad, CA, USA) were performed for all plasmid construction as instructed by the manufacturer. Escherichia coli DH5α was used for DNA manipulation. DNA fragments were amplified with Prime STAR HS DNA Polymerase (TaKaRa, Otsu, Japan). All of the primers used in this study are listed in Supplemental Table 1 (see http://dx.doi.org/10.1080/09168451.2014.917262).
Construction of the pyrG and adeB disruptants
For disruption of pyrG and adeB genes, the wild-type strain NSRKu70-1-1ACitation29) and ΔAoso strainCitation25,26) were used as hosts. In order to generate ΔAoso strain, the 1.5-kb upstream flanking region of the Aofus3 ORF (AO090003000402) was amplified with the primers (fus3-5UTR-F and fus3-5UTR-adeA-R) and the genomic DNA as template. The 1.5-kb downstream flanking region of the Aofus3 ORF was amplified with primers (adeA-fus3-3UTR-F and fus3-3UTR-R), and the adeA marker gene was amplified with primers (fus3-5UTR-adeA-F and adeA-fus3-3UTR-R). These three fragments were fused by PCR with primers (fus3-5UTR-F and fus3-3UTR-R) and introduced into the strain NSRKu70-1-1.Citation29) Disruption of the Aofus3 genes was confirmed by Southern blotting analysis (data not shown).
For disruption of the pyrG gene, the 1.5-kb upstream flanking region of the pyrG ORF was amplified with the primers (attB4-PYRG-F and attB1-PYRG-R) and the genomic DNA as template and inserted into pDONRTMP4-P1R (Invitrogen) by the BP recombination reaction, generating the 5′ entry clone pg5′pG. The 1.5-kb downstream flanking region of the pyrG gene was amplified with primers (attB2-PYRG-F and attB3-PYRG-R) and the genomic DNA as template, and then inserted into pDONRTMP2R-P3 (Invitrogen) by the BP recombination reaction, generating the 3′ entry clone pg3′pG. The obtained 5′/3′ entry clones and the center entry clone plasmid pgEsC were mixed for the LR recombination reaction together with the destination vector pDESTTMR4-R3 (Invitrogen), generating pgΔpGS. The fragment for pyrG gene disruption was amplified by primers (attB4-PYRG-F and attB3-PYRG-R) using pgΔpGS as template and introduced in the wild-type, ΔAoso, and ΔAofus3 strains. M medium containing uridine and uracil was used for selection of transformants. Genomic DNAs were used as template for PCR analysis using the primers for verification of the pyrG gene disruption, and the resulting ΔpyrG strains exhibited uridine/uracil auxotrophy (data not shown).
For disruption of the adeB gene, the 1.0-kb upstream flanking region of the adeB ORF was amplified with the primer (attB4-5aB-F and attB1-5aB-R) and the genomic DNA as template and inserted into pDONRTMP4-P1R (Invitrogen) by the BP recombination reaction, generating the 5′ entry clone pg5′aB. The 1.0-kb downstream flanking region of the adeB gene was amplified with primers (attB2-3aB-F and attB3-3aB-R) and the genomic DNA as template and inserted into pDONRTMP2R-P3 (Invitrogen) by the BP recombination reaction, generating the 3′ entry clone pg3′aB. The obtained 5′/3′ entry clones and the center entry clone plasmid pgEsC were mixed for the LR recombination reaction together with the destination vector pDESTTMR4-R3 (Invitrogen), generating pgΔaBS. The gene disruption fragment for adeB was amplified by primers (attB4-5aB-F and attB3-3aB-R) using pgΔaBS as template and introduced into the wild-type, ΔAoso, and ΔAofus3 strains. M medium containing adenine was used for selection of transformants. Genomic DNAs were used as template for PCR analysis using the primers for verification of the adeB gene disruption, and the resulting ΔadeB strains exhibited adenine auxotrophy (data not shown).
Method to evaluate hyphal fusion in mixed culture
Conidia of uridine/uracil auxotrophic strain (ΔpyrG) and adenine auxotrophic strain (ΔadeB) were collected from PD agar medium containing uridine/uracil or adenine. Equal numbers of conidia from both the strains were mixed, and 5 × 104 conidia/5 μL were spotted onto the agar media containing uridine/uracil and adenine. After this incubation at 30 °C for 5 d, the newly formed conidia were collected, and 1 × 104–1 × 106 conidia were spread onto the minimal agar medium without uridine/uracil and adenine. In order to count the number of colonies precisely, 0.25% Triton X-100 was added into the agar medium to make the colony size smaller. After incubation, colonies derived from the auxotrophically complemented heterokaryon were counted.
Fluorescence microscopy
Conidia and hyphae on a glass bottom dish (Asahi Techno Glass, Chiba, Japan) were observed by confocal microscopy using an IX71 inverted microscope (Olympus, Tokyo, Japan) equipped with a 100 × Neofluor objective lens (1.40 numerical aperture); 488-nm (Furukawa Electric, Tokyo, Japan) and 561-nm semiconductor lasers (Melles Griot, Carlsbad, CA, USA); GFP, DsRed, and DualView filters (Nippon Roper, Chiba, Japan); a CSU22 confocal scanning system (Yokogawa Electronics, Tokyo, Japan); and an Andor iXon cooled digital CCD camera (Andor Technology PLC, Belfast, UK). Fluorescent images were analyzed with Andor iQ software (Andor Technology PLC) and representative images are shown.
Results
Establishment of an assay for hyphal fusion efficiency in A. oryzae
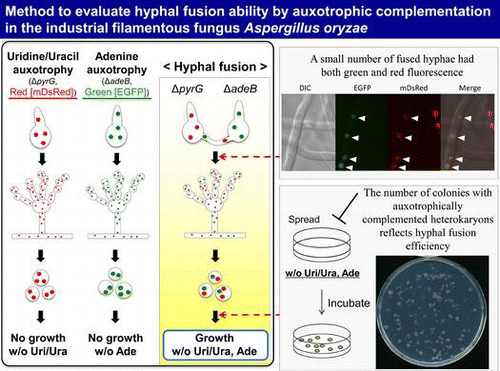
Two different auxotrophic strains were used for establishing an assay for hyphal fusion: the uridine/uracil auxotrophic strain PlD-HR1-c (ΔpyrG) and the adenine auxotrophic strain AblD-HG1-c (ΔadeB).Citation8) PlD-HR1-c cannot grow in the minimal medium without uridine and uracil, and AblD-HG1-c cannot grow without adenine. When hyphal fusion occurs between the two auxotrophic strains, the heterokaryon formed can grow on the minimal medium without uridine/uracil and adenine due to the auxotrophic complementation.Citation8)
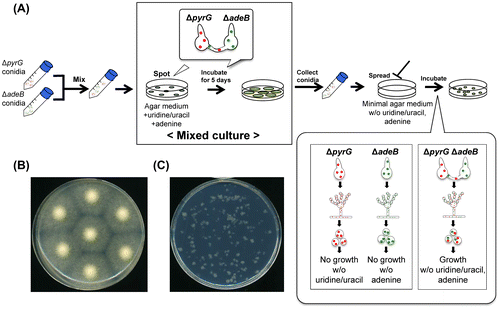
We established a method to evaluate the hyphal fusion in A. oryzae as illustrated in Fig. (A). Equal numbers of conidia from the uridine/uracil auxotrophic or adenine auxotrophic strains were mixed, and spotted onto an agar medium supplemented with uridine/uracil and adenine. In this culture, hereafter referred to as “mixed culture” (Fig. (A) and (B)), if hyphal fusion occurs between the two strains, auxotrophically complemented heterokaryon would be formed. The newly formed conidia were collected, and then spread onto a minimal agar medium without uridine/uracil and adenine, where only conidia from the auxotrophically complemented heterokaryon could grow. This method is possible since A. oryzae has multinucleate conidia,Citation30) which could keep heterokaryotic state. In order to count the number of colonies precisely, Triton X-100 was added to make the colony size smaller. When the conidia collected from mixed culture were spread onto the medium without uridine/uracil and adenine, heterokaryotic colonies with the auxotrophies complemented were found (Fig. (C)). This result suggested the occurrence of hyphal fusion in mixed culture.
Fig. 1. Outline of a method to evaluate hyphal fusion efficiency in A. oryzae.
Notes: (A) Mixed culture of two auxotrophic strains. Equal numbers of conidia from the two strains (ΔpyrG; uridine/uracil auxotrophic, ΔadeB: adenine auxotrophic) were mixed, and 5 × 104 conidia/5 μL were spotted onto the agar medium containing uridine/uracil and adenine. After incubation at 30 °C for 5 d, the newly formed conidia were collected, and they were spread onto the minimal agar medium without uridine/uracil and adenine. (B) The photo of an agar medium of mixed culture. Conidia suspensions mixed with the strains PlD-HR1-c (ΔpyrG) and AblD-HR1-c (ΔadeB) were spotted onto the CD agar medium containing uridine/uracil and adenine, and incubated at 30 °C for 5 d. (C) The photo of the agar medium without uridine/uracil and adenine, where 105 conidia from mixed culture with the strains PlD-HR1-c and AblD-HR1-c were spread and incubated at 30 °C for 3 d. Only heterokaryotic conidia with the auxotrophies complemented grew, and the colony size was restricted by addition of 0.25% Triton X-100.
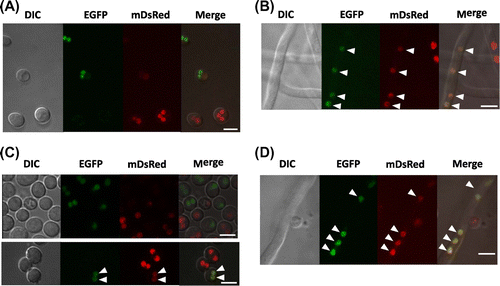
Fluorescence microscopy observation of heterokaryon formation was performed in mixed culture. The used strains PlD-HR1-c and AblD-HG1-c have red and green fluorescence in nuclei by expression of mDsRed and EGFP fused with histone H2B, respectively.Citation8) Conidia of the two strains with either green or red fluorescence in nuclei were mixed and inoculated onto the agar medium containing uridine/uracil and adenine (Fig. (A)). After 24 h of incubation, a few hyphae possessed both red and green fluorescence in nuclei (Fig. (B)), which were derived from the heterokaryon formed between the two strains. Among the conidia harvested from mixed culture, a few conidia had both red and green fluorescence in nuclei, while most of the conidia had either color of fluorescence (Fig. (C)). After 16 h of incubation of the conidia in a minimal medium without uridine/uracil and adenine, only auxotrophically complemented hyphae with both colors of fluorescence grew (Fig. (D)). These fluorescent images supported that hyphal fusion occurred in mixed culture.
Fig. 2. Fluorescence microscopy of heterokaryotic hyphae and conidia in mixed culture.
Notes: Fluorescence microscopic observation of heterokaryons was performed using the strains PlD-HR1-c and AblD-HG1-c with nuclei labeled in red and green, respectively. (A) Conidia spotted on the CD agar medium containing uridine/uracil and adenine. Conidia concentration was 5 × 104 conidia/5 μL/spot. The spotted agar media were cut off from the culture, put onto a glass base dish upside down, and then observed. (B) Hyphae in mixed culture on the CD agar medium containing uridine/uracil and adenine. After 24 h of growth, the incubated agar media were cut off and observed as described above. A heterokaryotic hypha with both green and red fluorescence was found as pointed by arrowheads. (C) Conidia formed in mixed culture. The conidia were collected after 5 d growth of mixed culture and observed. Conidia from the heterokaryons have both green and red fluorescence as pointed by arrowheads. (D) A growing hypha from the heterokaryotic conidium. The conidia formed in mixed culture were inoculated on the CD agar medium without uridine/uracil and adenine, and incubated at 30 °C for 16 h. The incubated agar media were cut off and observed as described above. Only heterokaryotic conidia grew on the minimal medium and showed both green and red fluorescence as pointed by arrowheads. Bars: 5 μm.
AoSO and AoFus3 are necessary for hyphal fusion in A. oryzae
In order to confirm the usability and validity of the method, we examined disruptants of genes involved in hyphal fusion. AoSO and AoFus3 are homologs of SOCitation19) and MAK-2,Citation18) which are required for hyphal fusion in N. crassa. We generated uridine/uracil or adenine auxotrophic strains from the ΔAoso and ΔAofus3 strains and used them in the hyphal fusion assay as performed in Fig. . First, conidia of the two wild-type strains with different auxotrophies (ΔpyrG and ΔadeB) were inoculated on the agar medium with uridine/uracil and adenine. After the growth for 5 d, newly formed conidia were harvested. When 106 conidia from mixed culture were spread onto the minimal medium without uridine/uracil and adenine, approximately 2.7 × 102 colonies appeared (Table ), which indicates the number of heterokaryotic conidia. In contrast, no heterokaryotic conidia were found from the conidia formed in mixed culture of the ΔAoso strains with uridine/uracil or adenine auxotrophies (Table ), which revealed no formation of auxotrophically complemented heterokaryon. Moreover, mixing the wild-type and ΔAoso strains with swapped combinations of auxotrophies did not result in growth of the heterokaryon on the minimal medium without uridine/uracil and adenine. These results indicated that the ΔAoso strain has lost the hyphal fusion ability. Similarly, no heterokaryotic conidia with the auxotrophies complemented were found from the conidia of mixed culture between the ΔAofus3 strains or between the ΔAofus3 and wild-type strains (Table ), which indicated that disruption of Aofus3 gene severely impaired the hyphal fusion ability.
Table 2. Effects of Aoso and Aofus3 gene disruptions on hyphal fusion.
Therefore, we concluded that AoSO and AoFus3 are required for hyphal fusion, which is consistent with those of N. crassa.Citation18,19) Given the fact that the number of heterokaryotic conidia was altered depending on hyphal fusion ability as in Table , it was supported that our method is effective to quantitatively evaluate hyphal fusion efficiency in A. oryzae.
Effects of nitrogen source on hyphal fusion efficiency
Next, we investigated the effects of media composition on hyphal fusion efficiency of A. oryzae by using the strains PlD-HR1-c and AblD-HG1-c. The number of heterokaryotic conidia out of the conidia formed in mixed culture was examined to evaluate hyphal fusion efficiency.
First, nitrogen source of the CD medium in mixed culture was changed from nitrate to leucine, ammonium glutamine, and tryptophan. Nitrate and leucine exhibited a higher number of heterokaryotic conidia than those of glutamine and ammonium (Fig. (A)). Therefore, it was indicated that the hyphal fusion efficiency was higher with nitrate and leucine, the nitrogen sources suggested not to be assimilated easily in A. nidulans,Citation31) but the efficiency was less with the preferred nitrogen sources, ammonium and glutamine. Tryptophan reduced the number of heterokaryotic conidia capable of growing on the medium without uridine/uracil and adenine (Fig. (A)). This result indicated that tryptophan reduced the hyphal fusion efficiency in A. oryzae, which was consistent with the report in N. crassa.Citation16)
Fig. 3. Effects of nitrogen source on hyphal fusion efficiency.
Notes: The assay of hyphal fusion was performed using strains PlD-HR1-c and AblD-HG1-c. The nitrogen source of the CD medium in mixed culture was changed from nitrate to leucine, ammonium, glutamine, and tryptophan (A), and the amount of sodium nitrate was changed from 0.3 to 0 and 3% (B). The CD medium in mixed culture contained uridine/uracil and adenine, which would be assimilated as another nitrogen source. The number of heterokaryotic conidia out of the conidia formed in mixed culture is shown in the graph. Error bars indicate standard deviations. Four independent experiments were performed. *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test.
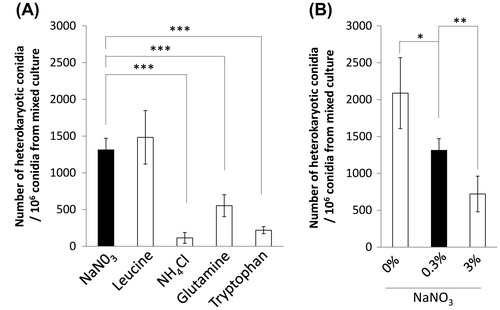
We checked whether the amount of nitrogen source affects the hyphal fusion efficiency. The CD media containing 0, 0.3, or 3.0% sodium nitrate were compared. High concentration of nitrate decreased the number of heterokaryotic conidia capable of growing on the medium without uridine/uracil and adenine (Fig. (B)). Although nitrate is a nitrogen source effective for inducing hyphal fusion, a large quantity of nitrate reduced the fusion efficiency. Depletion of nitrate increased the heterokaryotic conidia as compared to 0.3% sodium nitrate (Fig. (B)), indicating that shortage of nitrogen source increased the hyphal fusion efficiency.
Effects of carbon source in hyphal fusion efficiency
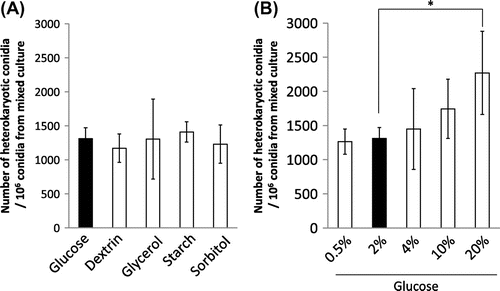
In order to examine the effect of carbon source in mixed culture on hyphal fusion efficiency, we changed carbon source in the CD medium. Replacement of glucose with dextrin, starch, glycerol, and sorbitol in mixed culture did not influence the number of heterokaryotic conidia capable of growing on the medium without uridine/uracil and adenine (Fig. (A)). We also changed the concentration of glucose in the CD medium. When 20% glucose was contained in mixed culture, a larger number of heterokaryotic conidia were obtained (Fig. (B)). Therefore, it was suggested that a larger amount of glucose increased the hyphal fusion efficiency.
Fig. 4. Effects of carbon source on hyphal fusion efficiency.
Notes: The carbon source of the CD medium in mixed culture was changed from glucose to dextrin, glycerol, starch, and sorbitol (A), and the amount of glucose was changed from 2 to 0.5, 4, 10, and 20% (B). The number of heterokaryotic conidia out of the conidia formed in mixed culture is shown in the graph. Error bars indicate standard deviations. Four independent experiments were performed. *p < 0.05, Student’s t test.
pH effect on hyphal fusion efficiency
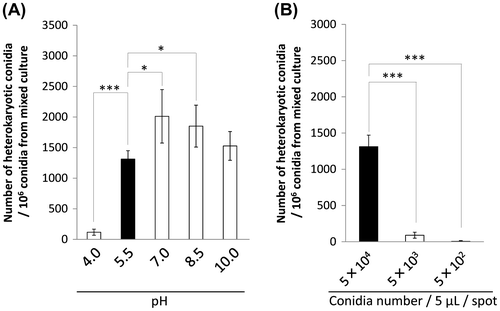
We tested if pH of the medium in mixed culture has effects on hyphal fusion efficiency of A. oryzae by changing pH of the CD medium from its initial 5.5 (Fig. (A)). At pH 4.0, a less number of heterokaryotic conidia were obtained than that of pH 5.5. At pH 7.0 and 8.5, the number of heterokaryotic conidia was higher than that of pH 5.5. Thus, it was suggested that neutral pH condition enhanced the hyphal fusion efficiency of A. oryzae, but extremely acidic condition decreased the efficiency.
Fig. 5 Effect of pH and conidia concentration on hyphal fusion efficiency.
Notes: (A) pH of the CD medium in mixed culture was changed from its initial 5.5. pH of the medium was adjusted before autoclave, and after sterilization, pH was checked again by pH test paper. (B) Three conidia concentrations for inoculation in mixed culture were examined. Conidia suspensions with 5 × 104, 5 × 103, and 5 × 102/5 μL were spotted onto the CD agar medium containing uridine/uracil and adenine. Conidia newly formed in mixed culture were harvested, and the 106 conidia were spread on the CD agar medium without uridine/uracil and adenine. The number of heterokaryotic conidia out of the conidia formed in mixed culture is shown in the graph. Error bars indicate standard deviations. Four independent experiments were performed. *p < 0.05, ***p < 0.001, Student’s t test.
Effects of conidia concentration on hyphal fusion efficiency
We changed the conidia concentration inoculated in mixed culture. Since initial concentration with 5 × 104 conidia/5 μL was the highest limit for accurately controlling the number of conidia in inoculation, lower concentration was examined. The number of heterokaryotic conidia was significantly decreased when 5 × 103 or 5 × 102 conidia/5 μL were inoculated for mixed culture (Fig. (B)). This result indicated that inoculation of highly concentrated conidia increased the hyphal fusion efficiency.
Inhibition of hyphal fusion in rich media
Effects of rich media such as PD (potato dextrose) and DPY (dextrin-polypeptone-yeast extract) media were examined. The numbers of heterokaryotic conidia/106 conidia from PD and DPY media were 6.3 ± 8.7 and 0.5 ± 0.9, respectively (n = 4). Out of the conidia harvested from the rich media, few heterokaryotic conidia were obtained, indicating that the hyphal fusion efficiency was significantly decreased in PD and DPY media. To investigate whether components of the rich media decrease the hyphal fusion efficiency, polypeptone and yeast extract were tested. The numbers of heterokaryotic conidia/106 conidia from CD + 0.5% yeast extract and CD + 1% polypeptone were 2.1 ± 2.4 and 0.1 ± 0.1, respectively (n = 4). Addition of polypeptone or yeast extract into the CD medium resulted in a marked decrease in the number of heterokaryotic conidia. The result indicated that these components significantly decreased the hyphal fusion efficiency.
We tested the possibility that ratios of surviving conidia are altered between the minimal and rich media. One hundred conidia harvested from the CD or PD medium in mixed culture were spread onto the CD agar medium containing uridine/uracil and adenine. However, there was no significant alteration in the number of colonies grown between the two media of mixed culture (data not shown). This suggested that difference in the number of heterokaryotic conidia was not caused by ratio of surviving conidia.
Optimization of media conditions for efficient hyphal fusion in A. oryzae
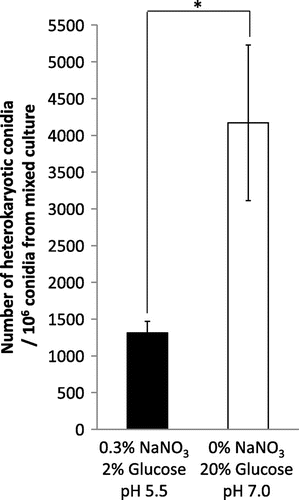
As described above, we found that the hyphal fusion efficiency was increased in some of the media conditions. In order to optimize media conditions for more efficient hyphal fusion, we examined the modified CD medium by depleting nitrate (Fig. (B)), including 20% glucose (Fig. (B)) and changing the pH to 7.0 (Fig. (A)). As expected, the number of heterokaryotic conidia was significantly increased, which is 3.2-fold higher than that of the original CD medium (Fig. ). This result indicated that the optimized condition is effective for increasing the hyphal fusion efficiency in A. oryzae.
Fig. 6. Optimization of media conditions for efficient hyphal fusion.
Notes: The original CD medium in mixed culture was modified by depleting nitrate, including 20% glucose, and changing pH to 7.0. The number of heterokaryotic conidia out of the conidia formed in mixed culture is shown in the graph. Error bars indicate standard deviations. Four independent experiments were performed. *p < 0.05, Student’s t test.
Discussion
In this study, we established a new method to quantitatively evaluate the hyphal fusion efficiency in A. oryzae. The method takes advantages of the feature that A. oryzae forms multinucleate conidia,Citation30) which allows a single conidium to contain nuclei from the two auxotrophic strains. Fluorescence microscopy demonstrating the co-existence of red and green fluorescence also supports heterokaryon formation by hyphal fusion (Fig. ). Moreover, we demonstrated that the two proteins AoSO and AoFus3 are necessary for hyphal fusion in A. oryzae (Table ), which agrees with the results of their homologous proteins in other filamentous fungi.Citation18,19,23,32) Taken together, these results indicated that our method is applicable to the analysis of hyphal fusion in A. oryzae.
By employing the method, we investigated the effects of media composition on hyphal fusion efficiency in A. oryzae. Our results indicated that kinds and amounts of nitrogen source affect the hyphal fusion efficiency (Fig. ). Tryptophan showed a suppressive effect on hyphal fusion in A. oryzae. Considering together with the previous report in N. crassa,Citation16) it is possible commonly in filamentous fungi that tryptophan inhibits hyphal fusion. Our data indicated that ammonium and glutamine decrease the hyphal fusion efficiency as compared with nitrate and leucine (Fig. (A)). Moreover, we found that depletion of nitrate increased the hyphal fusion efficiency (Fig. (B)). These preferences of nitrogen source for the hyphal fusion are well correlated with the report of areA mRNA stability.Citation31) The GATA transcription factor AreA has an important role in the expression of genes involved in nitrogen metabolism.Citation33) With preferred nitrogen sources such as ammonium and glutamine, the amount of areA mRNA is decreased, but the mRNA level is stable with other nitrogen sources such as nitrate and leucine and in nitrogen-depleted condition.Citation31) In Fusarium oxysporum, ammonium negatively regulates hyphal fusion via a nitrogen response pathway including the protein kinase TOR and the bZIP protein MeaB and AreA.Citation14) Our results also suggested the presence of a nitrogen response signaling for regulation of hyphal fusion ability in A. oryzae.
The kinds of carbon source did not have any significant effects on the hyphal fusion efficiency in A. oryzae (Fig. (A)). In contrast, large amounts of glucose increased the hyphal fusion efficiency (Fig. (B)), and high concentration (1.2 M) of sorbitol also had a positive effect (Supplemental Fig. 1). Hence, it might be possible to conclude that high concentration of carbon source promotes hyphal fusion. However, high concentration (0.6 M) of sodium chloride decreased the hyphal fusion efficiency (Supplemental Fig. S1). Thus, it could not be simply concluded that high osmolarity up-regulates the hyphal fusion efficiency. Another hypothesis is that the excessive amount of carbon source spuriously induces the nitrogen shortage by increasing carbon/nitrogen ratio.Citation34) Hence, some metabolic change by excessive amounts of carbon source might have a positive effect on hyphal fusion.
It was suggested that the neutral pH condition of mixed culture is effective for hyphal fusion in A. oryzae (Fig. (A)). However, in N. crassa, pH 5–6 is the optimal condition,Citation16) suggesting that optimal pHs for hyphal fusion are varied by fungal species. Molecular mechanism for pH-dependent regulation of hyphal fusion has not been studied in filamentous fungi. Figure (B) indicated that inoculation with lower conidia concentration in mixed culture decreased the hyphal fusion efficiency. The concentration is correlated with the distance between conidia; if the concentration is low, the distance would be too long for conidia to contact with each other. This gave a possibility that hyphal fusion in mixed culture occurred mainly during the germling phase as reported in CAT of N. crassa. To directly identify the growth phase of hyphal fusion in A. oryzae, more sensitive method of microscopic observation is required.
Rich media used in this study decreased the hyphal fusion frequency of A. oryzae. This is similar to the reports that the PD medium suppresses CAT formation in other filamentous fungi.Citation15,16) DPY medium had an inhibitory effect on hyphal fusion, and its components polypeptone and yeast extract also caused an inhibition. Polypeptone is probably recognized as a nitrogen source that can be easily assimilated, which decreases the hyphal fusion efficiency like ammonium and glutamine. Yeast extract and potato dextrose may commonly contain proteins, vitamins and minerals, and thus proteins or other components in the rich media might inhibit hyphal fusion in A. oryzae.
Finally, we achieved 3.2-fold higher efficiency of hyphal fusion in A. oryzae as compared to that of the original CD medium by optimizing carbon/nitrogen sources and pH (Fig. ). Appearance ratio of the heterokaryotic conidia was increased to 0.42%, which is still much lower than the hyphal fusion efficiency of N. crassa.Citation16) This report revealed that mixed culture started with A. oryzae conidia inoculated on the agar medium led to heterokaryon formation, raising a possibility that the hyphal fusion may occur in the rice-koji-making process. Collectively, further analysis of the molecular mechanism regulating hyphal fusion would help crossbreeding for sexual/asexual reproduction by increasing the fusion efficiency in A. oryzae.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.917262
Supplemental Fig. 1
Download PDF (67.7 KB)Supplemental Fig. 2 caption
Download MS Word (11.9 KB)Supplemental Fig. 2
Download PDF (285.4 KB)Supplemental Table 1
Download PDF (16 KB)Funding
This work was supported by Grant-in-Aid Challenging Exploratory Research and Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science. This was also supported by Research and development projects for application in promoting new policy of agriculture, forestry, and fisheries from National Agriculture and Food Research Organization, Japan, and by a fund from the Institute for Fermentation, Osaka (IFO), Japan.
References
- Kitamoto K. Molecular biology of the koji molds. Appl. Microbiol. 2002;51:129–153.10.1016/S0065-2164(02)51004-2
- Nakajima K, Asakura T, Maruyama J, Morita Y, Oike H, Shimizu-Ibuka A, Misaka T, Sorimachi H, Arai S, Kitamoto K, Abe K. Extracellular production of neoculin, a sweet-tasting heterodimeric protein with taste-modifying activity, by Aspergillus oryzae. Appl. Environ. Microbiol. 2006;72:3716–3723.10.1128/AEM.72.5.3716-3723.2006
- Nemoto T, Watanabe T, Mizogami Y, Maruyama J, Kitamoto K. Isolation of Aspergillus oryzae mutants for heterologous protein production from a double proteinase gene disruptant. Appl. Microbiol. Biotechnol. 2009;82:1105–1114.10.1007/s00253-008-1851-1
- Hara S, Jin FJ, Takahashi T, Koyama Y. A further study on chromosome minimization by protoplast fusion in Aspergillus oryzae. Mol. Genet. Genomics. 2012;287:177–187.10.1007/s00438-011-0669-1
- Wada R, Maruyama J, Yamaguchi H, Yamamoto N, Wagu Y, Paoletti M, Archer DB, Dyer PS, Kitamoto K. Presence and functionality of mating-type genes in the supposedly asexual filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2012;78:2819–2829.10.1128/AEM.07034-11
- Seishu seizo gijutsu. [Technique of sake production] Brew. Soc. Jpn. 2009.
- Ishitani C, Sakaguchi K. Hereditary variation and genetic recombination in Koji-molds (Aspergillus oryzae and Asp. sojae). V. Heterocaryosis. J. Gen. Appl. Microbiol. 1956;2:345–400.10.2323/jgam.2.345
- Wada R, Jin FJ, Koyama Y, Maruyama J, Kitamoto K. Efficient formation of heterokaryotic sclerotia in the filamentous fungus Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2014;98:325–334.10.1007/s00253-013-5314-y
- Roca MG, Arlt J, Jeffree CE, Read ND. Cell biology of conidial anastomosis tubes in Neurospora crassa. Eukaryot. Cell. 2005;4:911–919.10.1128/EC.4.5.911-919.2005
- Simonin A, Palma-Guerrero J, Fricker M, Glass NL. Physiological significance of network organization in fungi. Eukaryot. Cell. 2012;11:1345–1352.10.1128/EC.00213-12
- Roper M, Simonin A, Hickey PC, Leeder A, Glass NL. Nuclear dynamics in a fungal chimera. Proc. Nat. Acad. Sci. USA. 2013;110:12875–12880.10.1073/pnas.1220842110
- Palma-Guerrero J, Huang IC, Jansson HB, Salinas J, Lopez-Llorca LV, Read ND. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genet. Biol. 2009;46:585–594.10.1016/j.fgb.2009.02.010
- Ishikawa F, Souza EA, Read ND, Roca MG. Live-cell imaging of conidial fusion in the bean pathogen, Colletotrichum lindemuthianum. Fungal Biol. 2010;114:2–9.10.1016/j.funbio.2009.11.006
- López-Berges MS, Rispail N, Prados-Rosales RC, Di Pietro A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell. 2010;22:2459–2475.10.1105/tpc.110.075937
- Roca MG, Weichert M, Siegmund U, Tudzynski P, Fleißner A. Germling fusion via conidial anastomosis tubes in the grey mould Botrytis cinerea requires NADPH oxidase activity. Fungal Biol. 2012;116:379–387.10.1016/j.funbio.2011.12.007
- Fischer-Harman V, Jackson KJ, Muñoz A, Shoji JY, Read ND. Evidence for tryptophan being a signal molecule that inhibits conidial anastomosis tube fusion during colony initiation in Neurospora crassa. Fungal Genet. Biol. 2012;49:896–902.10.1016/j.fgb.2012.08.004
- Fu C, Iyer P, Herkal A, Abdullah J, Stout A, Free SJ. Identification and characterization of genes required for cell-to-cell fusion in Neurospora crassa. Eukaryot. Cell. 2011;10:1100–1109.10.1128/EC.05003-11
- Pandey A, Roca MG, Read ND, Glass NL. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell. 2004;3:348–358.10.1128/EC.3.2.348-358.2004
- Fleißner A, Sarkar S, Jacobson DJ, Roca MG, Read ND, Glass NL. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell. 2005;4:920–930.10.1128/EC.4.5.920-930.2005
- Fleißner A, Leeder AC, Roca MG, Read ND, Glass NL. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Nat. Acad. Sci. USA. 2009;106:19387–19392.10.1073/pnas.0907039106
- Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics. 2005;170:1091–1104.10.1534/genetics.104.036772
- Paoletti M, Seymour FA, Alcocer MJ, Kaur N, Calvo AM, Archer DB, Dyer PS. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr. Biol. 2007;17:1384–1389.10.1016/j.cub.2007.07.012
- Jun SC, Lee SJ, Park HJ, Kang JY, Leem YE, Yang TH, Chang M.-H. The MpkB MAP kinase plays a role in post-karyogamy processes as well as in hyphal anastomosis during sexual development in Aspergillus nidulans. J. Microbiol. 2011;49:418–430.10.1007/s12275-011-0193-3
- Bayram Ö, Bayram ÖS, Ahmed YL, Maruyama J, Valerius O, Rizzoli SO, Ficner R. The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 2012;8:e1002816.10.1371/journal.pgen.1002816
- Maruyama J, Escaño CS, Kitamoto K. AoSO protein accumulates at the septal pore in response to various stresses in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Commun. 2010;391:868–873.10.1016/j.bbrc.2009.11.154
- Huang HT, Maruyama J, Kitamoto K. Aspergillus oryzae AoSO is a novel component of stress granules upon heat stress in filamentous fungi. PLoS ONE. 2013;8:e72209.10.1371/journal.pone.0072209
- Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, Abe K, Gomi K, Horiuchi H, Kitamoto K, Kobayashi T, Takeuchi M, Denning DW, Galagan JE, Nierman WC, Yu J, Archer DB, Bennett JW, Bhatnagar D, Cleveland TE, Fedorova ND, Gotoh O, Horikawa H, Hosoyama A, Ichinomiya M, Igarashi R, Iwashita K, Juvvadi PR, Kato M, Kato Y, Kin T, Kokubun A, Maeda H, Maeyama N, Maruyama J, Nagasaki H, Nakajima T, Oda K, Okada K, Paulsen I, Sakamoto K, Sawano T, Takahashi M, Takase K, Terabayashi Y, Wortman JR, Yamada O, Yamagata Y, Anazawa H, Hata Y, Koide Y, Komori T, Koyama Y, Minetoki T, Suharnan S, Tanaka A, Isono K, Kuhara S, Ogasawara N, Kikuchi H. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161.10.1038/nature04300
- Maruyama J, Kitamoto K. Targeted gene disruption in koji mold Aspergillus oryzae. Methods Mol. Biol. 2011;765:447–456.10.1007/978-1-61779-197-0
- Escaño CS, Juvvadi PR, Jin FJ, Takahashi T, Koyama Y, Yamashita S, Maruyama J, Kitamoto K. Disruption of the Aopex11-1 gene involved in peroxisome proliferation leads to impaired woronin body formation in Aspergillus oryzae. Eukaryot. Cell. 2009;8:296–305.10.1128/EC.00197-08
- Maruyama J, Nakajima H, Kitamoto K. Visualization of nuclei in Aspergillus oryzae with EGFP and analysis of the number of nuclei in each conidium by FACS. Biosci. Biotechnol. Biochem. 2001;65:1504–1510.10.1271/bbb.65.1504
- Morozov IY, Galbis-Martinez M, Jones MG, Caddick MX. Characterization of nitrogen metabolite signalling in Aspergillus via the regulated degradation of areA mRNA. Mol. Microbiol. 2001;42:269–277.
- Prados Rosales RC, Di Pietro A. Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum. Eukaryot. Cell. 2008;7:162–171.10.1128/EC.00258-07
- Marzluf GA. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997;61:17–32.
- Geisseler D, Horwath WR, Joergensen RG, Ludwig B. Pathways of nitrogen utilization by soil microorganisms – A review. Soil Biol. Biochem. 2010;42:2058–2067.
