Abstract
Saccharomyces cerevisiae Ypq1p is a vacuolar membrane protein of the PQ-loop protein family. We found that ATP-dependent uptake activities of amino acids by vacuolar membrane vesicles were impaired by ypq1∆ mutation. Loss of lysine uptake was most remarkable, and the uptake was recovered by overproduction of Ypq1p. Ypq1p is thus involved in transport of amino acids into vacuoles.
Membrane transport proteins are functionally and phylogenetically categorized into a variety of superfamilies. Transporter–opsin–G protein-coupled receptor (TOG) superfamily, consisting of proteins putatively possessing seven or eight transmembrane helices, is exemplified by lysosomal cystine transporter, Ni/Co transporter, Pi/Na transporter, and ion-translocating microbial rhodopsin. The TOG superfamily is so far classified into nine families (Transporter Classification Database; http://www.tcdb.org/).Citation1) It is noteworthy that the G-protein coupled receptor (GPCR) family is included in this superfamily. A distinctive feature among the members of lysosomal cystine transporter is the conservation of a duplicated motif, termed PQ-loop motif after the presence of a conserved proline-glutamine dipeptide. The functional significance of PQ loops remains unclear, but some evidence being a contribution of lysosome targeting or core elements of transport mechanism.Citation2,3) Fig. . A shows the phylogenetic relationship among various PQ-loop proteins in micro-organisms, Caenorhabditis elegans (LAAT-1), rat (PQLC2) and human (cystinosin). Chung et al.Citation4) suggested that Schizosaccharomyces pombe Stm1p (Fig. (A)) acts as a GPCR that inhibits vegetative cell growth and induces sporulation in response to nitrogen starvation. They have also found two homologs YOL092 W (YPQ1) and YBR147 W (YPQ3) in Saccharomyces cerevisiae genome database (Fig. (A)) although these functions were not investigated. Very recently, Jézégou et al.Citation5) reported a new closest homolog YPQ2 (YDR352W) in S. cerevisiae (Fig. (A)). Ypq1p-GFP, Ypq2p-GFP, and Ypq3p-GFP were located at the vacuolar membrane when expressed in the sigma1278b strain background.Citation5) They found that ypq2∆ mutant acquired the resistance to canavanine (L-arginine analog). ypq1∆ mutant also displayed resistance to canavanine but to a lesser extent than ypq2∆ mutant. These results suggested that YPQ proteins are involved in homeostasis of basic amino acid. They also reported that rat PQLC2 (Fig. (A)) localized in the lysosomal membrane, and the electrophysiological experiment with voltage-clamped oocytes suggested that it is likely a transporter involving export of cationic amino acids from lysosomes. S. cerevisiae YPQ proteins are thus expected to be involved in transport of basic amino acids in vacuoles. Here we found that ATP-dependent uptake of amino acids by the vacuolar membrane vesicles was impaired by ypq1∆ mutation. Lysine uptake was totally lost. Ypq1p is thus involved in import of cationic amino acid into vacuoles.
Fig. 1. Phylogenetic relationship among the members of PQ-loop protein family and subcellular localization of Ypq1p-GFP.
Notes: (A) phylogenetic tree of PQ-loop proteins from micro-organisms (S. cerevisiae, Ashbya gossypii, Candida albicans, Aspergillus niger, Schizosaccharomyces pombe), C. elegans, rat, and human. The alignment was obtained from ClustalW and tree was build using GENETYX-Tree software. (B) subcellular localization of Ypq1p-GFP. Ypq1p-GFP overproduced by using GPD promoter was visualized using a fluorescent microscope. Images demonstrating colocalization of GFP fluorescence with the vacuolar membrane stained with FM4-64 are shown in a merged image. Bar, 5 μm.
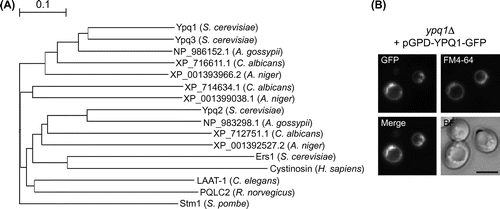
It is important to investigate the subcellular localization of Ypq1p in this study because protein localization is sometimes affected by genotype, strain background, culture condition, or expression level. We examined the localization of overexpressed Ypq1p fused to GFP in S. cerevisiae X2180-1B strain background. YPQ1 was deleted by PCR-based replacement with hphMX marker, in the wild-type strain STY3807.Citation6) To overproduce Ypq1p-GFP, GFP ORF was cloned into SalI and XhoI sites of p416GPDCitation7) to construct pGPD-C-GFP. Subsequently, YPQ1 ORF without stop codon was inserted into BamHI and SalI site of pGPD-C-GFP to construct pGPD-YPQ1-GFP. Constructed plasmid was introduced into the ypq1∆ strain. Cells were cultured in SD + CA medium (2% glucose, 0.5% ammonium sulfate, 0.5% casamino acid, 0.17% yeast nitrogen base w/o amino acids and ammonium sulfate, and 20 mg/liter of tryptophan). Overproduction of Ypq1p-GFP was confirmed by Western blot analysis using anti-GFP antibody (Molecular Probes) (data not shown). Overproduced Ypq1p-GFP exclusively localized to the vacuolar membrane as shown by colocalization with the fluorescence of vacuolar membrane specific dye FM4-64 (Fig. (B)).
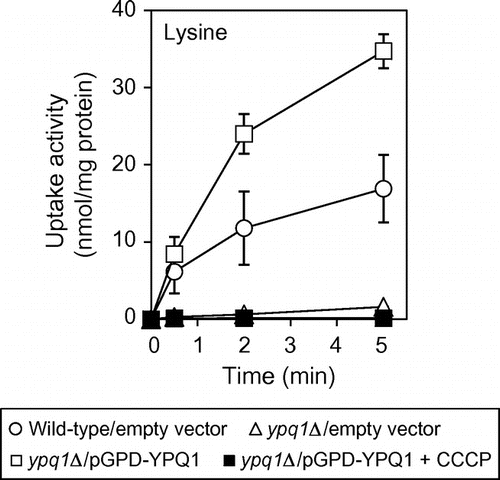
Vacuolar transport systems in S. cerevisiae were characterized with purified vacuolar membrane vesicles. ATP-dependent uptake activities of amino acids and several cations by the vesicles were driven by the proton electrochemical gradient, generated by the V-ATPase, across the vacuolar membrane.Citation8–10) The genes for these transport activities were identified by reverse genetics.Citation11,12) Fig. (A) shows the uptake activities of amino acids and 45Ca by vacuolar membrane vesicles of the parent and ypq1∆ mutant. Purification of vacuolar membrane vesicles was performed as described previously.Citation8,12) The uptake of [U-14C]arginine (0.1 mM; 12.9 GBq/mmol), [U-14C]lysine (0.1 mM; 11.8 GBq/mmol), [U-14C]histidine (final 0.1 mM; 11.6 GBq/mmol), [U-14C]tyrosine (0.1 mM; 16.9 GBq/mmol), and 45CaCl2 (0.5 mM; 18.5 GBq/mmol) by the membrane vesicles was performed as described previouslyCitation8,12) with minor modifications. All radioactive materials were obtained from PerkinElmer Japan. Cellulose acetate membrane filters (0.45 μm; ADVANTEC, Japan) were used to recover the vesicles, and the radioactivity retained on the filter was measured using a liquid scintillation counter with xylene scintillator. Protein concentration of the vesicles was determined by the method of Lowry et al.Citation13) with bovine serum albumin as standard. ATP-dependent uptake activities of arginine, lysine, histidine, tyrosine, and 45Ca observed in the parent were all inhibited by the V-ATPase inhibitor concanamycin A and the protonophore CCCP (data not shown). Among these amino acids, lysine uptake was severely impaired by ypq1∆ mutation. Arginine uptake partially decreased in ypq1∆ mutant, and uptake of histidine and tyrosine was slightly influenced by ypq1∆ mutation. Calcium uptake was little affected by ypq1∆ mutation. It is important to assess the generation of the proton electrochemical gradient by V-ATPase in ypq1∆ mutant. Vph1p, a major subunit of V-ATPase, and Pho8p, a vacuolar alkaline phosphatase, in the vacuolar membrane vesicles were detected by Western blotting with anti-Vph1p (10DA; Molecular Probes) and anti-Pho8p antibodies (1D3; Molecular Probes), respectively (Fig. (B)). Production Vph1p as well as Pho8p was not affected by ypq1∆ mutation. Generation of the pH gradient in the vacuolar vesicles was determined by measuring quenching of quinacrine as described previously.Citation14) The fluorescence signal of quinacrine was quenched by incubating the vesicles with ATP, reflecting uptake of protons and formation of pH gradient (Fig. (C)). Addition of 10 μM CCCP caused rapid and complete reversal of the fluorescence change. The pH gradient was thus generated in ypq1∆ mutant as well as the wild-type strain (Fig. (C)). Finally, Ypq1p-dependent lysine uptake was examined by the vacuolar membrane vesicles of the ypq1∆ strain overexpressing YPQ1 (Fig. ). For overexpression of YPQ1, YPQ1 ORF was cloned into BamHI and SalI sites of p416GPD. The constructed plasmid, pGPD-YPQ1, was introduced into ypq1∆ cells. Ypq1p-dependent lysine uptake was clearly observed, in which the activity was nearly twice than that of wild-type vesicles. This activity was totally inhibited by addition of CCCP. These results suggest that Ypq1p is involved in uptake of amino acids by vacuolar membrane vesicles. Ypq1p is likely a lysine-specific transporter of vacuoles.
Fig. 2. ATP-dependent uptake of amino acids and calcium by vacuolar membrane vesicles.
Notes: (A) Comparison of uptake activities between wild-type and ypq1Δ mutant vesicles. Wild-type (open circles) and ypq1Δ (open triangles) cells carrying empty vector were cultured in SD + CA medium and harvested at early logarithmic phase to isolate vacuolar membrane vesicles. Uptake assays for indicated amino acids and calcium were performed as described in the text. The results are means ± SD of three independent experiments. (B) Western blot analysis of vacuolar membrane vesicles. Vacuolar membrane vesicles (10 μg protein) isolated from wild-type and ypq1∆ cells were subjected to SDS-PAGE followed by Western blotting using anti-Vph1p and anti-Pho8p antibodies. For the detection of Vba1p-GFP, Western blotting using anti-GFP antibody was performed for vesicles isolated from wild-type and ypq1∆ cells carrying pVBA1GFP.Citation12) (C) Generation of the pH gradient by vacuolar membrane vesicles. The pH gradient established by the activity of V-ATPase in vesicles isolated from wild-type (left) and ypq1∆ (right) cells was measured as a quench of quinacrine fluorescence. The reaction was started with 0.5 mM ATP. After equilibrium was reached, 10 μM CCCP was added.
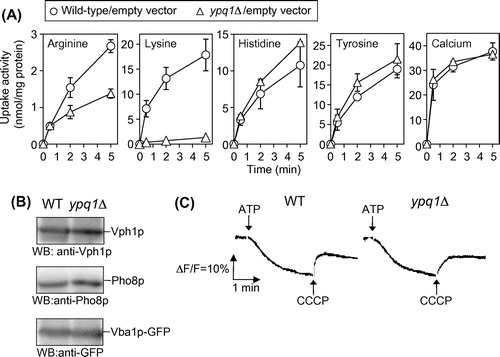
Fig. 3. Ypq1p-dependent lysine uptake by vacuolar membrane vesicles.
Notes: Vacuolar membrane vesicles isolated from ypq1∆ cells carrying pGPD-YPQ1 were treated with 10 μM CCCP for 10 min at 25 °C before addition of 0.5 mM ATP and assayed for ATP-dependent uptake of lysine as in Fig. (A) (closed squares). As control, vesicles isolated from wild-type cells carrying empty vector (open circles) and ypq1Δcells carrying empty vector (open triangles) or pGPD-YPQ1 (open squares) were treated with vehicle (ethanol) and assayed for lysine uptake.
Jézégou et al.Citation5) described in the previous paper that Ypq1p as well as Ypq2p is involved in export of basic amino acids from vacuoles, because ypq1∆ mutant acquired resistance to canavanine although it was weaker than ypq2∆ mutant. Accumulation of the drug into vacuoles might be accelerated in ypq1∆ mutant, resulting in decrease of its cytosolic level. In this study, however, we found that arginine uptake by vesicles was not increased but decreased by ypq1∆ mutation (Fig. (A)). Amino acid uptake by intact yeast cells involves active transport into vacuoles.Citation12,15,16) Therefore, it is possible that the uptake activity of amino acids into vacuoles is critical for an increase in the cytosolic concentration. The primary step for the maintenance of intracellular amino acid concentration is a transport across the cell membrane. In the case of basic amino acid transport across the cell membrane, several transport systems, such as Gap1p and Can1p, are involved. Activities of these transporters are regulated by the availability of amino acids.Citation17–20) Thus, the reason of drug (amino acid analog) sensitivity in cell growth is not simple, and it is too hasty to argue the involvement of Ypq1p in the drug sensitivity. Although the PQ-loop proteins in TOG superfamily are mainly characterized as symporter, uniporters, light-driven ion pumps, and GPCRs are also included as the member of this superfamily.Citation1) We expect Ypq1p as H+/Lys antiporter. In any case, an investigation with purification/reconstitution system of Ypq1p is important to clarify the characteristic of Ypq1p as a transporter.
Our previous reverse genetics on the transport activities of isolated vacuolar membrane vesicles has indicated that three VBA transporters (Vba1p, Vba2p, Vba3p) are involved in uptake of basic amino acids into vacuoles.Citation12) Vba1p works for histidine and lysine, Vba2p for histidine, arginine, and lysine, and Vba3p for histidine and lysine. The uptake activities of histidine, arginine, and lysine still slightly remained in the vesicles of vba1∆vba2∆vba3∆ mutant,Citation12) suggesting the existence of some other systems(s) involving uptake of basic amino acids into vacuoles. Ypq1p is likely this candidate. It is interesting that ATP-dependent lysine uptake activity was totally lost in ypq1∆ single mutant (Fig. (A)). This means some functional or regulatory interplay among Ypq1p and VBA proteins. Since we have not obtained antisera against these VBA proteins, effect of ypq1∆ mutation on the GFP-tagged Vba1p level was here tested (Fig. (B)). pVBA1GFPCitation12) with its own promoter region (1151 bp) and 3ʹ region (597 bp) was introduced into wild-type and ypq1∆ cells, and Western blot analysis using anti-GFP antibody (Molecular Probes) for vacuolar membrane vesicles was performed. The Vba1p-GFP level was not largely changed by ypq1∆ mutation (Fig. (B)). In any case, further investigation on expression of the VBA transporters, as well as the other vacuolar amino acid transporters, in ypq1∆ cells is an important issue for understanding the function of Ypq1p.
Acknowledgments
We thank Kana Hondo for technical help. This work was supported in part by Elizabeth Arnold Fuji Foundation (to Y.K.), Noda Institute for Scientific Research (to Y.K. and T.S.), and Takano Life Science Research Foundation (to T.S.).
Notes
Abbreviations: GPCR, G-protein coupled receptor; V-ATPase, vacuolar type H+-ATPase; GFP, green fluorescent protein; ORF, open reading frame; PCR, polymerase chain reaction; CCCP, carbonylcyanide m-chlorophenylhydrazone.
References
- Yee DC, Shlykov MA, Vastermark A, Reddy VS, Arora S, Sun EI, Saier MH Jr. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. FEBS J. 2013;280:5780–5800.10.1111/febs.12499
- Cherqui S, Kalatzis V, Trugnan G, Antignac C. The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J. Biol. Chem. 2001;276:13314–13321.10.1074/jbc.M010562200
- Ruivo R, Bellenchi GC, Chen X, Zifarelli G, Sagne C, Debacker C, Pusch M, Supplisson S, Gasnier B. Mechanism of proton/substrate coupling in the heptahelical lysosomal transporter cystinosin. Proc. Nat. Acad. Sci. USA. 2012;109:210–217.10.1073/pnas.1115581109
- Chung KS, Won M, Lee SB, Jang YJ, Hoe KL, Kim DU, Lee JW, Kim KW, Yoo HS. Isolation of a novel gene from Schizosaccharomyces pombe: stm1+ encoding a seven-transmembrane loop protein that may couple with the heterotrimeric Gα2 protein, Gpa2. J. Biol. Chem. 2001;276:40190–40201.
- Jézégou A, Llinares E, Anne C, Kieffer-Jaquinod S, O’Regan S, Aupetit J, Chabli A, Sagne C, Debacker C, Chadefaux-Vekemans B, Journet A, Andre B, Gasnier B. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Nat. Acad. Sci. 2012;109:3434–3443.10.1073/pnas.1211198109
- Sekito T, Chardwiriyapreecha S, Sugimoto N, Ishimoto M, Kawano-Kawada M, Kakinuma Y. Vacuolar transporter Avt4 is involved in excretion of basic amino acids from the vacuoles of Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. Forthcoming.
- Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122.10.1016/0378-1119(95)00037-7
- Ohsumi Y, Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1981;256:2079–2082.
- Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1983;258:5614–5617.
- Nishimura K, Igarashi K, Kakinuma Y. Proton gradient-driven nickel uptake by vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Bacteriol. 1998;180:1962–1964.
- Russnak R, Konczal D, McIntire SL. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 2001;276:23849–23857.10.1074/jbc.M008028200
- Shimazu M, Sekito T, Akiyama K, Ohsumi Y, Kakinuma Y. A family of basic amino acid transporters of the vacuolar membrane from Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:4851–4857.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275.
- Kakinuma Y, Ohsumi Y, Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 1981;256:10859–10863.
- Chardwiriyapreecha S, Shimazu M, Morita T, Sekito T, Akiyama K, Takegawa K, Kakinuma Y. Identification of the fnx1+ and fnx2+ genes for vacuolar amino acid transporters in Schizosaccharomyces pombe. FEBS Lett. 2008;582:2225–2230.10.1016/j.febslet.2008.05.017
- Chahomchuen T, Hondo K, Ohsaki M, Sekito T, Kakinuma Y. Evidence for Avt6 as a vacuolar exporter of acidic amino acids in Saccharomyces cerevisiae cells. J. Gen. Appl. Microbiol. 2009;55:409–417.10.2323/jgam.55.409
- Stanbrough M, Magasanik B. Transcriptional and posttranslational regulation of the general amino acid permease of Saccharomyces cerevisiae. J. Bacteriol. 1995;177:94–102.
- Merhi A, Andre B. Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol. Cell. Biol. 2012;32:4510–4522.10.1128/MCB.00463-12
- Cox KH, Rai R, Distler M, Daugherty JR, Coffman JA, Cooper TG. Saccharomyces cerevisiae GATA sequences function as TATA elements during nitrogen catabolite repression and when Gln3p is excluded from the nucleus by overproduction of Ure2p. J. Biol. Chem. 2000;275:17611–17618.10.1074/jbc.M001648200
- Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725.10.1016/j.cell.2008.09.025
