Abstract
Nicotinamide N-oxide is a major nicotinamide catabolite in mice but not in humans and rats. A high-performance liquid chromatographic method for the simultaneous measurement of nicotinamide, nicotinamide N-oxide, N1-methyl-2-pyridone-5-carboxamide, and N1-methyl-4-pyridone-3-carboxamide in mice urine was developed by modifying the mobile phase of a reported method for measurement of nicotinamide N-oxide.
Key words:
The number of nutritional experiments using mice has increased because of the ease of generating genetic knockout mice models compared with rats. Knockout mice have been developed for analysis of the components of the l-tryptophan (l-Trp) → nicotinamide (Nam) conversion pathway, including quinolinic acid phosphoribosyltransferase (QPRT)Citation1), l-Trp 2,3-dioxygenase,Citation2,3) indoleamine 2,3-dioxygenaseCitation4), and kynurenine 3-monooxygenaseCitation5). Nicotinamide N-oxide (Nam N-oxide) is a major catabolite of Nam that is excreted in the urine of mice, but not in humans and rats.Citation6) Urinary excretion of the sum of Nam and its catabolites (SUM) in mice, which reflects the amount of Nam synthesized from l-Trp when mice are fed a diet without preformed niacin, is calculated by Nam, N1-methyl-2-pyridone-5-carboxamide (2-Py), N1-methyl-4-pyridone-3-carboxamide (4-Py), N1-methylnicotinamide (MNA), and Nam N-oxideCitation1,2,6,7) (For metabolic pathway of l-Trp → Nam and its catabolites, see Shibata et al.Citation7) Current research focuses have increased the need for measurement of Nam N-oxide in urine.
In the late 1980s, Shibata reported simultaneous high-performance liquid chromatographic (HPLC) methods for Nam, 2-Py, and 4-PyCitation8), and HPLC measurement of Nam N-oxideCitation9). The extraction method of Nam N-oxide from urineCitation9) and that of Nam, 2-Py and 4-Py from urineCitation8) are very similar. Here, we have successfully developed a method for the simultaneous HPLC measurement of Nam, Nam N-oxide, 2-Py, and 4-Py in mice urine.
Nam, isonicotinamide (IsoNam), chloroform, potassium carbonate, potassium phosphate, methanol, and phosphoric acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). Nam N-oxide was purchased from Aldrich Chemical Co., Inc. (Milwaukee, WI, USA). The methods of Pullman and ColowickCitation10) and Shibata et al.Citation8) were used to synthesize 2-Py and 4-Py, respectively.
Vitamin-free milk casein and l-methionine were purchased from Wako Pure Chemical Industries. Sucrose was purchased from Dai-Nippon Meiji Sugar Co., Ltd (Tokyo, Japan) and corn oil was purchased from Ajinomoto (Tokyo, Japan). Gelatinized cornstarch, mineral mixture (AIN-93G mineral mixture), and vitamin mixture (nicotinic acid-free AIN-93 vitamin mixture containing 25% choline bitartrate) were purchased from Oriental Yeast Co., Ltd (Tokyo, Japan). A 20% casein diet without nicotinic acidCitation1,2,6,7) consisted of 20% vitamin-free milk casein, 0.2% l-methionine, 46.9% gelatinized cornstarch, 23.4% sucrose, 5% corn oil, 3.5% mineral mixture, and 1% vitamin mixture without nicotinic acid.
We conducted an experiment on animals to evaluate whether the proposed method could be applicable to urine samples from several strains of mice. The care and treatment of experimental animals conformed with the guidelines for the ethical treatment of laboratory animals set by the University of Shiga Prefecture (Shiga, Japan). The room temperature was 22 ± 2 °C and the humidity was 40–60%. A 12 h light–dark cycle was maintained. A total of 20 male 8-weak-old mice, five each of BALB/c, CBA, ICR, and C57BL/6 strains, were obtained from Charles River Japan (Tokyo, Japan). Each mouse was housed in a metabolic cage (CL-0355; CLEA Japan). All mice were fed ad libitum for 14 d with nicotinic acid free 20% casein diet and water.
QPRT is a key enzyme in the l-Trp → Nam conversion pathway.Citation1) Qprt knockout mice are a true experimental animal model for niacin deficiency when fed a diet without niacin. Seven-week-old Qprt knockout mice were housed in individual metabolic cages. Qprt knockout mice were fed ad libitum for 8 d with nicotinic acid free 20% casein diet and water. Urine samples (24 h; 09:00–09:00) were collected in vitreous bottles. Samples were treated with 1 mol/L HCl to make a final concentration 0.1 mol/L HCl and stored at -20 °C until needed.
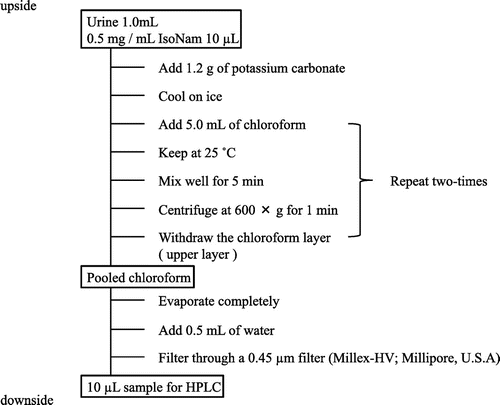
The extraction method of Nam, 2-Py, 4-Py, and Nam N-oxide in mice urine is shown in Fig. . The HPLC system used in the present experiment consisted of a L-7100 pump (Hitachi Instruments Service Co. Ltd., Tokyo, Japan), a L-7200 auto-sampler (Hitachi), a L-4000 UV detector (Hitachi), and a L-2350 column oven (Hitachi). The column was TSKgel ODS-80Ts (250 mm × 4.6 mm, i.d., average particle side 5 μm, Tosoh Co., Tokyo, Japan). The present mobile phase did not contain 1-octanesulfonic acid in the previous mobile phase, and was modified with the ratio of methanol: phosphateCitation9). The mobile phase was a mixture of 10 mmol/L potassium dihydrogen phosphate (pH was adjusted to 3.0 by addition of phosphoric acid): methanol (22:3, v/v) and was used at a flow rate of 0.8 mL/min. The wavelength was set at 260 nm and the column temperature was maintained at 30 °C. The UV detector was connected with a D-2500 (Hitachi) for data processing.
The limits of detection in Nam, 2-Py, 4-Py, and Nam N-oxide were 6.7, 1.5, 1.6, and 2.0 pmol per injections, respectively, at a signal-to-noise ratio of 5:1. The limits of determination in Nam, 2-Py, 4-Py, and Nam N-oxide were 27, 5.7, 6.2, and 7.8 pmol, respectively, per injections at coefficient of variance of 5%. The recoveries of Nam, 2-Py, 4-Py, and Nam N-oxide were investigated, and known standard amounts of Nam, 2-Py, 4-Py, and Nam N-oxide were added to urine samples prior to extraction. Each recovery was calculated by the following equation: recovery (%) = [integrated absorption area of (endogenous + added standard) – integrated absorption area of endogenous] / (integrated absorption area of added standard prior the extraction) × 100. The resulting recovery values of Nam, 2-Py, 4-Py, and Nam N-oxide from the urine samples were 80.7 ± 2.6% (mean ± SD; n = 3–5), 95.2 ± 3.3%, 99.4 ± 3.1%, and 77.7 ± 3.2%, respectively. IsoNam was used as an internal standard. When IsoNam was added to urine samples, its recovery was 79.7 ± 1.6 % (mean ± SD; n = 3–5). When the recovery was calculated using the recovery of IsoNam, the recoveries of Nam and Nam N-oxide became 96.0 ± 3.1% and 109 ± 2.0% (mean ± SD; n = 3–5), respectively, but the recoveries of 2-Py and 4-Py were over 150%. IsoNam did not reflect 2-Py and 4-Py in the present extraction procedure.
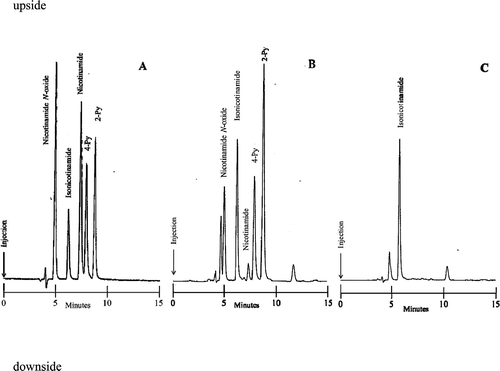
Fig. (A) shows a typical chromatogram of reference Nam N-oxide, IsoNam (internal standard), Nam, 4-Py, and 2-Py. The elution times were approximately 4.83, 6.08, 7.24, 7.80, and 8.69 min, respectively. The total analysis time was 15 min. Fig. (B) shows the elution profile obtained when the extract of C57BL/6 mouse urine was applied. Although the elution times of Nam and 4-Py were close (Fig. (A)), these can be clearly separated (Fig. (B)) since the peak height of Nam in mice urine is significantly lower compared to that of 4-Py. The mobile phase used in the reported method of Nam N-oxide contains sodium 1-octanesulfonic acid as an ion-pair reagent.Citation9) A mobile phase containing ion-pair reagent generally shortens the longevity of column. In a routine assay for the urinary excretion of Nam and its catabolites, the simultaneous measurement of Nam, 2-Py, 4-Py, and Nam N-oxide is more convenient.
Fig. 2. Chromatograms of a standard mixture of Nam, 2-Py, 4-Py, and Nam N-oxide (A) and extractions of C57BL/6 wild mouse urine (B) and Qprt knockout mouse urine (C).
Note. (A): Injection amounts; IsoNam, (internal standard) 164 pmol; Nam, 466 pmol; 2-Py, 108 pmol; 4-Py, 112 pmol; Nam N-oxide, 120 pmol; Hitachi chromatopac D-2500, attenuation 3. (B): Injection amounts; IsoNam, 655 pmol; Nam, 92.2 pmol; 2-Py; 371 pmol; 4-Py, 209 pmol; Nam N-oxide, 156 pmol; Hitachi chromatopac D-2500, attenuation 4. (C): Injection amounts; IsoNam, 652 pmol; Hitachi chromatopac D-2500, attenuation 4.
The urinary excretion percentage of SUM was different among mice strains.Citation7) The main urinary excretion of Nam metabolites in C57BL/6J, BALB/c, CBA, and ICR mice is listed in Table . The urinary excretion percentage of the SUM of Nam and its catabolites were nearly consistent with previous reportsCitation1,2,5,10), which had used the separate methods which are reported in Shibata et al.Citation8) to determine the urinary excretion of Nam, 2-Py, and 4-Py and in ShibataCitation9) to determine the urinary excretion of Nam N-oxide. The chromatograms of BALB/c, CBA, and ICR mouse are shown in Supplemental Fig. 1 (see http://dx.doi.org/10.1080/09168451.2014.918495). The present method for simultaneous measurements of Nam, 4-Py, 2-Py, and Nam N-oxide were applicable for all strains of mice.
Table 1. Urinary excretion of Nam and its catabolites and urinary excretion percentages.Table Footnotea
Fig. (C) shows the elution profiles obtained when the extract of Qprt knockout mice urine was applied. Only the peak of IsoNam was detected in Qprt knockout mice, as these mice cannot biosynthesize Nam from l-Trp. Therefore, each peak for Nam, 4-Py, 2-Py, and Nam N-oxide in the present chromatogram was free of contaminated substances.
In the previous extraction methodCitation9), the chloroform layer was the lower layer, which is slightly more inconvenient to remove in experimental analysis. We assessed the addition of various amounts of potassium carbonate to move the chloroform layer to an upper layer in the extraction solution. When 1.0 g of potassium carbonate was added to 1 mL urine and 5 mL chloroform, the chloroform layer was shifted to the upper layer. We next optimized the amount of added potassium carbonate with the aim of improving the recovery. The recovery became worse when 2.0 g of potassium carbonate was added and was unchanged when 1.0–1.5 g of potassium carbonate was added. Nam, 2-Py, 4-Py, and Nam N-oxide have stability in a low pH solution but these compounds become instable in a high pH solution. Then, addition of 1.2 g potassium carbonate was found to be the most suitable amount for the present extraction method (Fig. ).
The chloroform layer extracted from mice urine was evaporated at 70 °C for approximately 45–60 min (Fig. ). Nam and IsoNam are generally unstable at high temperatures, but 2-Py, 4-Py, and Nam N-oxide were stable. Evaporation at 70 °C for approximately 45–60 min somewhat broke down Nam and IsoNam to produce nicotinic acid and isonicotinic acid, respectively. Thus, IsoNam cannot be used as an internal standard in the present experiment, but it was useful for peak identification of Nam and its catabolites.
In conclusion, the proposed simultaneous measurement of Nam, 2-Py, 4-Py, and Nam N-oxide in mice urine presented here is more convenient than the previously reported method.Citation8,9) Furthermore, our results confirm that this method can be applicable for urine samples from several strains of mice in l-Trp → Nam conversion pathway.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.918495.
bbb-140155-File004_1_.ppt
Download MS Power Point (259 KB)Notes
Abbreviations: HPLC, high-performance liquid chromatography; IsoNam, isonicotinamide; l-Trp, l-tryptophan; MNA, N1-methylnicotinamide; Nam, nicotinamide; Nam N-oxide, nicotinamide N-oxide; QPRT, quinolinic acid phosphoribosyltransferase; 2-Py, N1-methyl-2-pyridone-5-carboxamide; 4-Py, N1-methyl-4-pyridone-3-carboxamide.
References
- Terakata M, Fukuwatari T, Sano M, Nakao N, Sasaki R, Fukuoka S, Shibata K. Truly niacin deficiency in quinolinic acid phosphoribosyltransferase (QPRT) knockout mice. J. Nutr. 2012;142:2148–2153.10.3945/jn.112.167569
- Terakata M, Fukuwatari T, Kadota E, Sano M, Kanai M, Nakamura T, Funakoshi H, Shibata K. The niacin required for optimum growth can be synthesized from L-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J. Nutr. 2013;143:1046–1051.10.3945/jn.113.176875
- Maeta A, Fukuwatari T, Funakoshi H, Nakamura T, Shibata K. Tryptophan-restriction diets help to maintain L-tryptophan homeostasis in tryptophan 2,3-dioxygenase knockout mice. Int. J. Trp. Res. 2013;6:55–65.
- Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, et al. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PLoS. One. 2013;8:e73404.10.1371/journal.pone.0073404
- Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M, Wu HQ, Schwarcz R, Muchowski PJ. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J. Biol. Chem. 2013;288:36554–36566.10.1074/jbc.M113.503813
- Kitamura J, Fukuwatari T, Ohta M, Higashida M, Sasaki R, Shibata K. Comparison of the metabolism of tryptophan to nicotinamide among humans, rats, and mice. J. Cre. Ap. Health. 2005;4:125–130.
- Shibata K, Morita N, Shibata Y, Fukuwatari T. Enzymes that control the conversion of L-tryptophan-nicotinamide and the urinary excretion ratio (N1-methyl-2-pyridone-5-carboxamide +N1-methyl-4-pyridone-3-carboxamide)/N1-methylnicotinamide in mice. Biosci. Biotechnol. Biochem. 2013;77:2105–2111.10.1271/bbb.130467
- Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J. Chromatogr. 1988;424:23–28.10.1016/S0378-4347(00)81072-5
- Shibata K. High-performance liquid chromatographic measurement of nicotinamide N-oxide in urine after extracting with chloroform. Agric. Biol. Chem. 1989;53:1329–1331.10.1271/bbb1961.53.1329
- Pullman ME, Colowick P. Preparation of 2- and 6-pyridones of N1-methylnicotinamide. J. Biol. Chem. 1954;206:121–127.
- Shibata K. Ultramicro-determination of N1-methylnicotinamide in urine by high-performance liquid chromatography. Vitamin (in Japanese). 1987;61:599–604.