Abstract
The predominant structure of the N-glycan on the prothoracicotropic hormone (PTTH) isolated from 1.8 million adult heads of silkmoths was determined to be Manα1-6Manβ1-4GlcNAcβ1-4(Fucα1-6)GlcNAc-OH, which is identical to that of the baculovirus-expressed recombinant PTTH. An ecdysis progression assay demonstrated that N-glycosylated PTTH exhibited a slightly higher activity than the recombinant PTTH without N-glycosylation produced by an Escherichia coli expression system.
Insect molting and metamorphosis are regulated by the sequential stimulation of peptide hormones and a repertoire of biologically active factors.Citation1,2) In particular, prothoracicotropic hormone (PTTH) exerts a pivotal stimulatory effect on postembryonic development and ecdysteroid biosynthesis and release by the prothoracic glands, eventually resulting in precisely timed ecdysis in insects.Citation3Citation−Citation5) PTTH was originally purified, isolated, and structurally determined from the head extract of a large number of adult Bombyx mori silkmoths using more than 15 purification steps.Citation6,7) Amino acid sequencing analysis revealed that B. mori PTTH is composed of 104 amino acids.Citation7) Molecular cloning of the cDNA encoding B. mori PTTH revealed several unsolved amino acid residues and the C-terminal portion of the PTTH from amino acid sequencing analyses.Citation8) Of the amino acids that were deduced following the molecular cloning of the cDNA encoding B. mori PTTH, the 41st amino acid was determined to be an asparagine residue, which generates a potential N-glycan posttranslational modification. Therefore, we aimed to identify and determine the N-glycan structure attached to PTTH extracted from its natural source.
Because PTTH is present in very small quantities in its natural source, we first analyzed baculovirus-expressed recombinant PTTH (B-rPTTH) using a B. mori nucleopolyhedrovirus (BmNPV) vector that contained a synthetic B. mori PTTH cDNA, which was infected into B. mori larvae.Citation9,10) After four steps of reversed-phase high-performance liquid chromatography (RP-HPLC), approximately 1.5 mg of B-rPTTH was purified from the hemolymph of six 5th-instar silkworm larvae 5 days after BmNPV infection. The purified B-rPTTH was digested with lysyl endopeptidase, and the digested peptides were compared with those from the non-glycosylated Escherichia coli-expressed recombinant PTTH (E-rPTTH)Citation11) to identify peptides with an N-glycan moiety. Separation of the digested peptides by RP-HPLC using an ODS column (4.6 id × 250 mm, Pegasil-ODS, Senshu Kagaku, Tokyo, Japan) resulted in peaks with different elution times between the E-rPTTH and B-rPTTH (peaks A and B in Fig. (A)) over a linear gradient of 10–40% acetonitrile with 220 nm UV detection. MALDI-TOF MS (Reflex-2, Bruker Japan, Tsukuba, Japan) analyses of these peptides revealed that the difference in the molecular weight of these peptides was 878, which almost corresponds to a sugar chain composed of five saccharide residues containing two N-acetyl hexosamines, two hexoses, and a deoxyhexose. Other peaks in the RP-HPLC profiles were determined to be fragment peptides from PTTH that had no glycoside according to MALDI-TOF MS analyses (data not shown).
Fig. 1. Analyses of peptides derived from the native and recombinant PTTHs.
Notes: (A) Comparison of the RP-HPLC profiles of lysyl endopeptidase-treated E-rPTTH and B-rPTTH. Peaks A in E-rPTTH and B in B-rPTTH were eluted at different retention times. The peptides corresponding to peaks A and B were further analyzed to determine the structure of the N-glycan. (B) MALDI-TOF MS analyses of the peptides derived from B-rPTTH and E-rPTTH. A possibly N-glycosylated peptide derived from B-rPTTH (a) and E-rPTTH (b) was analyzed. After PNGase F digestion, a peptide corresponding to peak B in Fig. (A) was also analyzed (c). The asterisk indicates an ion peak corresponding to the molecular weight of an N-glycosylated peptide without a deoxyhexose, possibly a fucose residue.
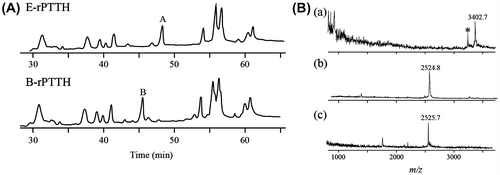
To determine if the peptides from peaks A and B in Fig. (A) were derived from the same peptide and if the larger peptide included a N-glycan, we digested these peptides with several N-glycanase-related enzymes. PNGase F (TaKaRa, Japan) digestion reduced the molecular weight of the peptide from 3402.7 to 2525.7 (Fig. (B)), which was approximately consistent with the weight of the corresponding non-glycosylated peptide derived from E-rPTTH (Fig. (A)). Additionally, EndoF2 (Sigma-Aldrich, MO, USA) treatment reduced the molecular weight of the peptide (data not shown). These enzymatic substrate specificities indicated that this peptide included a typical N-glycan structure composed of N-acetylchitobiose at the reducing end; thus, the possible structure of the N-glycan on B-rPTTH was Man-Man-GlcNAc-GlcNAc(Fuc)-OH. To determine the configuration of each linkage between the saccharide residues, we performed 2D PA (Pyridyl amidation) analyses of the N-glycan moieties.Citation12)
According to the conventional procedure, the N-glycan moiety was cleaved from the N-glycosylated peptide derived from B-rPTTH by hydrazine degradation, followed by N-acetylation.Citation13) The resulting N-glycan moiety was desalted using Bio-Gel P2 column gel filtration (14 mm id × 200 mm) with distilled water as a mobile phase to remove the remaining excess reagents. The fractions containing N-glycan were collected, lyophilized, and then pyridyl amidated with 2-aminopyridine in the presence of HCl. The resulting Schiff base was reduced by the addition of sodium borohydrate, as previously described.Citation14)
The PA derivative of N-glycan from B-rPTTH was subjected to HPLC using two different types of columns (ODS column, 4.6 id × 250 mm, Pegasil ODS, Senshu Kagaku; and TSK amide-80, 4.6 id × 250 mm, TOSOH, Tokyo, Japan) as previously reported.Citation12) The retention times of the peaks, as detected by a fluorescent detector (FA-80 Shimadzu, Kyoto, Japan) from two different columns, were mapped to a standard PA glucose oligomer (TaKaRa, Shiga, Japan). A number of N-glycan structures have been determined using this procedure referred as 2D map. The mapped point of the predominant PA-N-glycan of B-rPTTH coincided with that of an authentic PA derivative N-glycan composed of five saccharide residues, Manα1-6Manβ1-4GlcNAcβ1-4(Fucα1-6)GlcNAc-OH. We also observed some minor N-glycan moieties (Fig. (A) and (C)). The data, in which spots of the N-glycan migrated on the 2D map after α-fucosidase and α-mannosidase (Sigma-Aldrich) treatments (Fig. (A)), confirmed the presence of the terminal α1-6 mannose and α1-6 fucose residues in the N-glycan. The calculated molecular weight almost corresponded to that of the determined N-glycan structure attached to that N-glycosylated peptide. Additionally, a minor N-glycan structure without a fucose residue could be observed in the MALDI-TOF MS analysis data (asterisk in Fig. (B)).
Fig. 2. Structural analyses of PA-N-glycan on PTTHs.
Notes: (A) 2D PA-N-glycan map. The dots indicate the PA-N-glycan derived from B-rPTTH. The arrows indicate the migration on the map after treatment of the PA-N-glycan with α-fucosidase and α-mannosidase. Mapped glucose units were defined by the retention times of the commercially available PA-glucose oligomers. (B) HPLC profiles of PA-N-glycans derived from native PTTH and B-rPTTH using ODS column. Peaks corresponding to PA-N-glycans indicate numbers on peaks. (C) Structures of N-glycan on PTTHs. The percentages of N-glycans are indicated by each fluorescent peak intensity of PA-derivatives observed in (B).
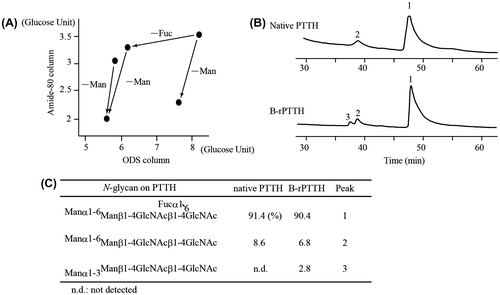
We next determined the structure of the N-glycan from its natural source. Almost identical purification steps allowed us to finally obtain 117 μg of native PTTH from 1.8 million B. mori adult heads. The identical procedure was used to determine the N-glycan attached to the purified native PTTH. As a result, the structure of the predominant N-glycan on native PTTH was determined to be identical to the N-glycan on B-rPTTH. Additionally, 2D mapping of PA-N-glycans revealed a somewhat different ratio of heterogeneity within the N-glycan compositions of B-rPTTH (Fig. (B) and (C)).
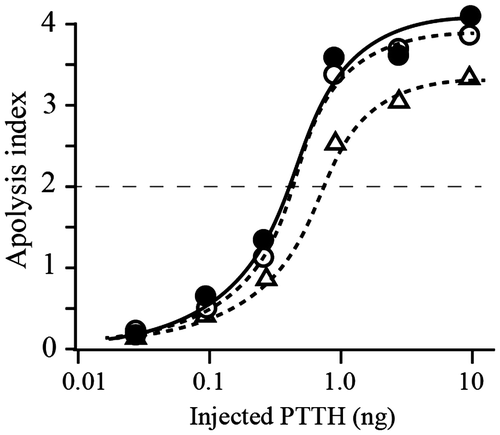
We next compared the biological activities of PTTH with and without the N-glycan moiety on the peptide. Two glycosylated PTTHs, native PTTH, and B-rPTTH, both of which were secreted as a dimer in B. mori larvae, and a non-glycosylated PTTH, E-rPTTH, which was precisely refolded into a soluble dimer in vitro, were subjected to the brainless pupal assay, which can detect the biological activity of PTTH.Citation15)As a result, injection of both PTTHs resulted in apolysis, which can be evaluated as ecdysis in the brainless pupae, in a dose-dependent manner (Fig. ). It is worth noting that slightly stronger activities at the ED50 level (0.32 ng/pupa vs. 0.59 ng/pupa) were observed following injection of N-glycosylated PTTH, native PTTH and B-rPTTH compared with the effect of injection of the non-glycosylated PTTH, E-rPTTH. This result suggests that the biological activity of PTTH is reinforced by N-glycosylation, but this posttranslational modification does not influence its biological activity.
Fig. 3. Biological activities of the native and recombinant PTTHs.
Notes: Dose-dependent activity was observed using three different PTTHs. The biological activity of PTTH was evaluated using the average of the apolysis index (N = 7); points 4, 3, 2, or 1 were observed if apolysis was observed on the 3rd, 4th, 5th, or 6th days after sample injection into the brainless pupae, respectively.Citation6) Closed circles, open circles, and open triangles indicate the effects of native PTTH, B-rPTTH, and E-rPTTH, respectively. The biological activities of these PTTHs were shown to be reproducible using a different preparation of brainless pupae.
Notes: During the preparation of this manuscript, the heterogeneous structures of N-glycan on the baculovirus-expressed recombinant PTTHs using insect cell lines were determined and reported.Citation9,16)
Acknowledgments
The brainless pupae were prepared using the hybrid strain J122 × C115, which was provided by Dr Fugo at the Tokyo University of Agriculture and Technology.
Funding
This work was partly supported by a Grants-in-Aid [grant number 08660407], [grant number 09265206] from the Japan Society for the Promotion of Science (A.S. and J.K.); and by a grant for Insect COE Project from the National Institute of Sericultural and Entomological Science (J.K.).
References
- Rybczynski R. Compr. Mol. Insect Sci. 2005;3:61–123.10.1016/B0-44-451924-6/00033-8
- Nagata S, Kataoka H, Suzuki A. Annals of NY Acad. Sci. 2005;1040:38–52.10.1196/annals.1327.004
- Dominic OS, Truman JM. J. Exp. Biol. 1985;117:45–68.
- Smith W, Rybczynski R. In: Gilbert LI (ed) Insect endocrinology. Academic Press, New York. pp. 1–62.
- Gilbert Li, Rybczynski R, Warren JT. Annu. Rev. Entomol. 2002;47:883–916.10.1146/annurev.ento.47.091201.145302
- Kataoka H, Nagasawa H, Isogai A, Tamura S, Mizoghuchi A, Fujiwara Y, Suzuki C, Ishizaki H, Suzuki A. Agric. Biol. Chem. 1987;51:1067–1076.10.1271/bbb1961.51.1067
- Kataoka H, Nagasawa H, Isogai A, Ishizaki H, Suzuki A. Agric. Biol. Chem. 1991;55:73–86.10.1271/bbb1961.55.73
- Kawakami A, Kataoka H, Oka T, Mizoguchi A, Kimura-Kawakami M, Adachi T, Iwami M, Nagasawa H, Suzuki A, Ishizaki H. Science. 1990;247:1333–1335.10.1126/science.2315701
- Nagaya M, Kobayashi J, Yoshimura T. Int. J. Wild Silkmoth Silk. 2002;7:59–68.
- O’reilly DR, Kelly TJ, Masler EP, Thyagaraja BS, Moy Robson RM, Shaw TC, Miller LK. Insect Biochem. Mol. Biol. 1995;25:475–485.10.1016/0965-1748(94)00087-F
- Ishibashi J, Kataoka H, Isogai A, Kawakami A, Saegusa H, Yagi Y, Mizoguchi A, Ishizaki H, Suzuki A. Biochemistry. 1994;33:5912–5919.10.1021/bi00185a031
- Tomiya N, Awaya J, Kurono M, Endo S, Arata Y, Yakahashi N. Anal. Biochem. 1988;171:73–90.10.1016/0003-2697(88)90126-1
- Takasaki S, Mizuochi T, Kobata A. Methods Enzymol. 1982;83:263–268.10.1016/0076-6879(82)83019-X
- Hase S, Sugimoto T, Takemoto H, Ikenaka T, Schmid K. J. Biochem. 1986;99:1725–1733.
- Ishizaki H, Mizoguchi A, Fujishita M, Suzuki A, Moriya I, O’oka H, Kataoka H, Isogai A, Nagasawa H, Tamura S. Dev. Growth Differ. 1983;25:593–600.10.1111/dgd.1983.25.issue-6
- Nagaya M, Kobayashi J, Takahashi N, Kato K, Yoshimura T. J. Insect Biotec. Sericol. 2003;72:79–86.
