Abstract
We purified several hundred mgs of four major theaflavins (theaflavin, theaflavin-3-O-gallate, theaflavin-3′-O-gallate, and theaflavin-3,3′-O-digallate). Among the 25 hTAS2Rs expressed in HEK293T cells, hTAS2R39 and hTAS2R14 were activated by theaflavins. Both hTAS2R39 and hTAS2R14 responded to theaflavin-3′-O-gallate. In addition, hTAS2R39 was activated by theaflavin and theaflavin-3,3′-O-gallate, but not by theaflavin-3-O-gallate. In contrast, hTAS2R14 responded to theaflavin-3-O-gallate.
Theaflavins (TFs) are red pigments contained in black tea that are produced by the oxidative condensation of catechins. The predominant TFs in black tea are theaflavin (TF1), TF-3-O-gallate (TF2A), TF-3′-O-gallate (TF2B), and TF-3,3′-O-digallate (TF3), which are synthesized with different combinations of precursors.Citation1) TFs have several catechin-like properties, such as antioxidativeCitation2,3) and antivirusCitation4) activities, with some being equal toCitation2) or strongerCitation4) than those of catechins.
Bitter taste receptors (TAS2Rs) are known to respond to bitter substances. Some medicines and food compounds are reported to activate TAS2Rs for example, caffeine, catechins, and other polyphenols in green tea or wine, sesquiterpene lactones in vegetables, and isoflavones in soybeans.Citation5–8) Recently, TAS2Rs have been found not only on the tongue but also in the small intestine, stomach, and trachea of mammals.Citation9–12) In the digestive tract, TAS2Rs are expressed in the endocrine cells of intestinal epithelial tissues and are known to participate in the secretion of incretins such as glucagon-like peptide-1 (GLP-1) and CCK.Citation13–15) Furthermore, it has been reported that bitter substances, such as denatonium, bind to tracheal smooth muscle, resulting in contraction of the bronchial tube.Citation16)
We have previously reported that among the 25 human TAS2Rs (hTAS2Rs), the bitterness of catechins, which are precursors of theaflavins, is perceived by hTAS2R39Citation8) and hTAS2R14.Citation17) Since black tea showed both bitterness and astringency by human sensory analysis,Citation18,19) we assumed that TFs are important compounds for bitterness of black teas. However, there is no report on the relationship between TFs and hTAS2R(s) response. TFs are oxidative condensates of catechins, therefore, we hypothesized that the bitterness of TFs may be sensed through hTAS2R39 and hTAS2R14, and investigated the responses of hTAS2Rs to four major theaflavins (theaflavin, theaflavin-3-O-gallate, theaflavin-3′-O-gallate, and theaflavin-3,3′-O-digallate).
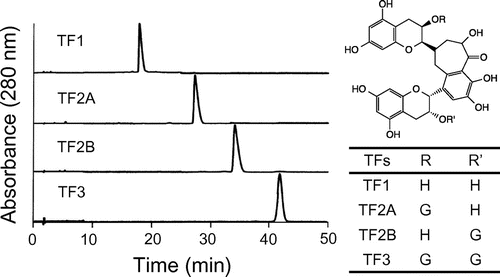
Crude TF mixtures (30.6 g, containing totally 42% TFs), prepared by incubating tea catechins in the presence of polyphenol oxidase were purchased from Yaizu Suisankagaku Industry Co. Ltd, Shizuoka, Japan. To obtain pure TFs, the mixtures were dissolved in water then, put through medium-pressure column chromatography using a reverse-phase preparative column with a gradient mobile phase (10–80% acetonitrile) to exclude catechins and caffeine. TFs obtained (2.1 g, >78% purity) were further purified through preparative high-performance liquid chromatography with the Capcellpak C18 (20 mm × 250 mm, Shiseido, Japan), using a mobile phase of 22% acetonitrile in the presence of 0.05% phosphoric acid to yield 131 mg of TF1 (20.0% yield from crude TF mixtures, >96% purity), 270 mg of TF2A (19.1% yield, >97% purity), 111 mg of TF2B (23.7% yield, >97% purity), and 383 mg of TF3 (21.6% yield, >97% purity) (Fig. ). The aqueous solutions containing the respective theaflavins were treated with the Sep-Pak C18 cartridge, eluted with ethanol, and lyophilized. The structure of the purified theaflavins was confirmed by NMR.
Fig. 1. HPLC chromatograms of purified theaflavins.
Notes: TF1, theaflavin; TF2A, theaflavin-3-O-gallate; TF2B, theaflavin-3′-O-gallate; TF3, theaflavin-3,3′-O-digallate; ECg, epicatechin gallate; EGCg, epigallocatechin gallate; G, galloyl group.
Expression plasmids for hTAS2Rs and the chimeric G-protein cDNA fragment encoding TAS2Rs were kindly gifted by Dr Misaka, and those for chimeric G-protein subunit Gα16gust44 were a kind gift provided by Dr Ueda.Citation20) The expression plasmids were subcloned into pEAK10 vector as previously described.Citation17)
For cell assays, either HEK293T cells were used until passage 30, or stably Gα16gust44-expressing Flp-In TREx 293T (Flp-In G) cells (Invitrogen, Carlsbad, CA) until passage 40. We used HEK293T for hTAS2R1, hTAS2R3, hTAS2R4, hTAS2R5, hTAS2R8, hTAS2R13, hTAS2R16, hTAS2R19, hTAS2R20, hTAS2R30, hTAS2R38, hTAS2R39, hTAS2R40, hTAS2R41, hTAS2R42, hTAS2R45, and others were applied for Flp-In G cells. The fluorescence assay was performed as described previously.Citation8) Briefly, we transfected hTAS2R and Gα16gust44 into HEK293T cells and hTAS2R into Flp-In G cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA). The cells were incubated for 60 min with 3 μM Fluo-8 AM (Molecular Probes, Eugene, OR, USA). Using the FlexStation II multimode fluorescent microplate reader (Molecular Devices, Sunnyvale, CA, USA), various concentrations of TFs were administered, and changes in the intracellular calcium concentration [Ca2+]i were measured. Average fluorescence values measured for 30 s before sample administration were used as baseline (F0). After administration of sample solutions, changes in fluorescence value were measured for 80 s, and the highest value (F) was employed to calculate the [Ca2+]i response according to the equation [(F − F0)/F0]. As a negative control, HEK 293T cells transfected with empty vector (mock) and Gα16gust44 were used. The response value was calculated by subtracting the response in mock cells from that in hTAS2R and Gα16gust44 co-transfected cells.
TF1-, TF2B-, and TF3-induced dose-dependent [Ca2+]i responses in HEK293T cells expressing hTAS2R39 (Fig. (A)), which is one of the major hTAS2Rs for catechins, especially epicatechin gallate (ECg) and epigallocatechin gallate (EGCg) (Fig. (B)).Citation8) The half effective (EC50) values for TF1, TF2B, and TF3 were 2.79, 0.67, and 1.55 μM, respectively. The response of TAS2R39 to TF2A was significantly lower than to the other TFs. Compared with catechins, TFs except for TF2A were much more sensitive: ECg (EC50 21.3 μM) and EGCg (EC50 112 μM) (Fig. (B)).
Fig. 2. Calcium response to theaflavins (A) and catechins (B) in HEK293T cells co-expressing hTAS2R39 with Gα16gust44 and calcium response to theaflavins (C) and aristolochic acid (D) in Flp-In G cells co-expressing hTAS2R14 with Gα16gust44.
Notes: TF1, theaflavin; TF2A, theaflavin-3-O-gallate; TF2B, theaflavin-3′-O-gallate; TF3, theaflavin-3,3′-O-digallate; ECg, epicatechin gallate; EGCg, epigallocatechin gallate. n = 3–4.
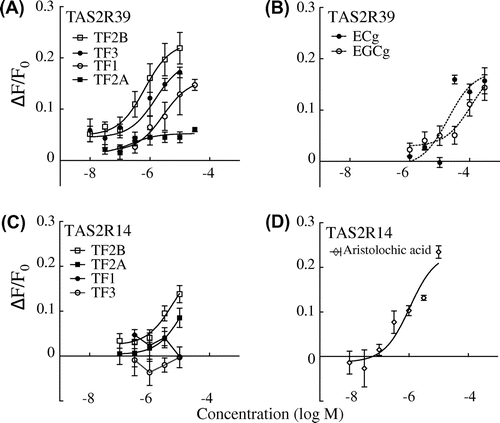
TF2B evoked a clear response (EC50 5.97 μM) from hTAS2R14, while TF2A weakly increased [Ca2+]i (EC50 14.0 μM) (Fig. (C)). TF2A and TF2B were also received by hTAS2R14 with higher affinity than catechins: ECg (EC50 70 μMCitation17)) and EGCg (EC50 34 μMCitation17)). In addition, a positive control of hTAS2R14, and aristolochic acid was received by the receptor at EC50 1.9 μM (Fig. (D)).
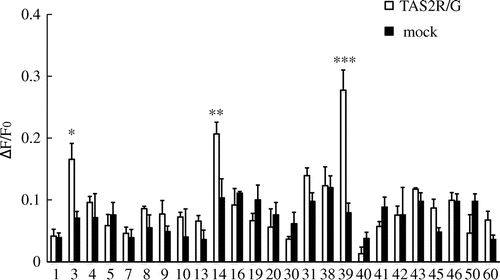
Since both hTAS2R39 and hTAS2R14 responded to TF2B, we applied TF2B to investigate its effects on the 23 other hTAS2Rs and found that hTAS2R3 was also activated by TF2B (Fig. ).
Fig. 3. Calcium response to 10 μM theaflavin-3′-O-gallate (TF2B) in HEK293T or Flp-In G cells co-expressing hTAS2Rs with Gα16gust44.
Notes: Black and white columns, respectively, show the calcium responses in hTAS2R- and Gα16gust44-expressing cells and mock cells. *, **, and *** respectively, indicate p < 0.05, 0.01 and 0.005 (t-test; n = 3–5).
The bitterness of catechins is known to be received by hTAS2R39Citation8) and hTAS2R14.Citation17) Therefore, we evaluated the possibility that TFs, which are oxidative condensates of catechins could also be received by these receptors. Our results clearly showed that TFs are strongly perceived by hTAS2R39 and hTAS2R14.
In general, bitter compounds with higher lipophilicity can bind the bitter receptor more easily.Citation21) Accordingly, TF2A, TF2B, and TF3 which contain the galloyl moiety in their structure are considered stronger agonists to bitter taste receptors than TF1, which possesses no galloyl group. However, our results showed that TF2A activated hTAS2R39 less than TF1 did. We believe that the perception of bitterness in TFs by hTAS2R39 is prevented by the position of the C-3 galloyl group. In fact, TF3-activated hTAS2R39 less than TF2B did. In addition, because both hTAS2R39 and hTAS2R14 responded to TF2B administration, we investigated whether TF2B activated the other 23 receptors. As a result, we determined that hTAS2R3 was also activated by TF2B (Fig. ). However, the reduced response of hTAS2R3 to TF2B in comparison with those of hTAS2R39 and hTAS2R14 indicates that hTAS2R3 may not function as a bitter receptor for TF2B. Moreover, we measured the responses of 25 hTAS2Rs to TF1, postulating that TF1 might show a distinct pattern of activation due to the lack of galloyl group. Among the 25 hTAS2Rs, no other receptor except for hTAS2R39 showed a drastic change of [Ca2+]i by treatment with TF1 (data not shown).
Although the content of total TFs in tea leaves varies by growing district, the concentration of TFs in black tea are generally higher than the EC50 of TFs-activating hTAS2Rs.Citation22) However, Scharbert et al. reported that theaflavins are not the key factor in the taste of black tea.Citation23,24) It has also been reported that while TF powder evokes a very rough taste, a solution of TF powder tastes tea-like.Citation25) These results are not entirely in accordance with ours. We believe that there are two reasons for this discrepancy. First, TFs are unstable compounds and degrade easily.Citation26) Second, because TFs are likely to interact with saliva protein,Citation27) the TF concentrations could decrease, causing difficulties in perceiving bitterness in the oral cavity. Therefore, the human threshold of bitterness perception might be higher than that in a cell-based assay.
Acknowledgments
We thank Dr Takumi Misaka (University of Tokyo, Tokyo, Japan) and Dr Takashi Ueda (Nagoya City University, Nagoya, Japan) for providing the hTAS2R genes and the Ga16gust44 construct, respectively. This work was supported by the Shizuoka Prefecture Collaboration of Regional Entities for the Advancement of Technological Excellence, JST.
Notes
Abbreviations: hTAS2R, human bitter taste receptor; TFs, theaflavins; TF1, theaflavin; TF2A, theaflavin-3-O-gallate; TF2B, theaflavin-3′-O-gallate; TF3, theaflavin-3,3′-O-digallate.
References
- Haslam E. Thoughts on thearubigins. Phytochemistry. 2003;64:61–73.10.1016/S0031-9422(03)00355-8
- Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001;131:2248–2251.
- Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VLW, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004;80:1558–1564.
- Liu S, Lu H, Zhao Q, He Y, Niu J, Debnath AK, Wu S, Jiang S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta. 2005;1723:270–281.10.1016/j.bbagen.2005.02.012
- Soares S, Kohl S, Thalmann S, Mateus N, Meyerhof W, De Freitas V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013;61:1525–1533.10.1021/jf304198k
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157–170.10.1093/chemse/bjp092
- Roland WS, Vincken JP, Gouka RJ, van Buren L, Gruppen H, Smit G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011;59:11764–11771.10.1021/jf202816u
- Narukawa M, Noga C, Ueno Y, Sato T, Misaka T, Watanabe T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011;405:620–625.10.1016/j.bbrc.2011.01.079
- Yamamoto K, Ishimaru Y. Oral and extra-oral taste perception. Semin. Cell Dev. Biol. 2013;24:240–246.10.1016/j.semcdb.2012.08.005
- Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 2011;105:4–13.10.1016/j.physbeh.2011.02.010
- Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun. 2011;406:146–151.10.1016/j.bbrc.2011.02.016
- Li F. Taste perception: from the tongue to the testis. Oxford J. 2013;19:349–360.
- Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AE, Choi HJ, Shaw H, Egan JM, Mitchell BD, Li X, Steinle NI, Munger SD. Bitter taste receptors influence glucose homeostasis. PLoS One. 2008;3:e3974.10.1371/journal.pone.0003974
- Nevé BL, Foltz M, Daniel H, Gouka R. The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am. J. Physiol. 2010;299:G1368–G1375.
- Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Invest. 2008;118:3693–3700.10.1172/JCI36461
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010;16:1299–1304.10.1038/nm.2237
- Yamazaki T, Narukawa M, Mochizuki M, Misaka T, Watanabe T. Activation of the hTAS2R14 human bitter-taste receptor by (−)-epigallocatechin gallate and (−)-epicatechin gallate. Biosci. Biotechnol., Biochem. 2013;77:1981–1983.10.1271/bbb.130329
- Hayashi N, Ujihara T, Chen R, Irie K, Ikezaki H. Objective evaluation methods for the bitter and astringent taste intensities of black and oolong teas by a taste sensor. Food Res. Int. 2013;53:816–821.10.1016/j.foodres.2013.01.017
- Scharbert S, Hofmann T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J. Agric. Food Chem. 2005;53:5377–5384.10.1021/jf050294d
- Ueda T, Ugawa S, Yamamura H, Imaizumi Y, Shimada S. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci. 2003;23:7376–7380.
- Kumazawa T, Kashiwayanagi M, Kurihara K. Contribution of electrostatic and hydrophobic interactions of bitter substances with taste receptor membranes to generation of receptor potentials. Biochim. Biophys. Acta. 1986;888:62–69.10.1016/0167-4889(86)90071-6
- Nishimura M, Ishiyama K, Watanabe A, Kawano S, Miyase T, Sano M. Determination of theaflavins including methylated theaflavins in black tea leaves by solid-phase extraction and HPLC analysis. J. Agric. Food Chem. 2007;55:7252–7257.10.1021/jf070312m
- Scharbert S, Holzmann N, Hofmann T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004;52:3498–3508.10.1021/jf049802u
- Scharbert S, Jezussek M, Hofmann T. Evaluation of the taste contribution of theaflavins in black tea infusions using the taste activity concept. Eur. Food Res. Technol. 2004;218:442–447.10.1007/s00217-004-0888-3
- Millin DJ, Crispin DJ, Swaine D. Nonvolatile components of black tea and their contribution to the character of the beverage. J. Agric. Food Chem. 1969;17:717–722.10.1021/jf60164a026
- Su YL, Leung LK, Huang Y, Chen Z-Y. Stability of tea theaflavins and catechins. Food Chem. 2003;83:189–195.
- Yao JW, Lin F, Tao T, Lin CJ. Affinity interactions between natural pigments and human whole saliva. Arch. Oral Biol. 2011;56:285–293.10.1016/j.archoralbio.2010.10.003
