Abstract
We previously reported that the two peroxisome proliferator-activated receptor-α agonists, 9- and 13-oxo-octadecadienoic acids (oxo-ODAs), were found in the tomato fruit. However, their localization remains unknown. Herein, we showed that oxo-ODAs localize primarily in the fruit peel and their amount increases after the homogenization of the tomato fruit.
Key words:
Tomatoes (Solanum lycopersicum) are one of the most extensively consumed crops worldwide. They contain many beneficial nutrients that are believed to suppress chronic conditions, such as cardiovascular diseases, cancer, and type-2 diabetes. Many previous studies have shown that dietary tomato intake is associated with a reduced risk of chronic diseases.Citation1–4) Recently, we reported that tomatoes contain the oxidized linoleic acid (LIA) derivatives 9-oxo-10,12-octadecadienoic acid (9-oxo-ODA) and 13-oxo-9,11-octadecadienoic acid (13-oxo-ODA), which can serve as peroxisome proliferator-activated receptor α (PPARα) agonists.Citation5,6) PPARα is a ligand-activated transcription factor that regulates lipid metabolism.Citation7–9) The activation of PPARα enhances fatty acid oxidation and decreases the levels of circulating and cellular lipids in obese diabetic patients,Citation10,11) suggesting that the intake of 9- or 13-oxo-ODA are valuable to maintain health.
Although we reported 9- and 13-oxo-ODAs (oxo-ODAs) in tomatoes,Citation12) little is known about their localization in the tomato fruit, which comprises the gelatinous, sarcocarp, and peel tissues. Furthermore, little information is available on the profile of the tomato’s free fatty acids (FFAs), which are possible precursors of oxo-ODAs. In this study, we performed a comparative analysis of the oxo-ODAs and FFAs in tomatoes by ultra performance liquid chromatography (UPLC) coupled with time-of-flight mass spectrometry (TOF-MS).
Authentic 9- and 13-oxo-ODAs were purchased from Larodan Fine Chemicals (Malmö, Sweden). Authentic cis-10-heptadecenoic acid (HDA) was obtained from Sigma (St. Louis, MO). All other chemicals used were obtained from Sigma or Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and were of High performance liquid chromatography (HPLC) or Liquid chromatography—mass spectrometry (LC-MS) grade.
The tomato fruit (eighth part of fruit, Momotaro variety), obtained from the local market, was first homogenized under liquid nitrogen or room temperature conditions, and then freeze-dried. The freeze-dried sample (100 mg) was homogenized in 1 mL of the extraction solvent (99.5% EtOH containing 1 μg/mL of HDA). After centrifugation (15,000 rpm, 10 min, 4 °C), the supernatant was collected. Using an additional 1 mL of the extraction solvent, the sample was re-extracted by the same procedure. The pooled extracts were filtered through a 0.2-μm-pore PVDF membrane (Whatman, Brentford, UK), and the filtrates were used in UPLC/TOF-MS analysis.
The contents of 9- and 13-oxo-ODAs in the tomato fruit were measured as previously described.Citation12) Briefly, LC-MS was performed using a Waters Acquity UPLC system coupled to a Xevo Quadrupole Time-of-Flight-MS system (Waters, Milford, MA), equipped with an electrospray source operating in the negative-ion mode with a lock-spray interface for accurate mass measurement. Leucine enkephalin was employed as the lock-mass compound. The data were acquired using the MassLynx software package (Waters). External mass calibration was performed following the manufacturer’s protocol. An aliquot of the extracted sample (3 μL) was injected into an Acquity UPLC BEH-C18 reversed-phase column (column size, 2.1 × 100 mm; particle size, 1.7 μm). Mobile phases A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile) were used. The column temperature was set at 40 °C. The buffer gradient consisted of 30–50% B for 0–4 min, 50–85% B for 4–14 min, and was held at 99% B for 14–17 min and returned to 30% B for 3 min before the next injection at a flow rate of 300 μL/min. The amounts of 9- and 13-oxo-ODAs were estimated from the calibration curves obtained using analytical-grade standards. The area of the peak at m/z [M − H] ± 0.05 Da was divided by the area of the internal standard, and the obtained value was used to generate the calibration curves.
Data are presented as the mean ± standard error of the mean (SEM). Differences between groups were compared with the Student’s t-test. Values of p < 0.05 were considered statistically significant.
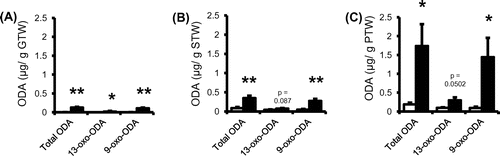
Quantitative analysis of 9- and 13-oxo-ODAs in each part of the tomato fruit homogenate under liquid nitrogen revealed that the total oxo-ODAs were primarily distributed in the peel and sarcocarp tissues (approximately 0.002 μg/g of gelatinous tissue weight, 0.1 μg/g of sarcocarp weight, and 0.2 μg/g of peel weight, Fig. white columns). Interestingly, we also found that the amount of the total oxo-ODAs increased markedly in the peel homogenate when kept for 30 min at 37 °C after room temperature homogenization (Fig. (C)).
Fig. 1. Contents of 9- and 13-oxo-ODAs in tomato fruit.
Notes: Quantitative analysis of 9- and 13-oxo-ODAs in the homogenates of (A) gelatinous, (B) sarcocarp, and (C) peel tissues. Data shown are the mean ± SEM (n = 6); *p < 0.05, and **p < 0.01 vs. control (extraction under liquid nitrogen conditions). □: homogenate under liquid nitrogen, ■: homogenate when kept for 30 min at 37 °C after room temperature homogenization. GTW: gelatinous tissue weight, STW: sarcocarp tissue weight, PTW: peel tissue weight.
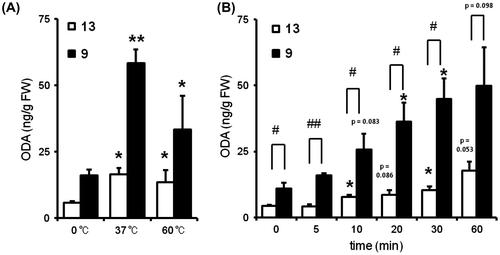
To elucidate the participation of heat treatment in the production of oxo-ODAs in the tomato fruit, we analyzed the contents of oxo-ODAs in the tomato-fruit homogenate incubated at 0, 37, and 60 °C for 30 min; the amounts of both 9- and 13-oxo-ODAs increased remarkably at 37 °C (Fig. (A)). Furthermore, both 9- and 13-oxo-ODAs in the homogenate sample incubated at 37 °C increased in a time-dependent manner (Fig. (B)). In the previous study, we demonstrated that authentic oxo-ODA is stable under thermal conditions.Citation12) The processing of tomatoes (e.g. juice) usually involves heat treatment. These findings suggest that the amount of oxo-ODAs in processed tomato products is increased by heat treatment.
Fig. 2. Contents of 9- and 13-oxo-ODAs in heat-treated tomato.
Notes: Quantitative analysis of 9- and 13-oxo-ODAs in heat-treated tomatoes in a (A) temperature and (B) time-dependent manner (temperature: 37 °C). Data shown are the mean ± SEM (n = 3); *p < 0.05, and **p < 0.01 vs. control (0 °C or 0 min); #p < 0.05, and ##p < 0.01 vs. 13-oxo-ODA. FW: fresh weight, 9: 9-oxo-ODA, 13: 13-oxo-ODA.
The compounds 9- and 13-oxo-ODAs are LIA derivatives. Recently, we reported an optimized LC-MS assay for the high-throughput and high-sensitivity analysis of 10 major long-chain FFAs (long-FFAs).Citation13) We applied this method to elucidate the profiles of LIA and other FFAs in the tomato fruit.
The long-FFA profiles in tomatoes were measured as previously described.Citation13) Briefly, analytical-grade myristic acid (MA), palmitic acid (PA), palmitoleic acid (POA), stearic acid (SA), oleic acid (OA), LIA, linolenic acid (LNA), arachidonic acid (AA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and ammonium formate were purchased for use as authentic samples from Sigma (St. Louis, MO, USA). The capillary, sampling cone, and extraction cone voltages were set at 2300, 40, and 1.5 V, respectively. The source and desolvation temperatures were 120 and 500 °C, respectively. The cone and desolvation gas-flow rates were set at 50 and 1000 L/h, respectively. Mobile phases A (90% acetonitrile, 10-mM ammonium formate, and 0.1% formic acid) and B (98% acetonitrile and 0.1% formic acid) were used. The buffer gradient consisted of 0.1% B for 0–5 min, 0.1–99.9% B for 5–6 min, 99.9% B for 6–11 min, 99.9 to 0.1% B for 11–12 min, and 0.1% B for 3 min before the next injection at a flow rate of 400 μL/min. The amounts of FFAs were estimated from the calibration curves obtained using analytical-grade standards.
The profile analysis of the long-FFAs revealed that PA and SA were the main FFA components in the homogenate of tomato fruit under both liquid nitrogen and room temperature conditions (Table (A), (B)). Both, total weight and composition of FFA varied markedly depending on the homogenizing temperature (Table (A), (B)). There is a possibility that this phenomenon participates in the activation of many enzymes. In the analysis of LIA, compared with the extraction under liquid nitrogen conditions, LIA tended to be abundant in the extraction of gelatinous tissue under room temperature conditions (approximately 6.2 and 23.8 μg/g of fresh weights, respectively, Table (A), (B)). In contrast, the amounts of LIA tended to be low in the sarcocarp and peel-tissue extractions under room temperature conditions (approximately 1.9 and 4.5 μg/g of fresh weights, respectively, Table (B)). It is suggested that LIA is metabolized to oxo-ODAs and other LIA derivatives. These data raise the possibility that the different localizations of FFAs, including LIA, influence the production of oxo-ODAs. In tomato fruit, fatty acids exist in free (FFA) or esterified form. The possible precursors of oxo-ODAs are not only FFA but also esterified fatty acid (EFA). It has been reported that EFAs in the soybean-seed homogenate are the substrates for lipoxygenases (LOXs).Citation14) Further examination is necessary to determine the precursors of oxo-ODAs.
Table 1. Quantitative analysis of FFAs in tomato fruit. (A) homogenate under liquid nitrogen. (B) homogenate when kept for 30 min at 37 °C at room temperature. (µg/g fresh weight).
There is a possibility that non-enzymatic (auto-oxidation reaction) and/or enzymatic reaction participates in the production of oxo-ODAs. In the enzymatic reaction, LOXs oxidize LIA at the C9 (9-LOX activity) or C13 (13-LOX activity) positions to produce 9- or 13-hydroperoxy octadecatrienoic acids,Citation15,16) which are possible precursors of 9- and 13-oxo-ODAs, respectively. The ratio of 9-oxo-ODA/13-oxo-ODA is much larger than 1 (Figs. and ). There is a possibility that LIA at the C9 is susceptible to oxidation under auto-oxidation reaction and/or that the activity of 9-LOX is higher than that of 13-LOX in the tomato fruit.
In this study, we revealed that oxo-ODAs were primarily distributed in the peel or sarcocarp tissues in freeze-dried tomato-fruit homogenates. In particular, oxo-ODAs markedly increased in the peel compared with other tissues by homogenization at room temperature. We also showed that the oxo-ODAs in the homogenate sample incubated at 37 °C increased in a time-dependent manner. These findings suggest that oxo-ODAs in processed tomato products are increased by heat treatment.
Acknowledgments
This work was supported in part by funding from the Research and Development Projects for Application in Promoting New Policies in Agriculture, Forestry, and Fisheries of Japan; by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [22228001]; and by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists [24521].
Notes
Abbreviations: FFA, free fatty acid; HAD, cis-10-heptadecenoic acid; HPOD, hydroperoxy octadecatrienoic acid; LIA, linoleic acid; LOX, lipoxygenase; long-FFA, long-chain free fatty acid; PPARα, peroxisome proliferator-activated receptor α; 9-oxo-ODA, 9-oxo-10,12-octadecadienoic acid; 13-oxo-ODA,13-oxo-9,11-octadecadienoic acid.
References
- Pandey DK, Shekelle R, Selwyn BJ, Tangney C, Stamler J. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The western electric study. Am. J. Epidemiol. 1995;142:1269–1278.
- Lazarus SA, Bowen K, Garg ML. Tomato juice and platelet aggregation in type 2 diabetes. JAMA-J. Am. Med. Assoc. 2004;292:805–806.10.1001/jama.292.7.805
- Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. JNCI J. Natl. Cancer Inst. 1999;91:317–331.10.1093/jnci/91.4.317
- Blum A, Monir M, Wirsansky I, Ben-Arzi S. The beneficial effects of tomatoes. Eur. J. Intern. Med. 2005;16:402–404.10.1016/j.ejim.2005.02.017
- Kim YI, Hirai S, Takahashi H, Goto T, Ohyane C, Tsugane T, Konishi C, Fujii T, Inai S, Iijima Y, Aoki K, Shibata D, Takahashi N, Kawada T. 9-oxo-10(E),12(E)-Octadecadienoic acid derived from tomato is a potent PPAR α agonist to decrease triglyceride accumulation in mouse primary hepatocytes. Mol. Nutr. Food Res. 2011;55:585–593.10.1002/mnfr.v55.4
- Kim YI, Hirai S, Goto T, Ohyane C, Takahashi H, Tsugane T, Konishi C, Fujii T, Inai S, Iijima Y, Aoki K, Shibata D, Takahashi N, Kawada T. Potent PPARα activator derived from tomato juice, 13-oxo-9,11-octadecadienoic acid, decreases plasma and hepatic triglyceride in obese diabetic mice. PLoS One. 2012;7:e31317.10.1371/journal.pone.0031317
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000;448:121–138.10.1016/S0027-5107(99)00231-6
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688.
- Chinetti G. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000;49:497–505.10.1007/s000110050622
- Goldenberg I, Benderly M, Goldbourt U. Update on the use of fibrates: focus on bezafibrate. Vasc. Health Risk Manag. 2008;4:131–141.10.2147/vhrm.2008.04.01.131
- Kim S, Shin HJ, Kim SY, Kim JH, Lee YS, Kim DH, Lee MO. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARα. Mol. Cell Endocrinol. 2004;220:51–58.10.1016/j.mce.2004.03.011
- Takahashi H, Kim YI, Hirai S, Goto T, Ohyane C, Tsugane T, Konishi C, Fujii T, Inai S, Iijima Y, Aoki K, Shibata D, Takahashi N, Kawada T. Comparative and stability analyses of 9- and 13-Oxo-octadecadienoic acids in various species of tomato. Biosci. Biotechnol. Biochem. 2011;75:1621–1624.10.1271/bbb.110254
- Takahashi H, Suzuki H, Suda K, Yamazaki Y, Takino A, Kim Y-I, Goto T, Iijima Y, Aoki K, Shibata D, Takahashi N, Kawada T. Long-chain free fatty acid profiling analysis by liquid chromatography–mass spectrometry in mouse treated with peroxisome proliferator-activated receptor α agonist. Biosci. Biotechnol. Biochem. 2013;77:2288–2293.10.1271/bbb.130572
- Pulvera ZM, Kitamura K, Hajika M, Shimada K, Matsui K. Oxylipin metabolism in soybean seeds containing different sets of lipoxygenase isozymes after homogenization. Biosci. Biotechnol. Biochem. 2006;70:2598–2603.10.1271/bbb.60121
- Kuo JM, Hwang A, Yeh DB, Pan MH, Tsai ML, Pan BS. Lipoxygenase from Banana Leaf: purification and characterization of an enzyme that catalyzes linoleic acid oxygenation at the 9-position. J. Agric. Food Chem. 2006;54:3151–3156.10.1021/jf060022q
- Mariutto M, Fauconnier ML, Ongena M, Laloux M, Wathelet JP, du Jardin P, Thonart P, Dommes J. Reprogramming of fatty acid and oxylipin synthesis in rhizobacteria-induced systemic resistance in tomato. Plant Mol. Biol. 2013;84:455–467.
