Abstract
Sublethal doses of surfactants as exemplified by NP-40 clearly induce premature senescence in normal human cells. To understand molecular basis for this phenomenon, we tried to suppress it with use of various inhibitors. An inhibitor of p38 of the MAPK family almost completely suppressed growth arrest and morphological changes induced by surfactants; however, other inhibitors tested had no effect. Oleic acid, a weak inducer of premature senescence, was found to suppress the effect of NP-40. Fluorescein-labeled oleic acid rapidly bound to the cell surface, and this binding was clearly blocked by pre-treatment with surfactants, suggesting that surfactants and oleic acid compete for binding to the cell surface. Moderate concentrations of cycloheximide, an inhibitor of protein synthesis, also suppressed the senescent features induced by NP-40. These results suggest that surfactants activate p38 signaling pathway by binding to the cell surface, and induce cellular senescence.
Graphical Abstract
Fluorescein-labeled oleic acid rapidly bound to the cell surface, and this binding was clearly blocked by pre-treatment with surfactants.
Normal human cells in culture gradually lose division potential, a phenomenon termed “replicative senescence.”Citation1) Normal cells also show a similar phenomenon, termed “premature senescence,” at any stages of culture by treatment with various agents such as reactive oxygen species, DNA damaging agents, lipids, cell cycle inhibitors, chromatin-destabilizing agents, and modulators of cell signalingCitation2,3) and cardiolipin.Citation4) Occasionally, tumor-derived immortal cell lines, which are thought to be escaped from senescence, show a senescence-like phenomenon by treatment with specific agents such as excess thymidine and 5-bromodeoxyuridine.Citation5–7)
Morphological alterations and retardation of DNA replication are always coupled with replicative senescence, premature senescence, and various forms of cellular senescence-like phenomena. These facts imply that cellular senescence is directly linked to morphological alterations due to delay in progression of S-phase of the cell cycle, a phenomenon historically known as “unbalanced growth.” In fact, the aforementioned inducers of premature senescence in normal human cells and those of senescence-like phenomena in immortal cell lines, all seem to delay DNA replication allowing for normal synthesis of RNA and protein. Under these conditions, impermeant and negatively charged RNA and proteins accumulate in cells, giving rise to osmotic pressure to the cells.Citation8) This generates influxes of water and/or permeant ions across the water-permeable plasma membrane, and results in cell swellingCitation9) and eventually nuclear swelling.Citation10)
Recently, we have found that sublethal doses of surfactants, as low as micro-molar levels, induce premature senescence in normal human cells.Citation11) Our results warn that dishwashers, body soaps, facial foams, and shampoos can be a risk factor for aging of the skin and scalp if they are not used properly.
In this study, we assessed molecular basis for the premature senescence induced by surfactants in normal human fibroblasts. We investigated three facets, namely a signaling pathway activated by surfactants, binding properties of surfactants to the cell surface, and suppression of unbalanced growth induced by surfactants.
Among signaling pathways involved in senescence, the MAPK cascades have major roles in responding to senescence inducers and arresting DNA replication. It is generally thought that ERK is involved in cellular proliferation or differentiation, and p38 and JNK are involved in apoptosis or cell cycle arrest although there are many exceptions. Upon addition of excess thymidine, ERK1/2 mediates senescence in normal human fibroblasts and HeLa cells.Citation10,Citation12,Citation13) H2O2 or oncogenic transfection phosphorylates p38 in WI-38 cells.Citation14) Also, some reports describe phosphorylation of MAPK by membrane damage.Citation15–19) Prolonged activation of MAPK cascades in response to long-term cellular damages can arrest DNA replication and cell cycle progression.
To date, several reports describe the mode of binding of surfactants to the cell surface. EGFR and ERK1/2 are phosphorylated by addition of oleic acid to human endothelial ECV-304 cells.Citation20) Mitochondrial oxidative stress is decreased by oleic acid via EGFR-dependent activation of glutathione peroxidase.Citation21) Polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid phosphorylate EGFR and p38, resulting in apoptosis in MDA-MB-231 human breast cancer cells.Citation22)
Materials and methods
Materials
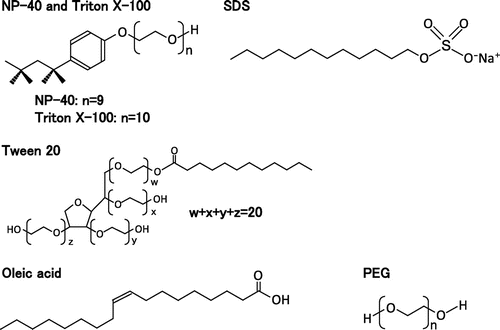
U0126, SP600125, Oleic acid, and fluoresceinamine were purchased from Sigma (Saint Louis, USA). SB203580 was purchased from LC Laboratories (Woburn, USA). NP-40 [Polyoxyethylene (9) octylphenyl ether], Cycloheximide, Sodium oleate, Triton X-100 [Polyoxyethylene (10) octylphenyl ether], SDS, and Wakopak NAVI C18 5 μm column were purchased from Wako (Osaka, Japan). Tween 20, Tween 80 (Fig. ), and HPLC grade acetonitrile were purchased from Kanto Chemical (Tokyo, Japan). Anti-p38 (pT180/pY182), anti-ERK1/2 (pT202/pY204), anti-ERK1/2, and anti-JNK (pT183/pY185) antibodies were purchased from Cell signaling (Danvers, USA). Anti-p38 α/SAPK2α and anti-β-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Anti-pan-JNK/SAPK1 was purchased from BD (Franklin Lakes, USA). Dishwashing detergents, shampoos, and facial cleansing foams were purchased from shops.
Cell culture
Normal human fibroblasts (TIG-7) and cervical tumor cells (HeLa) were obtained from Japanese Collection of Research Bioresources. Human cervical keratinocyte (CK) cells were described previously.Citation23) Human breast keratinocyte (BK) cells and human follicle dermal papilla cell (FDPC) cells were obtained from TOYOBO, Osaka. Immortalized human keratinocytes (HaCaT) were obtained from Cell Lines Service, Eppelheim, Germany.
TIG-7 cells and HaCaT cells were cultured at 37 °C in plastic Petri dishes (Nunc) containing Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum under 5% CO2 and 95% humidity as described previously.Citation7) HeLa cells were cultured in ES medium supplemented with 5% calf serum. CK and BK cells were cultured in collagen type I-coated plastic Petri dishes (AGC TECHNO GLASS, Shizuoka) containing mixture of MCDB153 medium (Cosmo Bio, Tokyo) and Serum Free Medium for human keratinocyte KJB-200 (DS Pharma Biomedical, Osaka) (2:1). FDPC cells were cultured in collagen-coated dishes containing PCGM medium (TOYOBO, Osaka).
Senescence-associated β-galactosidase assay
Assay was performed as described previously.Citation7) Cells were fixed in 2% formaldehyde/0.2% glutaraldehyde at room temperature for 5 min, and incubated at 37 °C with a fresh staining solution [1 mg/mL of 5-bromo-4-chloro-3-indolyl ß-D-galactoside, 40 mM citric acid-sodium phosphate (pH 6.0), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 150 mM NaCl, and 2 mM MgCl2].
Colony forming assay
TIG-7 cells (1000 cells) were plated in 60 mm plastic Petri dishes (Nunc) containing DMEM supplemented with 10% fetal calf serum and added with agents on the next day. The cells were cultured with agents for one week and then cultured for one or two additional weeks in normal growth medium. Cells were replenished with the same fresh medium for every 3 or 4 days. Colonies formed were stained with Coomassie Brilliant Blue R-250 (CBB).
Western blot analysis
Cells were suspended in a lysis buffer [40 mM Tris–HCl, pH 7.5, 1 mM EDTA, pH 7.5, 10 μM Na3VO4, 100 nM Okadaic acid, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL each of leupeptin and aprotinin, 10 mM DTT, and 5% CHAPS], and disrupted by sonication for a total of 8 s on ice. After centrifugation of the cell lysates at 15,000 rpm at 4 °C for 20 min, the resulting supernatants were used as cell extracts. The cell extracts were subjected to 10% SDS–polyacrylamide gel electrophoresis, blotted onto a PVDF-membrane and probed with specific antibodies against ERK1/2, pERK1/2 (pT202/pY204), p38a/SAPK2a, pp38 (pT180/pY182), pan-JNK/SAPK1, pJNK (pT183/pY185), or β-tubulin. The specific mouse or rabbit antibodies were detected with a chemiluminescence detection kit (GE Healthcare). Protein concentrations were determined with Bio-Rad Protein Assay Kit (Bio-Rad).Citation7)
Synthesis of fluorescein-labeled oleic acid
A solution of 380 μL of 5 mg fluoresceinamine/mL acetonitrile was mixed with 140 μL of 10 mg oleic acid/mL acetonitrile, 70 μL of 20 mg 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride/mL acetonitrile, and 410 μL of acetonitrile. This mixture was incubated at room temperature for 24 h,Citation24) and the resultant fluorescein-labeled oleic acid was purified by HPLC. The reaction mixture (400 μL) was loaded with a Rheodyne loop injector onto a Wakopak NAVI C18 5 μm column (250 × 4.6 mm internal diameter). The column was eluted with a linear gradient of 0–100% acetonitrile/water over 30 min and then 100–100% acetonitrile over additional 60 min at a flow rate of 0.5 mL/min. Elution was monitored with a UV detector (210 nm). The fractions containing fluorescein-labeled oleic acid eluted after 50 min were collected. The labeled oleic acid was diluted with acetonitrile and used in the competition experiments described below.
Competition experiments
Cells were collected by trypsinization, washed with serum-free medium, and 106 cells were suspended in 1 mL of the same medium. The cell suspension was added with a test surfactant for 5 min, and then added with 5 μL of fluorescein-labeled oleic acid solution for additional 5 min. The cells were pelleted down by centrifugation (300 × g for 1 min), washed with PBS, and resuspended in 200 μL of PBS. The cells were placed onto a haemocytometer, and observed under a fluorescence microscope to take photographs.
Results
Morphology and senescence-associated β-galactosidase assay
Various cell types were cultured in the presence of sublethal concentrations of genuine surfactants (NP-40, Triton X-100, SDS, Tween 20, etc) and commercially available products (dishwasher, shampoo, or facial cleansing foam) for 1–2 weeks. Normal type of cells examined, namely normal human fibroblasts (TIG-7), CK, BK, and FDPC, and all exhibited typical senescence features, flat and enlarged cell shape, and were clearly stained with the senescence-associated β-galactosidase. All of the surfactants behaved similarly, although their effective concentrations differed depending on their solubilization powers. As representatives of surfactants, the effects of NP-40 and Triton X-100 were shown (Fig. (A)).
Fig. 2. Effects of surfactants on morphology and senescence-associated β-galactosidase in various cell types.
Notes: Proliferating cells indicated were cultured for three weeks in the presence of NP-40 (TIG-7, 5 μg/mL; CK, 2 μg/mL; BK, 2 μg/mL; FDPC, 1 μg/mL; HaCaT, 10 μg/mL; HeLa, 5 μg/mL), or Triton X-100 (TIG-7, 10 μg/mL; CK, 5 μg/mL; BK, 2 μg/mL; FDPC, 5 μg/mL; HaCaT, 10 μg/mL; HeLa, 10 μg/mL) and stained with senescence-associated β-galactosidase as described in Materials and Methods.
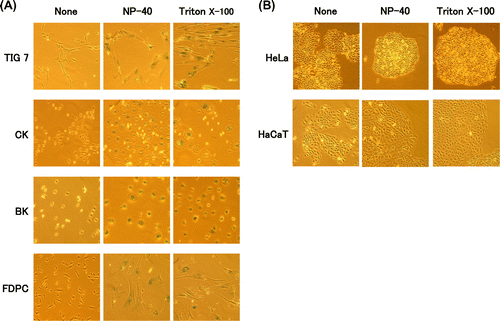
In sharp contrast, immortalized human keratinocyte (HaCaT) and cervical epithelial tumor cells (HeLa) did not show senescence features under any conditions tested (Fig. (B)). They shrank or lysed at higher concentrations of surfactants. Therefore, induction of the senescence features by surfactants is specific to normal types of cells.
Effects of oleic acid
We examined some properties of three compounds– oleic acid, benzoic acid, and polyethylene glycol. These compounds correspond to one of the three molecular moieties of NP-40 (alkyl chain, benzene ring, and polyoxyethylene chain), respectively. Among them, oleic acid weakly induced the senescence features in TIG-7 cells. At higher concentrations, it completely induced the senescence features (Fig. (A)). Benzoic acid and polyethylene glycol were unable to induce any senescence features.
Fig. 3. Suppression of the cytotoxicity of NP-40 by oleic acid in TIG-7 cells.
Notes: (A) Effect of oleic acid on morphology and senescence-associated β-galactosidase as observed as in Fig. . (B) Cells were cultured in the presence of NP-40 with or without oleic acid for 2 weeks to yield photographs. (C) Colonies formed in (B) were stained with CBB.
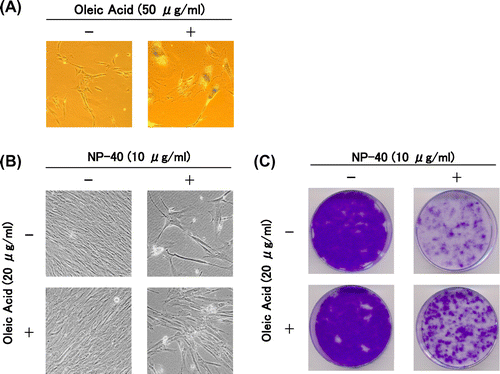
Then we examined how oleic acid, benzoic acid, and polyethylene glycol affect the senescence features induced by NP-40 in TIG-7 cells. Oleic acid partially suppressed the effect of NP-40 to induce the senescence features (Fig. (B) and (C)). The colony forming assay was performed in the presence of oleic acid (10 or 20 μg/mL) with NP-40. Oleic acid inhibited NP-40 in a dose-dependent manner (Yamakami Y et al., unpublished results). Benzoic acid increased the cytotoxicity of NP-40, and PEG had no effect (Yamakami Y et al., unpublished results). From these data, it is suggested that oleic acid and NP-40 bind to a common cell surface structure and oleic acid, a less cytotoxic compound, and NP-40, a strongly active compound, compete for binding to such structures.
Competition between oleic acid and surfactants for binding to the cell surface
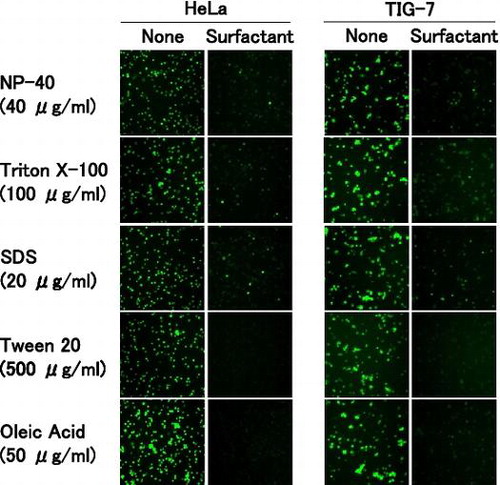
To examine competitive binding of oleic acid and surfactants to the cell surface, we synthesized fluorescein-labeled oleic acid and used it for subsequent experiments. The labeled oleic acid quickly bound to the entire region of the cell surface in both TIG-7 and HeLa cells. When the cells were pre-treated with surfactants, the labeled oleic acid was unable to bind to the cell surface in the two cell types (Fig. ). All of the surfactants examined similarly blocked binding of the labeled oleic acid. Especially, pre-treatment with high concentrations of Tween 20 or oleic acid completely blocked its binding. NP-40 or Triton X-100 (HeLa cells) and NP-40 or SDS (TIG-7 cells) inhibited oleic acid in a dose-dependent manner (Yamakami Y et al., unpublished results).These results demonstrate that the surfactants and oleic acid compete for binding to the cell surface.
Fig. 4. Competition between oleic acid and surfactants for binding to the cell surface.
Notes: HeLa and TIG-7 cells were pre-incubated with the surfactant indicated, added with fluorescein-labeled oleic acid, and observed under a fluorescence microscope as described in Materials and Methods.
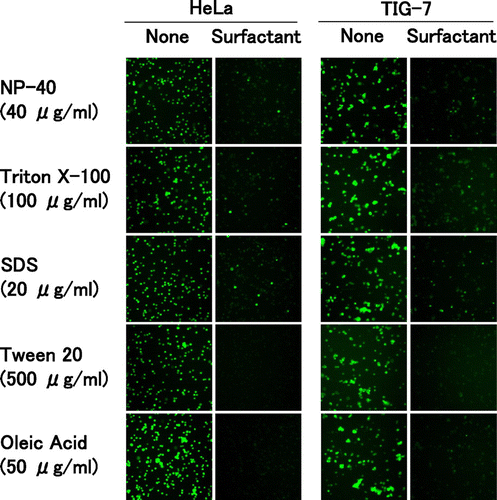
HeLa cells behaved similarly to TIG-7 cells as regards the binding of the labeled oleic acid to the cell surface. Therefore, signaling pathways downstream of the binding of the surfactants may be different from normal types of cells.
Signal pathways induced by surfactants
We added various inhibitors of signal pathways, e.g. the MAPK family, checkpoint kinase 1 and 2, PKC δ, and PI3 K, to TIG-7 cells during culture with NP-40, and investigated which inhibitor suppresses the senescence induced by NP-40. For inhibitors of the MAPK family, we used U0126 (MEK inhibitor, an upstream kinase of ERK1/2), SB203580 (p38 inhibitor), and SP600125 (JNK inhibitor).
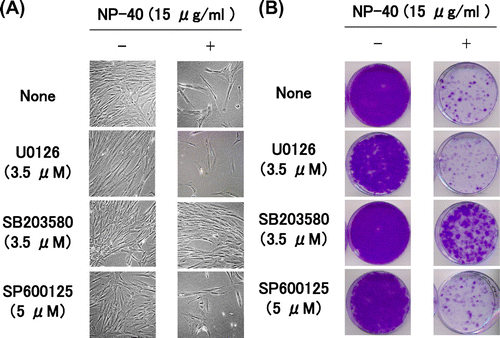
TIG-7 cells were cultured in the presence of NP-40 with and without each kinase inhibitor for 1 week, and then allowed to form colonies in normal growth medium for 1–2 weeks. Among the above inhibitors, only p38 inhibitor SB203580 clearly suppressed the senescence induced by NP-40 (Fig. ). It suppressed the senescence induced by oleic acid or SDS as well as NP-40 (Yamakami Y et al., unpublished results).
Fig. 5. Suppression of the cytotoxicity of NP-40 by MAPK inhibitors in TIG-7 cells.
Notes: (A) Cells were cultured in the presence and absence of NP-40 together with the inhibitor indicated for 1 week to yield photographs. (B) Cells cultured as in (A) were replenished with normal growth medium and cultured for additional 2 weeks to form colonies.
We investigated by Western blot analysis whether MAPKs are actually phosphorylated by NP-40 in TIG-7 cells. ERK1/2 was significantly phosphorylated, p38 was strongly phosphorylated, and JNK1 was extremely phosphorylated during treatment with NP-40 after 1 day and after 7 days, reaching 3.2, 4.1 (ERK1/2), 5.4, 7.4 (p38), and 11, 21 (JNK1) folds above the control level after 1 day and after 7 days, respectively (Fig. ). However, they were rather inactivated after 3 days.
Fig. 6. Western blot analysis of MAPKs.
Notes: TIG-7 cells were cultured in the presence of 15 μg/mL NP-40 with or without 3.5 μM SB203580. At intervals, the cells were harvested and subjected to Western blot analysis with the specific antibody indicated as described in Materials and Methods. (A) Chemiluminescence was detected by a Chemiluminescence Analyzer. (B) Chemiluminescence signals were quantitated by the Software Quantity One, normalized with that of β-tubulin, and expressed relative to controls.
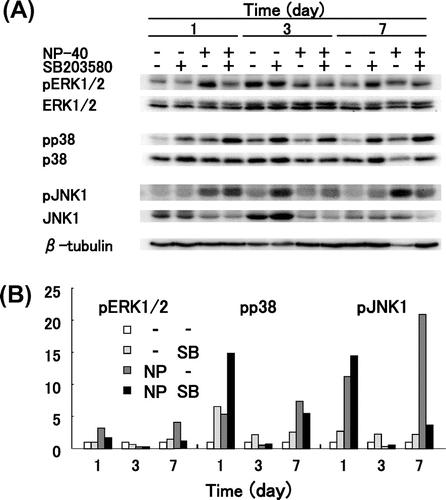
Addition of SB203580 further increased the phosphorylated p38 and JNK1 but not ERK1/2 levels after 1 day and after 3 days. It also increased the phosphorylated ERK1/2, p38, and JNK1 levels after 7 days without NP-40 but not with NP-40. SB203580 was reported to compete with ATP for binding to p38 and inhibit its activity but does not decrease or slightly increases the phosphorylation of p38.Citation25) Therefore, it does not mean failure that the pp38 levels of some samples in Fig. (B) were not decreased or increased by SB203580. Fluctuation of phosphorylated MAPK levels during treatment with NP-40 is not known not due to an experimental error because oleic acid and Tween 20 phosphorylated MAPKs similarly to NP-40. These results suggest that MAPKs are phosphorylated by treating cells with NP-40. Although the activation pattern was different depending on MAPKs, all MAPKs were phosphorylated by treating cells with NP-40.
Suppression of senescence features induced by surfactants
We examined whether inhibitors of the MAPK family can suppress the senescence features induced by surfactants in TIG-7 cells as mentioned above. SB203580 allowed the cells to continuously grow in the presence of NP-40 and kept the cell shape almost normal (Fig. ). The colony-forming assay was performed in the presence of SB203580 (1.75 or 3.5 μM) with NP-40. SB203580 inhibited NP-40 in a dose-dependent manner (Yamakami Y et al., unpublished results). U0126 and SP600125 had no effect. These results show that p38 mediates the signaling pathway induced by surfactants to lead to senescence.
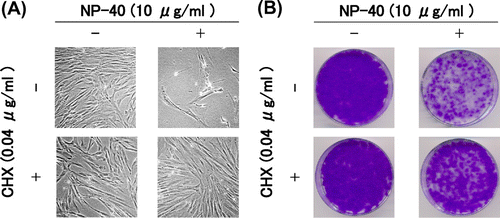
Then, we examined the effects of CHX on TIG-7 cells cultured in the presence of NP-40. Limitation of protein synthesis by modest concentrations of CHX is shown to prevent unbalanced growth and suppress senescence features induced by excess thymidine in various cell types.Citation10,12,13) When CHX was added to TIG-7 cells with NP-40 for 1 week and continued to culture in normal growth medium for additional 2 weeks to form colonies, colony-forming ability of the cells did not decrease significantly (Fig. (B)). The appearance of the cells cultured with NP-40 and CHX was kept normal (Fig. (A)). These results suggest that the cellular senescence induced by surfactants is due to unbalanced growth.
Fig. 7. Suppression of the cytotoxicity of NP-40 by CHX in TIG-7 cells.
Notes: (A) Cells were cultured in the presence and absence of NP-40 with or without CHX for 1 week to yield photographs. (B) Cells cultured as in (A) were replenished with normal growth medium and cultured for additional 2 weeks to form colonies.
Discussion
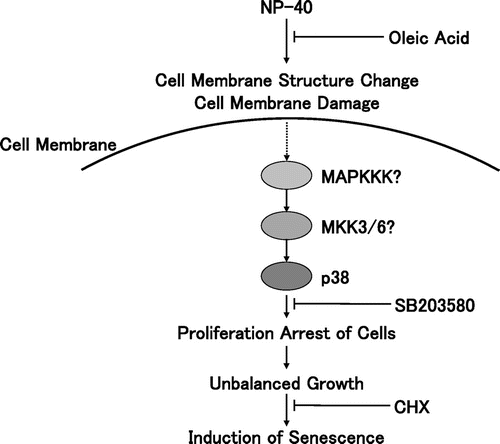
All of the synthetic surfactants and commercial products examined are found to induce cellular senescence at low concentrations in normal types of human cells. In contrast, they showed only cytotoxicity against immortal or tumor cells. These cells often have a defect in cell cycle checkpoint or stress response mechanisms. Therefore, surfactants may act through a stress response pathway working in normal cells. If so, the surfactants can constitute a new class of inducers of cellular senescence although their structures differ significantly (Fig. ).
Many surfactants used in this study have polyoxyethylene chains in their molecules. It is well known that polyethylene glycols, which are used for various biological experiments, contain polyoxyethylene chains. The polyoxyethylene chains were shown to induce cellular senescence more easily than other kinds of surfactants. This suggests that polyoxyethylene chains interact with the cell membrane with a high affinity. A target of surfactants during cell lysis is suggested to be the cell membrane.Citation26) It is therefore interesting to reveal a cell surface target of surfactants, which may activate a stress-activated signaling pathway leading cellular senescence.
Among the various inhibitors tested, only the inhibitor of p38 was found to suppress the senescence induced by surfactants. Although JNK1 was strongly phosphorylated, an inhibitor of JNK did not suppress the senescence. These results suggest that the p38 pathway was shown to mediate the stress induced by surfactants. In our previous studies, it has been found that ERK1/2 is phosphorylated and involved in the cellular senescence induced by excess thymidine in TIG-7 cells.Citation12,13) Therefore, different members of the MAPK family are phosphorylated to arrest DNA replication to induce senescence in the same cells.
In our binding competition assays, pre-incubation of cells with surfactants almost completely eliminated the binding of fluorescein-conjugated oleic acid. Tween 20 most clearly competed out the binding of the fluorescein-labeled oleic acid to the cell surface among the surfactants used probably due to its lowest cytotoxic effect. Oleic acid also suppressed the capacity of surfactants to induce senescence in the colony-forming assays. These results suggest that surfactants compete with oleic acid for binding to the cell surface. The various surfactants and oleic acid differ in their structures but similarly activate the p38 pathway and compete with each other for binding to the cell surface. Therefore, hydrophobic chains of the surfactants seem to be main structures responsible for their biological effects reported here.
As regards the binding assay of the fluorescein-labeled oleic acid, a question may arise that surfactants simply washed out the labeled oleic acid bound to the cells. The labeled oleic acid did not bind to the cell surface already bound to oleic acid, and also not to the hydrophobic surface of plastic Petri dishes. This implies that the labeled compound binds to the cell surface thorough specific interaction. Usually more than 0.1% of detergent is necessary for breaking hydrophobic interactions, while much lower concentrations can suppress interaction between the labeled oleic acid and the cell surface. Therefore, surfactants used here are thought to act as a ligand but not as a detergent. Low doses of surfactants may perturb the cell surface structures or alter conformation of receptors. Consequently, receptors such as those for epidermal growth factor (EGF) and cytokines could be activated as reported in the case of UVB radiation or osmotic stress.Citation15) In support of this, fatty acids are reported to phosphorylate EGF receptor and MAPK.Citation20,22) It may be also possible that surfactants activate their downstream signal pathway as well.
Alternatively, surfactants may simply cause membrane damages. UV irradiation or osmotic stress are shown to change the structure of the cell membrane, inducing clustering, internalization, and activation of cell surface receptors for EGF and cytokines, followed by strong phosphorylation of JNK and p38.Citation15–18) Pore-forming toxins are shown to induce efflux of potassium ion followed by phosphorylation of p38 and ERK but not JNK to restore plasma membrane integrity and ion homeostasis.Citation19) The MAPK phosphorylation induced by surfactants can lead to retardation of DNA replication causing premature senescence.
Finally, surfactants penetrate the skin rather easily than other chemicals. This suggests that low doses of surfactants cause deleterious effects to cells through a novel mechanism. Excess or insufficient use of shampoos may cause residual contamination of surfactants in the hair follicles, leading hair loss due to damages to hair follicle cells. The same may be true in epidermal cells when we use cosmetics improperly.
Notes
Abbreviations: MAPK, mitogen-activated protein kinase; MKK6, mitogen-activated protein kinase kinase 6; ERK, extracellular signal regulated kinase; JNK, mitogen-activated protein kinase 8; MEK, mitogen-activated protein kinase kinase; SAPK, stress-activated protein kinase; EGFR, epidermal growth factor receptor; CHX, Cycloheximide; DMEM, Dulbecco’s modified Eagle’s medium; CK, human cervical keratinocyte; BK, human breast keratinocyte; FDPC, human follicle dermal papilla cell.
References
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621.10.1016/0014-4827(61)90192-6
- Young J, Smith JR. Epigenetic aspects of cellular senescence. Exp. Gerontol. 2000;35:23–32.10.1016/S0531-5565(99)00076-5
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602.10.1016/S0092-8674(00)81902-9
- Arivazhagan P, Mizutani E, Fujii M, Ayusawa D. Cardiolipin induces premature senescence in normal human fibroblasts. Biochem. Biophys. Res. Commun. 2004;323:739–742.10.1016/j.bbrc.2004.08.177
- Thelander L, Reichard P. Reduction of ribonucleotides. Annu. Rev. Biochem. 1979;48:133–158.10.1146/annurev.bi.48.070179.001025
- Ayusawa D, Iwata K, Seno T. Alteration of ribonucleotide reductase in aphidicolin-resistant mutants of mouse FM3A cells with associated resistance to arabinosyladenine and arabinosylcytosine. Somatic Cell Genet. 1981;7:27–42.10.1007/BF01544746
- Michishita E, Nakabayashi K, Suzuki T, Kaul SC, Ogino H, Fujii M, Mitsui Y, Ayusawa D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J. Biochem. 1999;126:1052–1059.
- Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. Int. Rev. Cytol. 1995;161:173–262.10.1016/S0074-7696(08)62498-5
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740.
- Kobayashi Y, Sakemura R, Kumagai A, Sumikawa E, Fujii M, Ayusawa D. Nuclear swelling occurs during premature senescence mediated by MAP kinases in normal human fibroblasts. Biosci. Biotechnol. Biochem. 2008;72:1122–1125.10.1271/bbb.70760
- Yamakami Y, Miki K, Endoh M, Yonekura R, Ukekawa R, Kobayashi Y, Fujii M, Ayusawa D. Sublethal doses of surfactants induce premature senescence in normal human skin cells. Biosci. Biotechnol. Biochem. 2011;75:1395–1398.10.1271/bbb.110179
- Sumikawa E, Matsumoto Y, Sakemura R, Fujii M, Ayusawa D. Prolonged unbalanced growth induces cellular senescence markers linked with mechano transduction in normal and tumor cells. Biochem. Biophys. Res. Commun. 2005;335:558–565.10.1016/j.bbrc.2005.07.106
- Kobayashi Y, Lee SS, Arai R, Miki K, Fujii M, Ayusawa D. ERK1/2 mediates unbalanced growth leading to senescence induced by excess thymidine in human cells. Biochem. Biophys. Res. Commun. 2012;425:897–901.10.1016/j.bbrc.2012.08.006
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144.10.1046/j.1365-2443.2003.00620.x
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197.10.1126/science.274.5290.1194
- Decraene D, Smaers K, Gan D, Mammone T, Matsui M, Maes D, Declercq L, Garmyn M. A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytes. J. Invest. Dermatol. 2004;122:484–491.10.1046/j.0022-202X.2004.22215.x
- Peus D, Meves A, Vasa RA, Beyerle A, O’Brien T, Pittelkow MR. H2O2 is required for UVB-induced EGF receptor and downstream signaling pathway activation. Free Radic. Biol. Med. 1999a;27:1197–1202.10.1016/S0891-5849(99)00198-7
- Peus D, Vasa RA, Beyerle A, Meves A, Krautmacher C, Pittelkow MR. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J. Invest. Dermatol. 1999b;112:751–756.10.1046/j.1523-1747.1999.00584.x
- Gonzalez MR, Bischofberger M, Frêche B, Ho S, Parton RG, van der Goot FG. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol. 2011;13:1026–1043.10.1111/cmi.2011.13.issue-7
- Vacaresse N, Lajoie-Mazenc I, Augé N, Suc I, Frisach MF, Salvayre R, Nègre-Salvayre A. Activation of epithelial growth factor receptor pathway by unsaturated fatty acids. Circ. Res. 1999;85:892–899.10.1161/01.RES.85.10.892
- Duval C, Augé N, Frisach MF, Casteilla L, Salvayre R, Nègre-Salvayre A. Mitochondrial oxidative stress is modulated by oleic acid via an epidermal growth factor receptor-dependent activation of glutathione peroxidase. Biochem. J. 2002;367:889–894.10.1042/BJ20020625
- Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007;137:548–553.
- Kikuchi K, Yasumoto S. Retention of cell adhesion and growth capability in human cervical cancer cells deprived of cell anchorage. Jpn. J. Cancer Res. 1999;90:867–873.10.1111/cas.1999.90.issue-8
- Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, Kawashima Y. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 2002;19:132–139.10.1023/A:1014260513728
- Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 1999;263:825–831.10.1006/bbrc.1999.1454
- Aránzazu PM, Ostolaza H, Goñi FM, Barberá GE. Surfactant-induced cell toxicity and cell lysis. A study using B16 melanoma cells. Biochem. Pharmacol. 1990;40:1323–1328.