Abstract
Grifola frondosa (GF), distributed widely in far east Asia including Korea, is popularly used as traditional medicines and health supplementary foods, especially for enhancing the immune functions of the body. To extend the application of GF polysaccharides (GFP) for atopic dermatitis (AD), we investigated the effects of GFP on the 2,4-dinitrochlorobenzene-induced AD-like skin lesion in NC/Nga mice. GFP treatment significantly reduced the dorsa skin dermatitis score and combination treatment with GFP, and dexamethasone has a synergistic effect in AD-like skin lesion by reduced Serum IgE, mast cells infiltration, and cytokines expression. These results indicate that GFP suppressed the AD-like skin lesions by controlling the Th-1/Th-2-type cytokines in NC/Nga mice. These findings strongly suggest that GFP can be useful for AD patients as a novel therapeutic agent and might be used for corticosteroids replacement or supplement agent.
Graphical Abstract
This study suggests that GFP modulates the Th-1/Th-2 response and mast cell infiltration is a major mechanism of the AD like skin lesion in NC/Nga mice.
Recently, atopic dermatitis (AD) became a major health topic for the young age children, worldwide. In recent years, 10–20% of children and 1–2% of adults suffer from AD.Citation1,2) AD is a chronically relapsing inflammatory skin disease, accompanied by erythematous skin, severe itching, and lichenification with cutaneous hypersensitivity.Citation3) AD is a multifactorial skin disease, with complex interactions of innate and adaptive immune responses based on a strong genetic predisposition triggered by various environmental and psychological factors, allergen factors, like dust mites, and pharmacological factors.Citation4,5) Inappropriate treatment of AD can lead to severe after effect throughout a lifetime, such as lack of self-confidence and physiological problems.
For typical therapeutic trials to moderate AD, topical corticosteroids have been the standard treatment of choice for acute outbreaks of AD, but prolonged application of high dose can be associated with atrophogenic side effects. An alternative, steroid-free, immune-modulatory agent, like the medicinal plant, including edible mushrooms that could provide a safe and effective long-term treatment for AD patients, would add to the treatment options for this complicated disease.Citation6−Citation8)
Grifola frondosa (GF) is a Basidiomycete fungus that belongs to the order Aphyllopherales and family Polyporeceae. GF has been recognized as an edible mushroom and enhanced immune function in the eastern Asia. Previous studies demonstrated that GF has ameliorated hypertension,Citation9) decreased cholesterol level from the serumCitation10) and prevented cardiovascular disease.Citation11) A major component of GF is polysaccharides, which are distributed in mushroom, and have been reported to possess beneficial effects such as antitumor and immunoregulatory effects. Moreover, recent studies have reported that β-Glucan and mannan, which are the most abundant components of GF, are strongly correlated with their immunomodulation effects. These polysaccharides are recognized by TLRs and mannose receptors, and activate antigen-presenting cells.Citation12) What is interesting is that GF enhances the immune system via oral administration, same as an intra-peritoneal injection.Citation13) A phase I/II trial using GF polysaccharides (GFP) extract affected the host immune system without dose-limiting toxicity.Citation14) An oral administration of GFP may be a useful dietary supplement for healthy individuals to modulate an immune response.
In this study, we investigated how an oral administration of GFP inhibits AD-like skin lesion. In addition, we evaluated the efficacy of GFP and combination effect of glucocorticoid in AD-like skin lesion.
Materials and methods
Chemicals
2,4-dinitrochlorobenzene (DNCB) and histological analysis materials were obtained from Sigma-Aldrich (Milwaukee, WI, USA). Enzyme-linked immunosorbent assay (ELISA) kits for immunoglobulin (Ig)E, interleukin (IL)-1β,-4,-6, and TNF-α were purchased from Shibayagi (Shibayagi co., Ltd, Gunma, Japan) and Enzo Lifescience. All chemicals were of the highest purity grade.
Preparation of polysaccharides from Grifola frondosa
Briefly, 1000 g of GF fruiting body was purchased from Animal Bio Resources Bank affiliated in the Korean National Animal Resources Research Center. The clean, washed fruiting body of GF was extracted with 5000 mL double distilled water at 110 °C pressurization condition for 4 h in a reflux apparatus. After filtration with perlite-coating filter, an extract was concentrated with a concentrator, and sterilized for 30 min at 90 °C. The sterilized extract was frozen at −70 °C for three days and lyophilized until fully dried (Samwon Freezing Engineering, Korea). The final yield from fruiting bodies was 76.75 g. The dried extract yield from crude materials was approximately 2.4% (w/w).
Determination of β-glucan concentration in polysaccharides
The content of β-glucan in the polysaccharides was quantitatively determined with a β-glucan assay kit for mushrooms (Megazyme, Bray, Ireland) according to the manufacturer’s instruction. Briefly, for the determination of total (α- and β-) glucan content, dried extract from the mushrooms fruiting bodies were hydrolyzed with 7 M HCl at 30 °C for 45 min. Then, 10 mL of water was added and incubated at 100 °C for 2 h. After being neutralized with 2 M KOH, an aliquot was dissolved with exo-1,3-β glucanase (20 U/mL) plus β-glucosidase (4 U/mL) in 200 mM sodium acetate buffer (pH 5.0). The hydrolysates were incubated with a mixture of glucose oxidase and peroxidase at 40 °C for 1 h. The absorbance of the mixture was measured at 510 nm. For determining the α-glucan (phytoglycogen and starch) content, the extracts were dissolved in 2 M KOH, hydrolyzed, and treated with 1.2 M sodium acetate buffer (pH 3.8). Amyloglucosidase (1630 U/mL) and invertase (500 U/mL) were added to the solution and the mixture incubated at 40 °C for 30 min. The aliquot was incubated with a mixture of glucose oxidase and peroxidase at 40 °C for 20 min. The absorbance of the solution was measured at 510 nm. The content of β-glucan was determined by subtracting α-glucan from total glucan (Table ).
Table 1. Total glucan, α-Glucan, and β-Glucan contents of a range of GFP.
Animals
Female NC/Nga mice (15–18 g body weight, four weeks of age) were purchased from SLC, Inc. (Shizuoka, Japan). Animals were housed in specific pathogen-free conditions in a controlled room with 12/12 h light and dark cycle for two weeks before the start of experiments. Mice pellet food was provided ad libitum. After two weeks, the mice were randomly divided into seven groups (n = 3 per group). All animal experimental study and protocols were approved and followed by the Animal Ethics Committee, Gyeong Sang National University (Approved Number: GNU-130118-M0005).
Induction of AD and oral administration of GFP
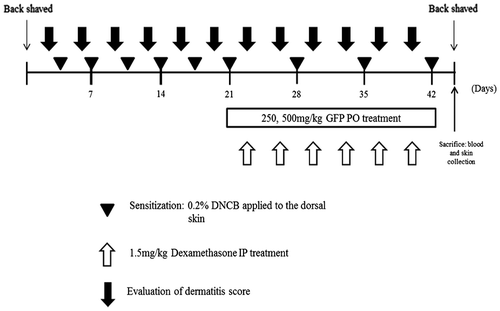
To induce AD-like skin lesions, DNCB was applied to the dorsal skin lesions. After completely shaving the dorsal hair of approximately 8 cm2, a 200 μL of 0.2% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) was spread two times a week for three weeks. Once in a week, we shaved DNCB applied area with razor. After inducing AD, the seven groups of mice (two control groups and five test groups) were treated three times a week for four weeks as follows:
Negative control (NC) group – only vehicle (3:1 mixture of acetone and olive oil) treatment.
Positive control (PC) group – application of 0.2% DNCB.
250 mg/kg GFP treatment group (GFP1) – oral administration of GFP (normal saline) based on the body weight.
500 mg/kg GFP treatment group (GFP2)–oral administration of GFP (normal saline) based on the body weight.
Dexamethasone group (Dex) – 1.5 mg/kg Dexamethasone IP injection three times a week.
Combination group 1 (C1) – 1.5 mg/kg Dexamethasone IP injection and 250 mg/kg GFP (normal saline) treatment group.
Combination group 2 (C2) – 1.5 mg/kg Dexamethasone IP injection and 500 mg/kg GFP (normal saline) treatment group.
Animals were sacrificed 43 days after the first application of DNCB (Fig. ). Whole blood was collected from the vena cava, and the dorsal skin tissues were removed and subjected to histopathological analysis.
Evaluation of dermatitis lesion
The severity of dermatitis was evaluated using the method described by Yamamoto et al.Citation15) The severity of dermatitis is evaluated three times a week. The development of erythema/hemorrhage, scarring/dryness, edema, and excoriation/erosion was scored as 0 (none), 1 (mild), 2 (moderate), and 3 (severe). The sum of the individual list scores was taken as the dermatitis score.
Measurement of IgE concentration in serum
Mice serum samples were obtained from whole blood, and levels of IgE in the serum were measured using an IgE ELISA kit for mice (Shibayagi co., Ltd, Gunma, Japan), according to the manufacturer’s protocols. IgE concentrations were calculated using a linear regression equation obtained from the standard absorbance values.
Measurement of cytokines production
The cytokine levels were determined using ELISA. Mice dorsal skin samples (100 mg) of the AD region were homogenized in 1 mL of T-PER tissue protein extraction buffer (Pierce, Rockford, IL, USA) containing protease inhibitor cocktail. Homogenized samples were centrifuged at 15,000 × g for 30 min at 4 °C and the supernatant was collected. The amount of IL-1β, IL-6, TNF-α, and IL-4 were determined using a murine ELISA kit (Enzo life science).
Histological analysis of skin lesions
The dorsal skin lesions were sliced and tissues were fixed in 8% buffered-neural formalin for 48 h. The fixed skin tissues were embedded in paraffin wax, sectioned, deparaffinized, and rehydrated using a standard technique with a minor modification. Skin sections with thickness of 5 μm were subjected to hematoxylin and eosin (H–E stain) for the detection of the inflammation cells, periodic acid Schiff stain (PAS stain) for basement membrane detection, and immunohistochemistry for the detection of cytokeratin 10 protein. Selected sections were stained with toluidine blue staining dye for the detection of mast cell infiltration. Histopathological changes and epidermal thickness were examined by a light microscopy (Leica). For measuring the epidermal thickness, three nonsequential sections were evaluated, and for each section, the epidermal thickness was determined by five sequential measurements. An arbitrary scope was given to each microscopic field, viewed at a magnification of 200×.
Statistical analysis
The present data are expressed as the mean ± standard error. Statistical significance was determined using one-way analysis of variance (ANOVA), followed by the Dunnet multiple comparison test using Instat® software (Graphpad, San Diego, CA). P values < 0.05 were considered statistically significant.
Results
Glucan contents from GFP
GF is an abundant source of polysaccharide such as β-glucan. After the extraction of polysaccharide from GF, we measured total glucan, α- and β-glucan contents in the GFP and summarized in Table . The β-glucan content in GFP was 18.35 ± 0.59% and α-glucan was 8.86 ± 0.33%.
GFP-suppressed DNCB-induced AD-like skin lesion in NC/Nga mice
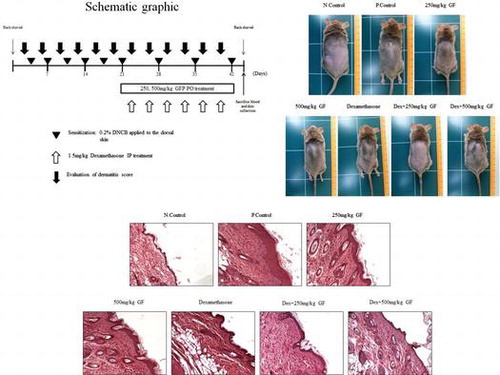
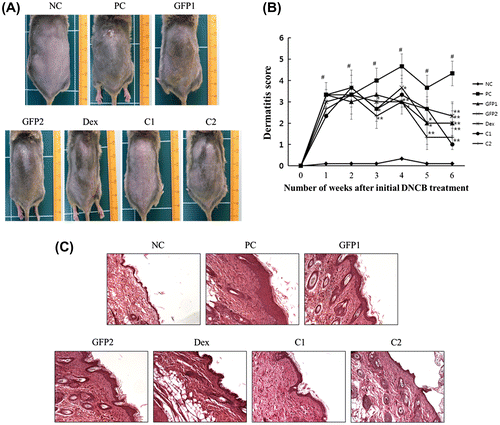
The AD-like skin lesions induced by the dermal application of DNCB resulted in severe itching, mild hemorrhage, and erythema on the dorsal region, which was followed by skin edema, abnormal keratinization, dryness, and excoriation of the skin. In all groups of NC/Nga mice, except the NC group, dermatitis sign and symptoms were rapidly observed during the two weeks of repeat DNCB topical application. However, after GFP and combination treatment with Dex application for three weeks, these AD-like clinical symptoms were controlled, except the Dex group. In case of the Dex group, continued itching behavior lead to an excoriation of the skin. Lesions of excoriation were limited on the hind-leg claw action radius. A photograph of the dorsal skin lesions of NC/Nga mice from each group, which was taken at the end of the experiment, is shown in Fig. (A). The dermatitis severity was assessed twice a week for six weeks. As shown in Fig. (B), the dermatitis score of the PC group increased rapidly and became significantly different from that of the NC group by the first week after the first application of DNCB. Whereas, the dermatitis scores of other groups began to decrease from the third week after the first DNCB treatment, and decreased markedly at the sixth week. Following H–E staining, hypertrophy of the epidermis was observed in the PC group, while they were not detected in the NC group. However, the GFP and Dex treatment groups showed a decrease in this inflammatory change (Fig. (C)).
Fig. 2. Effect of GFP on DNCB-induced AD-like skin lesions in NC/Nga mice.
Notes: (A) Photography example of the clinical severity of AD-like skin lesions. (B) The dermatitis score was defined as the sum of scores for seven different clinical criteria: erythema/hemorrhage, scarring/dryness, edema, and excoriation/erosion. (C) Dorsal skin lesions were removed and fixed in 4% para-formaldehyde solution. Skin sample was cut with 5 μm and stained with H–E. The photographs were taken on the 42nd day after the sacrifice of animals. Magnification, 200×. Data are presented as means ± SD (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test.
*p < 0.05, **p < 0.01 vs. NC; #p < 0.05 NC vs. PC.
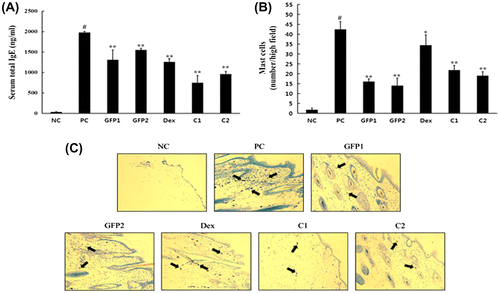
GFP-inhibited DNCB-induced serum IgE and mast cell infiltration in NC/Nga mice
The serum total IgE level of each group is shown in Fig. (A). The elevation of the serum total IgE level is closely related to the severity in AD disease.Citation16) The extrinsic type of AD shows a high level of IgE as a consequence of skin barrier damage and feasible allergen infiltration. Application of DNCB for six weeks significantly increased the serum total IgE levels in the PC group, as compared with the NC group. However, the serum total IgE levels were significantly reduced in all the test groups (GFP1 to C2) as compared with the PC group. The C1 and C2 groups exhibited a greater significant decrease in the IgE levels compared with the GFP1, GFP2, and Dex groups. To investigate whether the inflammatory cells had infiltrated into the dermis after DNCB application, dorsal skin sections were stained with toluidine blue-staining dye for detection of the mast cells. Mast cells in the dermis were markedly increased in the PC group after DNCB application than in the NC group; whereas, the test groups significantly reduced the number of mast cells in the dermis. However, mast cells number in the Dex group was higher than the other test groups (Fig. (B) and (C)).
Fig. 3. Effect of GFP on the serum total IgE level and mast cells in dorsal skin lesion.
Notes: (A) Serum total IgE was measured using ELISA method. (B) and (C) Mast cells were stained with toluidine blue stain method and marked as black arrow. Data are presented as means ± SD (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test.
*p < 0.05, **p < 0.01 vs. NC; #p < 0.05 NC vs. PC.
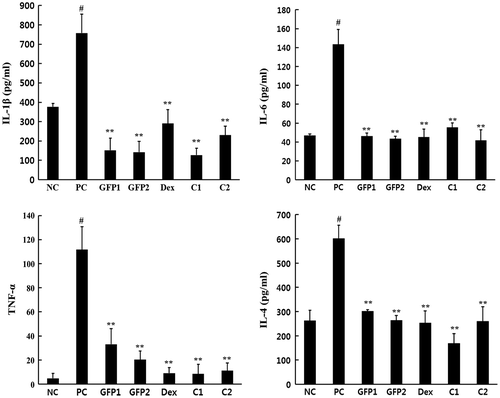
GFP-suppressed IL-1β, IL-6, TNF-α, and IL-4 cytokine production in NC/Nga mice
Pro-inflammatory cytokines are contributed to the AD pathogenesis in the chronic stage, and Th2-type cytokines are dominant in the acute phase AD. As shown in Fig. , repeat application of DNCB treatment significantly elevated the level of IL-1β, IL-6, TNF-α, and IL-4 in the PC group compared with the NC group. GFP1, GFP2, and Dex groups significantly inhibited the production of these cytokines. The C1 and C2 groups also significantly reduced the cytokine production. These results demonstrate that GFP decreased DNCB-induced inflammatory cytokines, leading to the suppression of dorsal skin inflammation caused by an invasion of the inflammatory cells.
Fig.4. Effect of GFP on Th-1/Th-2-type cytokines production quantitative ELISA assay of mouse dorsal skin tissue harvested at the end of the experiment.
Notes: Data are presented as means ± SD (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test.
*p < 0.05, **p < 0.01 vs. NC; #p < 0.05 NC vs. PC.
GFP decreased hyperkeratosis and cytokeratin 10 differentiations at dorsal skin lesion in NC/Nga mice
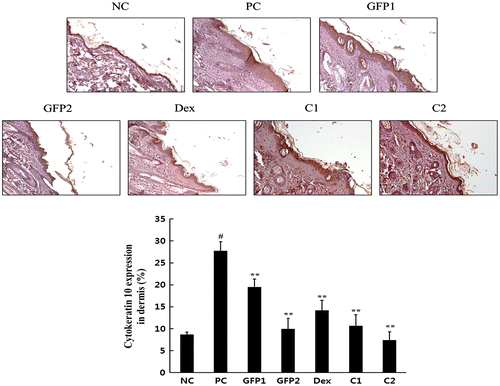
Hyperkeratosis is the main symptom of AD patients, and cytokeratin 10 is closely associated with epidermolytic hyperkeratosis.Citation17,18) As shown in Fig. , we stained the skin tissue with PAS stain methods for the basement membrane detection. Epidermis was significantly thickened with repeated application of DNCB treatment compared with the NC group. However, daily supply with GFP and Dex significantly reduced the thickness of the basement membrane. To gain a better understanding of the hyperkeratosis, we assessed the cytokeratin 10 expression. As shown in Fig. , a repeat application of DNCB significantly increased cytokeratin differentiation in the PC group compared with that of the NC group. The test groups significantly inhibited cytokeratin 10 differentiation in the skin dermis.
Fig. 5. Effect of GFP on histological changes of the dorsal skin lesions.
Notes: Skin tissues were stain with PAS basement membrane stain method. Magnification, 200×. Data are presented as means ± SD (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test.
*p < 0.05, **p < 0.01 vs. NC; #p < 0.05 NC vs. PC.
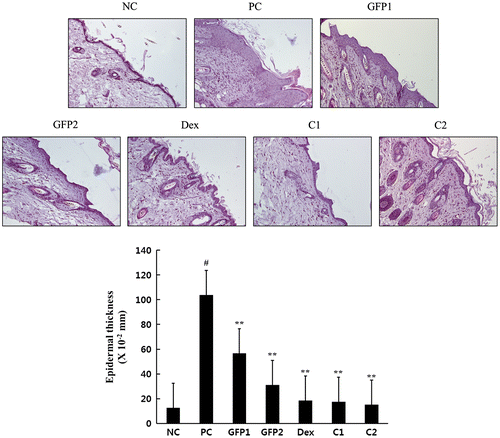
Fig. 6. Effect of GFP on histological changes of the dorsal skin lesions.
Notes: Skin tissues were applied with anti-cytokeratin 10 antibody. Magnification, 200×. Data are presented as means ± SD (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test.
*p < 0.05, **p < 0.01 vs. NC; #p < 0.05 NC vs. PC.
Discussion
In the present study, we demonstrated that oral administration of GFP inhibits AD-like skin lesions in NC/Nga mice with repeat application of DNCB. Interestingly, DNCB induced the clinical symptom within one week. This is very convenient for induced AD like the skin lesions, because we control the experiment start point and, hence, easy to evaluate the dermatitis score at one time. Indeed, we found that oral administration of GFP exhibited a significant suppression in AD-like skin lesions. Moreover, combination treatment of GFP with Dex inhibited AD-like skin lesions more significantly than those of a Dex single treatment. These findings strongly suggest that GFP can be useful for AD as a novel therapeutic agent and might be used for corticosteroids replacement or as a supplemental agent.
AD is the most common chronically relapsing inflammatory skin disease present from infancy or childhood.Citation19) Most AD patients suffer from not only its clinical symptoms, but their hideous appearance. A topical application of corticosteroids is the most common treatment for AD. These anti-inflammatory drugs bind to the glucocorticoid receptor and activate its downstream signal pathway. Due to the concerns regarding the potential side effects associated with long-term use of corticosteroids and possibility of corticosteroid resistance microbe appearances, corticosteroid rescue therapy is necessary for AD patients. For this reason, phytomedicine therapy can be an excellent complementary method for AD patients. Edible mushroom, especially GF, has been used as a medicinal herb for a long time in an Asian country, including China, Korea, and Japan.Citation20) Recent studies also show that many edible mushrooms apply to AD-like skin lesions.Citation21,22) GF increased urine volume temporary, but there are no severe side effects. Particularly, its immune-modulating effects through oral administration were studied in normal mice and humans.Citation14,23) Here, we try to focus on this mushroom not for its nutritional properties, but as a therapeutic agent. The major active polysaccharide of GF is β-glucans, and its medicinal properties are strongly dependent on high molecular weight, ranging from 500 to 2000 kDa. Our extract might be consisting of high molecular weight β-glucan. For this reason, our next study will focus on the identification of β-glucan molecular weight. It is important to realize that chemical modification is necessary to improve clinical qualities, water solubility, and the ability of stomach wall permeability.
The serum IgE level is one of the most important factors in AD patients since high level of IgE mediates the clinical characteristics in extrinsic AD patients. A high level of IgE binds to the mast cells and releases the inflammatory mediators.Citation24) We found that GFP and Dex significantly inhibited the IgE level after DNCB treatment. However, the serum IgE level and number of mast cells were not matched in the Dex treatment group. Both were significantly decreased compared with the PC group, and the number of mast cells was still higher than other treatment groups (Fig. (B)). It might be related with continuous itching behavior and local skin excoriation (Fig. (B)). While, combination treatment inhibits mast cell infiltration and the IgE level was also decreased in comparison to the Dex single treatment, these results so far show that GFP might counteract the side effects of glucocorticoid.
IL-1β, IL-6, and TNF-α promote inflammation and are called pro-inflammatory or Th-1-type cytokines. These cytokines promote T-cell activation and activate inflammation cascade reaction. IL-1β and TNF-α act synergistically in this process and trigger the expression of inflammatory mediators.Citation25) This acute-phase reaction causes fever and stimulates leukocyte to release the inflammatory mediators. On the contrary to this, Th2-type cytokines called anti-inflammatory cytokines block the inflammatory process or suppress the intensity of the inflammation cascade.Citation26) The human immune system is regulated as a highly complex and intricate network of signal pathways. From the balance point of view, anti-inflammatory cytokines should be up regulated to inhibit pro-inflammatory cytokines. However, AD patients show a high level of IgE that are released from B-cells and will bind to mast cells. This reaction triggers the release of prostaglandin and histamines,Citation27,28) and it makes a typical AD symptom. GFP treatment significantly inhibited IL-1β, IL-6, TNF-α, and IL-4. These results suggest that the therapeutic effects of GFP in NC/Nga mice are associated with both the control of the pro-inflammatory cytokines and Th-2-type cytokines (Fig. (A)).
Histologically, AD patients exhibit hypertrophy and hyperkeratosis of the epidermis infiltration of the inflammatory cells.Citation29) In our study, hypertrophy of the epidermis was confirmed with PAS staining, and hyperkeratosis was also checked with immunohistochemistry, using anti-cytokeratin 10 antibody. Epidermal thickness and cytokeratin 10 differentiation were followed as a similar pattern. Also, the combination-treatment groups show a more significantly decreased tendency than that of the GFP single-treatment groups.
In conclusion, our study suggests that oral administration of GFP modulates the Th-1/Th-2 response and that mast cell infiltration might be a major mechanism of the AD-like skin lesion in NC/Nga mice. Also, we tested a histological analysis and provided evidence that GFP inhibited differentiation of the cytokeratin 10 and control of the basement membrane after DNCB treatment. Furthermore, a combination treatment with Dex and GFP inhibited AD-like skin lesion more effectively. These findings strongly suggest that GFP can be useful for AD patients as an excellent corticosteroids replacement or supplement agent.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347:1151–1160.10.1056/NEJMoa021481
- Williams, H, Stewart, A, von Mutius, E, Cookson, W, Anderson, HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide?J. Allergy Clin. Immunol. 2008;121:947–954.10.1016/j.jaci.2007.11.004
- Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000;105:860–876.10.1067/mai.2000.106484
- Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, Ikeda T, Hirota N, Nakagawa H, Ishii A. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003;58:139–145.10.1034/j.1398-9995.2003.23790.x
- McNally NJ, Phillips DR, Williams HC. The problem of atopic eczema: aetiological clues from the environment and lifestyles. Soc. Sci. Med. 1998;46:729–741.10.1016/S0277-9536(97)00174-3
- Han C, Cui B. Pharmacological and pharmacokinetic studies with agaricoglycerides, extracted from Grifola frondosa, in animal models of pain and inflammation. Inflammation. 2012;35:1269–1275.10.1007/s10753-012-9438-5
- Kang JS, Yoon WK, Han MH, Lee H, Lee CW, Lee KH, Han SB, Lee K, Yang KH, Park SK, Kim HM. Inhibition of atopic dermatitis by topical application of silymarin in NC/Nga mice. Int. Immunopharmacol. 2008;8:1475–1480.10.1016/j.intimp.2008.06.004
- Yang G, Lee K, Lee MH, Kim SH, Ham IH, Choi HY. Inhibitory effects of Chelidonium majus extract on atopic dermatitis-like skin lesions in NC/Nga mice. J. Ethnopharmacol. 2011;138:398–403.10.1016/j.jep.2011.09.028
- Preuss HG, Echard B, Bagchi D, Perricone NV. Maitake mushroom extracts ameliorate progressive hypertension and other chronic metabolic perturbations in aging female rats. Int. J. Med. Sci. 2010;7:169–180.10.7150/ijms.7.169
- Fukushima M, Ohashi T, Fujiwara Y, Sonoyama K, Nakano M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. (Maywood). 2001;226:758–765.
- Martin KR. Both common and specialty mushrooms inhibit adhesion molecule expression and in vitro binding of monocytes to human aortic endothelial cells in a pro-inflammatory environment. Nutr. J. 2010;9:29. DOI:10.1186/1475-2891-9-2910.1186/1475-2891-9-29
- Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and nods. Immunol. Rev. 2007;219:75–87.10.1111/imr.2007.219.issue-1
- Masuda Y, Inoue H, Ohta H, Miyake A, Konishi M, Nanba H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer. 2013;133:108–119.10.1002/ijc.v133.1
- Deng G, Lin H, Seidman A, Fornier M, D’Andrea G, Wesa K, Yeung S, Cunningham-Rundles S, Vickers AJ, Cassileth B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J. Cancer Res. Clin. Oncol. 2009;135:1215–1221.10.1007/s00432-009-0562-z
- Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, Deguchi M, Arimura A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007;56:139–148.10.2332/allergolint.O-06-458
- Ott H, Stanzel S, Ocklenburg C, Merk HF, Baron JM, Lehmann S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta Derm. Venereol. 2009;89:257–261.10.2340/00015555-0627
- Cheng J, Syder AJ, Yu QC, Letal A, Paller AS, Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992;70:811–819.10.1016/0092-8674(92)90314-3
- Morais P, Mota A, Baudrier T, Lopes JM, Cerqueira R, Tavares P, Azevedo F. Epidermolytic hyperkeratosis with palmoplantar keratoderma in a patient with KRT10 mutation. Eur. J. Dermatol. 2009;19:333–336.
- Leung DY, Bieber T. Atopic dermatitis. The Lancet. 2003;361:151–160.10.1016/S0140-6736(03)12193-9
- Wu MJ, Cheng TL, Cheng SY, Lian TW, Wang L, Chiou SY. Immunomodulatory properties of Grifola frondosa in submerged culture. J. Agric. Food Chem. 2006;54:2906–2914.10.1021/jf052893q
- Ukawa, Y, Izumi, Y, Ohbuchi, T, Takahashi, T, Ikemizu, S, and Kojima, Y. Oral administration of the extract from Hatakeshimeji (Lyophyllum decastes sing.) mushroom inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice J. Nutr. Sci. Vitaminol.2007;53:293–296.10.3177/jnsv.53.293
- Choi JH, Kim HG, Jin SW, Han EH, Khanal T, Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, Jeong TC, Jeong HG. Topical application of Pleurotus eryngii extracts inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by the regulation of Th1/Th2 balance. Food Chem Toxicol. 2013;53:38–45.10.1016/j.fct.2012.11.025
- Wang L, Ha CL, Cheng TL, Cheng SY, Lian TW, Wu MJ. Oral administration of submerged cultivated Grifola frondosa enhances phagocytic activity in normal mice. J. Pharm. Pharmacol. 2008;60:237–243.10.1211/jpp.60.2.0013
- Amin K. The role of mast cells in allergic inflammation. Respir. Med. 2012;106:9–14.10.1016/j.rmed.2011.09.007
- Dinarello CA. Proinflammatory cytokines. Chest J. 2000;118:503–508.10.1378/chest.118.2.503
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest J. 2000;117:1162–1172.10.1378/chest.117.4.1162
- Amin K, Janson C, Boman G, Venge P. The extracellular deposition of mast cell products is increased in hypertrophic airways smooth muscles in allergic asthma but not in nonallergic asthma. Allergy. 2005;60:1241–1247.10.1111/all.2005.60.issue-10
- Naclerio RM. Pathophysiology of perennial allergic rhinitis. Allergy. 1997;52:7–13.10.1111/all.1997.52.issue-s36
- Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009;21:666–678.10.1016/j.coi.2009.09.006