Abstract
An allergen-stimulating cytokine, interleukin-13 (IL-13), plays a significant role in allergic inflammation. Benzyl isothiocyanate (BITC), derived from several cruciferous vegetables, significantly suppressed the IL-13 expression in the calcium ionophore-stimulated human basophilic KU812 cells. Down-regulation of phosphorylated mitogen-activated protein kinases as well as nuclear transcriptional factors might be involved in the underlying mechanism.
Interleukin-13 (IL-13) is a T helper 2 (Th2) cytokine that plays important immunoregulatory roles related to the pathogenesis of allergic disorders in a wide variety of cell types.Citation1) In human B cells, IL-13 shares many functional properties with IL-4 by promoting B-cell proliferation and inducing class switching to immunoglobulin G4 and immunoglobulin E (IgE) in combination with CD40/CD40 ligand co-stimulation.Citation2) The allergic reaction can be further enhanced by IL-13 via up-regulation of the high affinity receptor for IgE (FcεRI) expression on mast cells and basophils.Citation3) Human basophilic cells, which are activated by IgE and various allergens, have roles in allergic disorders such as bronchial asthma, atopic dermatitis, and allergic rhinitis. The release of IL-13 by human basophils after IgE-dependent stimulation also suggests that these cells maintain the Th2 dominance of the established allergic-immune responses through a positive feedback loop system.Citation4–6) Thus, targeting IL-13 may be one of the most potential therapeutic approach to the treatment of asthma and/or allergy.Citation7)
Isothiocyanates (ITCs), which are derived from cruciferous vegetables such as broccoli, watercress, cabbage, and Japanese radish, may be the agents responsible for the supposed cancer chemopreventive potential of these vegetables.Citation8) One of them is benzyl ITC (BITC), which we isolated from an extract of papaya (Carica papaya),Citation9) and which induces phase 2 enzyme and apoptosis.Citation8) More recently, BITC, as well as phenethyl ITC and sulforaphane, were identified as metabolites in serum from a human subject eating broccoli, garden cress, and watercress, suggesting that BITC can be consumed from a diet containing cruciferous vegetables.Citation10) Although BITC has also been reported to possess an anti-inflammatory effect on mouse skin,Citation11) the modulating effect of ITCs on Th2 cytokine expression in basophilic cells has not yet been investigated.
In the present study, we analyzed the influence of BITC on the calcium ionophore-induced IL-13 expression in human basophilic KU812 cells, because IgE-induced IL-13 production depends on the intracellular calcium mobilizationCitation12) and a calcium ionophore, A23187, also induces Th2 cytokine production in basophil cells.Citation13) Unlike primary basophils, KU812 cells are not reactive enough to allergen-IgE complexes because of low expression of FcεRI.Citation14) Thus, A23187 was used for the activation of KU812 cells to analyze the effects of chemicals on signal transduction. Here, we report that BITC dose-dependently down-regulates IL-13 expression in the A23187-activated human basophilic KU812 cells. In addition, our results suggest that not only c-Jun-N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) but also nuclear factor-κB (NF-κB) and nuclear factor of activated T cells c1 (NFATc1) have roles in the down-regulation of IL-13 expression by BITC.
BITC was purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). RPMI-1640 medium and fetal bovine serum were purchased from Gibco-Invitrogen (Carlsbad, CA, USA). Protease and phosphatase inhibitor cocktails were obtained from Sigma (St. Louis, MO, USA). Antibodies to phospho-JNK, phospho-p38 MAPK, phospho-ERK, JNK, ERK, and p38 MAPK were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies to β-actin, NF-κB p65, and lamin B1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and an antibody specific for NFATc1 (7A6) was from Affinity Bioreagents (Golden, CO, USA). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
The human basophilic leukemia cell line KU812 (RIKEN Cell Bank, Tsukuba, Ibaraki, Japan) was maintained in RPMI 1640 medium and supplemented with 15% (v/v) FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37 °C under 5% CO2 and 95% air. For experiments, the cells were seeded in complete medium and treated with each reagent or vehicle (final 0.1%, v/v). Cell viability was evaluated using a trypan blue exclusion assay as previously reported.Citation15)
RT-PCR was performed as previously reported.Citation15) The forward and reverse primers and the expected PCR product sizes are as follows: IL-13, 5′-TTGACCACGGTCATTGCTCT-3′, and 5′-TCGATTTTGGTGTCTCGGACA-3′ (317 bp); NFATc1, 5′-GCCGCAGCACCCCTACCAGT-3′, and 5′-TTCTTCCTCCCGATGTCCGTCTCT-3′ (502 bp) and β-actin, 5′-GTCACCCACACTGTGCCCATCTA-3′ and 5′-GCAATGCCAGGGTACATGGTGGT-3′ (455 bp). The PCR products were then subjected to agarose gel electrophoresis (1.5%), stained with ethidium bromide, and photographed. Band intensities were measured with Multi Gauge software v. 3.0 (Fuji Film Life Science, Tokyo, Japan).
The preparation of total cell lysates and nuclear lysates from the cytoplasmic pellets and Western blotting was performed as previously reported.Citation15)
All values are expressed as means ± SD. Data were analyzed by a one-way analysis of variance (ANOVA) followed by multiple comparisons among means (Tukey’s HSD) using XLSTAT software version 2014.3.04 (Addinsoft, Paris, France). Different letters above the bars indicate significant differences among treatments for each compound (p < 0.05).
We assessed the cytotoxic effect of BITC in KU812 cells before investigating the IL-13 mRNA expression. Cells were incubated with different doses of BITC for 3 h and 21 h with or without A23187 stimulation. The BITC concentration needed to inhibit the KU812 cell growth by 50% was approximately 20 μM for 3 h-incubation and 7.5 μM for 21 h-incubation (Fig. (A) and (C)). Thus, for IL-13 experiments, KU812 cells were treated with BITC for 3 h or 21 h, washed with fresh medium, and additionally incubated with A23187, a representative calcium ionophore, for 3 h (so that the total incubation periods were 6 h and 24 h). A23187 slightly decreased the numbers of viable KU812 cells, independently of BITC. As shown in Fig. (B), A23187 treatment of KU812 cells resulted in an approximately 5-fold increase in IL-13 mRNA expression compared with control. Pretreatment of BITC for 3 h significantly inhibited the A23187-induced IL-13 up-regulation at concentrations higher than 10 μM (Fig. (B)). The effective concentration of BITC, for IL-13 down-regulation by the longer pre-incubation, (21 h, Fig. (D)) was lower than that of the 3-h incubation (Fig. (B)) and was comparable with that of anti-allergic flavones such as apigenin.Citation16)
Fig. 1. BITC inhibits cell proliferation and IL-13 mRNA expression in a time-and dose-dependent manner in KU812 cells.
Notes: KU812 cells (1 × 106) were pretreated with BITC for 3 h ((A) and (B)) or 21 h ((C) and (D)), washed with fresh medium, and additionally incubated with A23187 for 3 h. Cell viability was determined by a trypan blue exclusion assay ((A) and (C)). IL-13 mRNA was analyzed by RT-PCR ((B) and (D)). The quantitative data are expressed as means ± SD of three independent experiments. Bars with the same letters are not significantly different at p < 0.05.
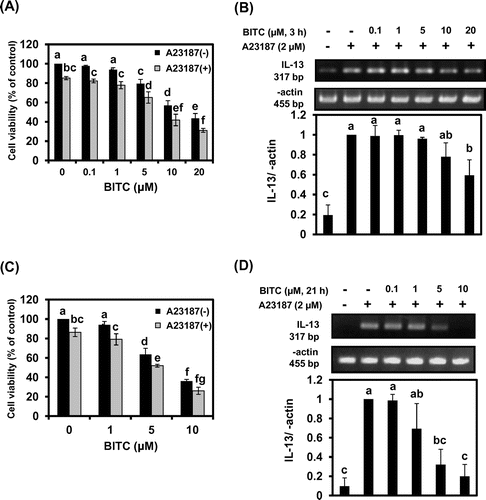
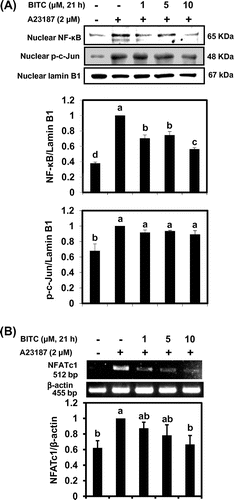
We next tried to clarify the signal transduction pathways involved in BITC-modulated IL-13 expression. Many factors are involved in IL-13 mRNA expression in basophils, such as MAPK signaling cascade as well as transcription factors including activator protein-1 (AP-1), NF-κB, and NFATs.Citation17,18) To determine the role of MAPKs on the inhibitory effects of BITC on IL-13 expression, the total cellular protein was subjected to Western blotting using specific antibodies for phosphorylated MAPKs such as ERK, JNK, and p38. As shown in Fig. , pretreatment of BITC at each concentration for 21 h significantly suppressed A23187-induced phosphorylation of JNK and p38 MAPK. In contrast, BITC at the concentrations required for IL-13 down-regulation did not significantly inhibit the level of phospho-ERK. Next we performed Western blotting to examine whether BITC affects the nuclear expression of transcription factors known to modulate the expression of IL-13. Although BITC did not affect the nuclear level of c-Jun, a major component of AP-1, in KU812 cells, the nuclear translocation of NF-κB was significantly suppressed in response to BITC treatment (Fig. (A)). We have recently found that pretreatment with the JNK-specific inhibitor SP600125 significantly decreased not only nuclear translocation but also mRNA level of the NFATc1 in KU812 cells.Citation15) Because BITC showed the tendency to inhibit the nuclear NFATc1 level (data not shown), we examined the effects of BITC on NFATc1 mRNA expression using an RT-PCR. As shown in Fig. (B), the mRNA expression of NFATc1 was significantly suppressed by BITC (10 μM). These results suggest that nuclear NFATc1 protein level could be transcriptionally regulated by JNK in KU812 cells.
Fig. 2. BITC inhibits the phosphorylation of JNK and p38 MAPK.
Notes: KU812 cells (5 × 106) were treated with BITC at the indicated concentrations for 21 h, washed with fresh medium, and additionally incubated with A23187 for 30 min for phosphorylated MAPK detection. Bars with the same letters are not significantly different at p < 0.05.
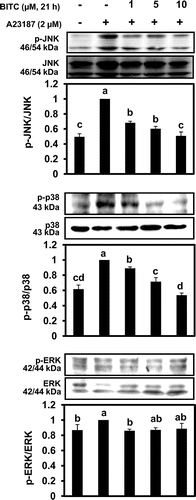
Fig. 3. BITC inhibits the nuclear translocation of transcriptional factors.
Notes: KU812 cells (5 × 106) were treated with BITC at the indicated concentrations for 21 h, washed with fresh medium, and additionally incubated with A23187 for 1 h for nuclear proteins (A) and NFATc1 mRNA (B). Nuclear levels of NF-κB, and c-Jun were analyzed by Western blotting, and NFATc1 mRNA was determined by RT-PCR. The quantitative data are expressed as means ± SD of three independent experiments. Bars with the same letters are not significantly different at p < 0.05.
Our results showed that BITC, an ITC present in several cruciferous vegetables, markedly suppressed A23187-induced IL-13 in the cultured basophilic cells. We considered that inhibition of JNK and p38 MAPK, along with a resultant inhibition of NF-κB and NFATc1, was involved in the underlying mechanism. There is huge evidence indicating that MAPKs regulate the NF-κB pathway in various ligand-stimulated cellular systems. For example, lipopolysaccharide-triggered NF-κB activation is due to the activation of ERK1/2 and p38 MAPK.Citation19) Although the mechanism by which inhibition of MAPKs negatively regulates NF-κB activation is not fully understood, it should be noted that p38 MAPK has been reported to phosphorylate Ser276 of p65 via MSK1 activation.Citation20) NFATc1 is also one of the key transcriptional factors for IL-13 expression,Citation17) possibly activated by JNK.Citation15,21) Taken together, dual suppression by BITC of both JNK and p38 MAPK probably contributes to decrease in nuclear NF-κB and NFATc1, and thus down-regulation of IL-13 expression.
Animal studies have shown that at the earlier stage of metabolism, glutathione conjugates are the major products of ITCs (reviewed in Ref. Citation8). However, biologically free available ITCs have been found in urine and serum of humans.Citation22) Thus, reaction products of thiols with ITCs are not stable, since thiol conjugates may partly regenerate an ITC molecule and then react with other nucleophiles.Citation8) The effective concentration of the glutathione conjugate of BITC for IL-13 down-regulation (20 μM; unpublished data) was higher than that of the free BITC (5 μM, Fig. (D)). These results suggested that BITC suppresses excessive IL-13 expression in vivo, possibly through deconjugation of thiol adducts.
ITCs are potent inducers of nuclear factor erythroid 2-related factor 2 (Nrf2)-regulated phase 2 enzymes, which includes, in addition to the classical conjugating enzymes (e.g. glutathione S-transferases; GSTs, and UDP-glucuronosyltransferases), many other proteins such as antioxidant enzymes (e.g. heme oxygenase and catalase) and other protective proteins that can affect redox status (thioredoxin, etc.).Citation8) We previously reported that BITC significantly induces GST expression at a transcription level in rat hepatocytes.Citation8) Our finding that the longer incubation of BITC was more effective in suppressing IL-13 suggests the involvement of de novo induction of inhibitory factors such as antioxidants/phase 2 detoxifying enzymes. This idea is in line with a report showing that pretreatment of sulforaphane, an abundant ITC in broccoli, for 6 h, but not its simultaneous treatment with lipopolysaccharide, showed Nrf2-dependent inhibition of pro-inflammatory cytokine expression.Citation23) GSTP1, one of the major GST isoforms, regulates stress-induced cell signaling by inhibition of JNK through direct protein-protein interaction of the GSTP1 monomer.Citation24) In addition, the thioredoxin system, including thioredoxin and thioredoxin reductase, negatively regulates apoptosis signal-regulating kinase 1 (ASK1), a MAPK kinase kinase upstream of JNK.Citation25) Taken together, these findings strongly suggest that redox-dependent JNK signaling inhibition might contribute to IL-13 down-regulation by BITC.
In conclusion, the present data demonstrate that BITC significantly down-regulates IL-13 expression in the human basophil cell line KU812. These results indicate that BITC is a promising natural agent for the prevention and treatment of basophil-mediated allergic disorders. Further studies are necessary to elucidate its anti-allergic mechanisms not only using in vitro models for IL-13 secretion but also using in vivo animal models in terms of involvement of phase 2 enzymes.
Acknowledgements
We thank Mayuko Yasunaga and Toshihiko Osawa (Prof. Emer.) at Nagoya University for their technical support.
Additional information
Funding
Notes
Abbreviations: IL-13, Interleukin-13; Th2, T helper 2; IgE, immunoglobulin E; FcεRI, high affinity receptor for IgE; ITC, Isothiocyanate; BITC, benzyl ITC; JNK, c-Jun-N-terminal kinase; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; NFATc1, nuclear factor of activated T cells c1; AP-1, activator protein-1; GST, glutathione S-transferase.
References
- Romagnani S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. 2000;105:399–408.10.1067/mai.2000.104575
- Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int. Rev. Immunol. 1998;17:51–52.
- Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J. Immunol. 1995;155:2688–2694.
- Brunner T, Heusser CH, Dahinden CA. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J. Exp. Med. 1993;177:605–611.10.1084/jem.177.3.605
- Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J. Immunol. 1996;156:4833–4838.
- Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J. Allergy Clin. Immunol. 2003;111:677–690.10.1067/mai.2003.1333
- Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr. Opin. Investig. Drugs. 2010;11:527–534.
- Nakamura Y, Miyoshi N. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Biosci. Biotechnol. Biochem. 2010;74:242–255.10.1271/bbb.90731
- Nakamura Y, Yoshimoto M, Murata Y, Shimoishi Y, Asai Y, Park EY, Sato K, Nakamura Y. Papaya seed represents a rich source of biologically active isothiocyanate. J. Agric. Food Chem. 2007;55:4407–4413.10.1021/jf070159w
- Kumar A, Sabbioni G. New biomarkers for monitoring the levels of isothiocyanates in humans. Chem. Res. Toxicol. 2010;23:756–765.10.1021/tx900393t
- Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575.
- Redrup AC, Howard BP, MacGlashan DW Jr, Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J. Immunol. 1998;160:1957–1964.
- Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J. Immunol. 1996;156:4833–4838.
- Hara T, Yamada K, Tachibana H. Basophilic differentiation of the human leukemia cell line KU812 upon treatment with interleukin-4. Biochem. Biophys. Res. Commun. 1998;247:542–548.10.1006/bbrc.1998.8816
- Wu H, Qi H, Iwasaki D, Zhu B, Shimoishi Y, Murata Y, Nakamura Y. JNK-dependent NFATc1 pathway positively regulates IL-13 gene expression induced by (-)-epigallocatechin-3-gallate in human basophilic KU812 cells. Free Radical Biol. Med. 2009;47:1028–1038.10.1016/j.freeradbiomed.2009.07.011
- Hirano T, Higa S, Arimitsu J, Naka T, Shima Y, Ohshima S, Fujimoto M, Yamadori T, Kawase I, Tanaka T. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int. Arch. Allergy Immunol. 2004;134:135–140.10.1159/000078498
- Lorentz A, Klopp I, Gebhardt T, Manns MP, Bischoff SC. Role of activator protein 1, nuclear factor-κB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J. Allergy Clin. Immunol. 2003;111:1062–1068.10.1067/mai.2003.1342
- Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M, Kawase I, Tanaka T. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003;111:1299–1306.10.1067/mai.2003.1456
- Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003;243:153–160.10.1023/A:1021624520740
- Calleros L, Lasa M, Toro MJ, Chiloeches A. Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell Signal. 2006;18:2292–2301.10.1016/j.cellsig.2006.05.012
- Fujii T, Onohara N, Maruyama Y, Tanabe S, Kobayashi H, Fukutomi M, Nagamatsu Y, Nishihara N, Inoue R, Sumimoto H, Shibasaki F, Nagao T, Nishida M, Kurose H. G 12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J. Biol. Chem. 2005;280:23041–23047.10.1074/jbc.M409397200
- Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2003;323:39–47.10.1016/j.ab.2003.08.011
- Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008;76:967–973.10.1016/j.bcp.2008.07.036
- Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001;276:20999–21003.10.1074/jbc.M101355200
- Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol. Nutr. Food Res. 2009;53:87–103.10.1002/mnfr.v53:1
