Abstract
Cystine is formed from two molecules of the cysteine under oxidized conditions, but is reversibly converted to cysteine by reduction. Growth of Escherichia coli is retarded in the presence of excess cystine. Transcriptome analysis showed 11 up-regulated and 26 down-regulated genes upon exposure to excess cystine. The reporter assay confirmed regulation by cystine of the expression of one up-regulated membrane gene, yijE, and two down-regulated membrane genes, yhdT and yihN. In order to identify the as yet unidentified gene encoding cystine efflux transporter, the putative cystine efflux candidate, yijE gene, was over-expressed. Expression of the yijE gene suppressed the slow growth of E. coli in the presence of high concentration of extracellular cystine. In good agreement, the knock-out of yijE gene increased the sensibility to cystine. These observations altogether imply that the yijE gene is involved in response to cystine in E. coli.
Graphical Abstract
The Escherichia coli yijE gene, induced by cystine, is involved in response to excess cystine.
Cystine, a sulfur-containing amino acid, is formed by oxidation of two molecules of cysteine, and is reversibly converted to cysteine under intracellular reduced conditions. The inter-conversion system between cystine and cysteine plays an important role in defense of Escherichia coli against oxidative stress, such as in the presence of hydrogen peroxide.Citation1) For maintenance of the intracellular concentration of cysteine at an optimum level, E. coli is known to possess multi-transporters including Bcr,Citation2) CydDC,Citation3) EamA (YdeD),Citation4,5) and EamB (YfiK).Citation6) In contrast, the transport system of cystine has never been identified in E. coli. For searching the putative cystine-transport system, we performed transcriptome analysis of the genes affected in the presence of cystine, and then reporter analyses of some candidate membrane genes. Among the genes tested, the hitherto uncharacterized yijE gene was found to confer tolerance to cystine.
Materials and methods
E. coli strains and growth conditions
E. coli BW25113 was used as a test strain throughout this study.Citation7) JW4303 is the yijE knock-out strain of BW25113 was provided by NRBP of Japan.Citation8) Cells were grown at 37 °C in LB medium or M9-glucose minimum medium in an elbow glass tube with reciprocal shaking for aerobic condition or without shaking in N2-saturating chamber for anaerobic condition. For the analysis of gene expression, cystine was added to a final concentration of 10 μM, which will not affect cell growth.
Construction of plasmids and lysogens
For construction of the yijE expression plasmid, a fragment of the yijE gene was amplified by PCR using the genome of E. coli W3110 type-A strainCitation9) as a template and a pair of primers, pQE-YIJE-LF (5′-CTGGAGGATCCATGTCTGCCGCAGGAAAGA-3′) and pQE-YIJE-LR (5′-GAAGAAAGCTTTCAGATCCTTTTTACACTG-3′). The PCR product was digested with BamH I and Hind III and then ligated into pQE80L (Qiagen) at the corresponding sites. The recombinant plasmid, pYijE, was confirmed by DNA sequencing.
A single copy of test promoter-lacZ translational fusion gene on the genome was constructed according to the published procedure.Citation10) In brief, a fragment of the test promoter, −500 and +150 with respect to the initiation codon, including the promoterCitation11,12) and the N-terminal proximal coding sequences, was amplified by PCR using the genome of E. coli W3110 type-A strain as a template and a pair of following primers: YIJE-P-LF (5′-TTTCCGAATTCGAGTCACTGCTGATCGACA-3′) and YIJE-P-LR (5′-TGAATGGATCCAGAGCGCCGAAAATGCAGC-3′) for yijE promoter; YIHN-P-LF (5′-CGCGGGAATTCGTTCCTCGGTCACGGTGC-3′) and YIHN-P-LR (5′-CGAGGGGATCCAACGCCTGGCGCAGCGTCA-3′) for yihN promoter; and YHDT-P-LF (5′-TGCCGGAATTCCGCTGTCGATCAAGCAAGA-3′) and YHDT-P-LR (5′-GCGGCGGATCCATGCAGGCCATCTCAAACC-3′) for yhdT promoter. The PCR product was digested with BamH I and EcoR I and then ligated into pRS552Citation10) at the corresponding sites. The recombinant plasmids, pTW02 (yijE-lacZ), pHN01 (yihN-lacZ), and pDT04 (yhdT-lacZ), were confirmed by DNA sequencing using Lac30R primer.Citation13) Each lacZ translational fusion plasmid was transformed into MC4100,Citation14) and the transformant was infected with λ RS45 to prepare λ recombinant phage containing the lacZ fusion gene. E. coli BW25113 was infected with the λ lysate, and the lysogen containing the recombinant λ phage was selected by resistance to kanamycin. The isolated lysogens are JE30617 (λyijE-lacZ Kmr), HN81108 (λyihN-lacZ Kmr), and DT31108 (λyhdT-lacZ Kmr).
Transcriptome analysis
E. coli BW25113 was grown at 37 °C in M9 medium with and without 10 µM cystine. At the middle of exponential phase, cells were harvested, and total RNAs were prepared with hot phenol method as previously described.Citation15,16) Preparation of Cy3 or Cy5-labeled cDNA was performed by RNA fluorescence labeling core kit (Takara Bio) using Cy3-dUTP or Cy5-dUTP (GE Healthcare) as a substrate. Hybridization on DNA chip, scanning microarrays, and data analysis were performed as described.Citation17) E. coli CHIPs (Takara Bio) used were Takara Ver. 2 products. All fluorescent intensity data were statically analyzed as described.Citation15–17)
The lacZ reporter assay of E. coli
Overnight cultures were diluted 1:1000 into fresh medium, and cells were grown at appropriate time and then subject for β-galactosidase activity. The activity of β-galactosidase was measured according to the standard Miller method.Citation18) The measurement was performed four times to get the average values with standard deviation.
Results and discussion
Search for the genes affected in the presence of excess cystine
For search of the whole set of cystine-responsive genes, we performed the DNA microarray analysis of E. coli cells growing in M9-glucose medium in the presence or absence of 10 μM cystine. In the middle of exponential growth phase, total RNAs were prepared and subjected to the microarray analysis using an E. coli DNA chip. Among a total of 4000 genes on the DNA chip, transcription of a total of 37 genes exhibited more than three fold change in the presence of cystine (Table ). A set of 11 genes including 5 hitherto uncharacterized genes were up-regulated, while another set of 26 genes including 13 uncharacterized genes were down-regulated. The set of cystine-responded genes are related to transport and utilization of metals such as copper and flagella formation.
Table 1. The genes affected by excess of extracellular cystine in E. coli.
As an approach for identification of the putative gene involved in cystine transport, we focused the hitherto uncharacterized membrane genes. We selected three targets: one up-regulated gene, yijE encoding a drug/metabolite exporter (DME) familyCitation17), and two down-regulated genes, yhdT encoding the major facilitator superfamily of transporterCitation18) and yihN encoding a short peptide consisting of 80 amino acid residues with two transmembrane domains.
Influence of excess cystine on expression of the putative cystine-transport gene(s)
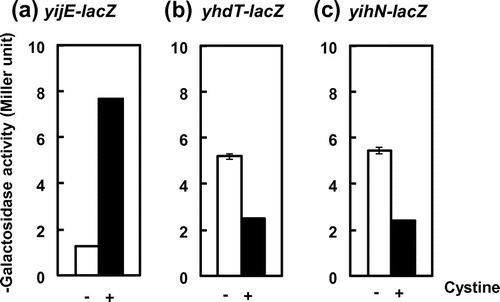
To examine possible influence of excess cystine on expression of three putative cystine-transport genes, yijE, yhdT, and yihN, we performed a reporter assay using the promoter-lacZ fusions. Transformants of E. coli carrying the respective reporter assay vectors, JE30617 (λyijE-lacZ), HN81108 (λyihN-lacZ), and DT31108 (λyhdT-lacZ), were grown under aeration in M9-glucose minimum medium in the presence or absence of cystine until logarithmic phase and then subjected to measure the activity of β-galactosidase. As expected, the promoter for yijE was significantly induced by cystine (Fig. (a)), whereas the promoters for yhdT and yihN were repressed (Fig. (b) and (c)). Interestingly, the changes in cystine-dependent expression of these three promoters were not observed when E. coli were grown under anaerobic condition (data not shown). One possible is that cystine is converted to cysteine under anaerobic condition. Previous reports suggest that anaerobic conditions would shift this equilibrium toward cysteine.Citation19,20) These results indicated that the yijE promoter is activated by cystine, while the yhdT and yihN promoters are repressed. Specific response to cystine rather than cysteine was supported by a microarray experiment looking at cysteine-responsive genes where none of these genes was significantly altered in expression upon cysteine shock (fold change of all the three genes in duplicated data ranged within 1.5 at 5 min after addition of 100 μM cysteine; data not shown).
Fig. 1. Induction of yijE promoter and repression of yhdT and yihN promoters by addition of cystine.
Notes: The reporter strains containing yijE-lacZ (a), yhdT-lacZ (b), and yihN-lacZ (c) (see Materials and methods) were grown in M9-glucose with (black bars) and without (white bars) cystine (10 μM) until log phase, and then subjected to β-galactosidase assay.
Influence of excess cystine on the sensitivity of E. coli lacking the putative cystine-transporter gene
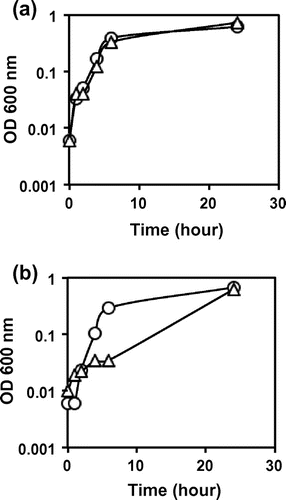
Based on the prediction that knock-out of the gene involved in cystine transport should influence the sensitivity to excess cystine, the inducible expression system was constructed for each of the yijE, yhdT, and yihN genes under the control of the IPTG-inducible lacZ promoter. These strains were grown in M9-glucose medium with and without excess cystine, and the cell growth was compared in the presence and absence of IPTG induction. The growth of wild-type E. coli BW25113 was significantly reduced in the presence of 80 μM cystine, but after prolonged culture, reached to the same level as measured by optical density at 600 nm (Fig. (a)). Among the three candidates of cystine transporter examined, the growth retardation of E. coli BW25113 by the addition of cystine was completely impaired when the yijE gene on the expression plasmid pQE80L was induced in the presence of 0.1 mM IPTG (Fig. (b)). In good agreement of these finding, the yijE-deficient mutant did not completely grow in the presence of 20 μM cystine whereas the wild type did (Fig. ). This finding indicates that the yijE gene expression confers E. coli on the tolerance to excess cystine.
Fig. 2. Resistance to cystine of E. coli by expression of yijE gene.
Notes: BW25113 harboring pQE80L (a) and pYijE (b) were grown in M9-glucose with (triangle) and without (circle) cystine (80 μM). The growth of cultures with reciprocal shaking of an elbow glass tube was automatically measured by TVS062CA (Advantec). This measurement of growth curve was performed three times for each experiment with independent cultures. The fluctuation level among three determinations was less than 20%.
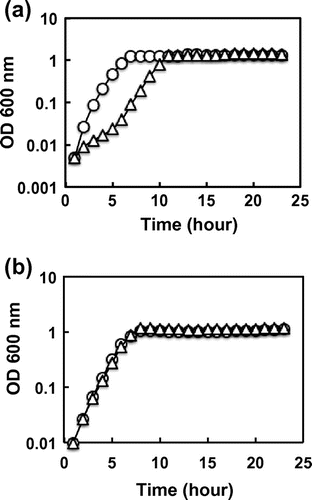
Fig. 3. Sensibility to cystine of the yijE-deficient mutant.
Notes: BW25113 (a) and JW4303 (b) were grown in M9-glucose with (triangle) and without (circle) cystine (20 μM). The growth of cultures with reciprocal shaking of a glass tube was measured by its turbidity at OD 600 nm. This measurement of growth curve was performed three times for each experiment with independent cultures. The fluctuation level among three determinations was less than 15%. The p-values from Student’s t-distribution were 0.29, 0.33, 021, 0.04, <0.01, and 0.39 at 0, 1, 2, 4, 6, and 24 h, respectively, shown in (b).
The drug/metabolite transporter superfamily consists of 14 phylogenetic families,Citation21,22) of which the DME family is the largest, including more than 100 known members for the export of drugs and metabolites. YijE belongs to this DME family of inner membrane protein.Citation21,22) As in the case of other known DME family transporter, YijE carries 10 transmembrane domains and C-terminal domain exposed to the cytoplasm, predicated using C-terminal tagging by Daley et al.Citation23) In this study, we found the induction of the yijE gene in E. coli upon exposure to excess cystine, and moreover the over-expression of the yijE gene confers E. coli on resistance to growth inhibition by excess cystine. Multiple cysteine transporters are known to export cysteine that convert into cystine in the periplasm because high concentration of cysteine rather than cystine is toxic to cells.Citation1,24) The cysteine/cystine shuttle system with multiple cysteine transporters is proposed to play an important role in oxidative stress tolerance.Citation1) YijE may function for maintenance of reducing equivalents by cysteine/cystine balance of the periplasm in the presence of excess cystine.
Acknowledgments
We thank Takahiro Katayama, Takashi Wada, Ayano Tomiyama, and Eri Ishii (Hosei University) for technical assistance. We also thank the National BioResource Project (NBRP) of Japan for providing E. coli JW4303.
Additional information
Funding
References
- Ohtsu I, Wiriyathanawudhiwong N, Morigasaki S, Nakatani T, Kadokura H, Takagi H. The L-cysteine/L-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J. Biol. Chem. 2010;285:17479–17487.10.1074/jbc.M109.081356
- Yamada S, Awano N, Inubushi K, Maeda E, Nakamori S, Nishino K, Yamaguchi A, Takagi H. Effect of drug transporter genes on cysteine export and overproduction in Escherichia coli. Appl. Environ. Microbiol. 2006;72:4735–4742.10.1128/AEM.02507-05
- Pittman MS, Corker H, Wu G, Binet MB, Moir AJ, Poole RK. Cysteine is exported from the Escherichia coli cytoplasm by CydDC, an ATP-binding cassette-type transporter required for cytochrome assembly. J. Biol. Chem. 2002;277:49841–49849.10.1074/jbc.M205615200
- Desai KK, Miller BG. Recruitment of genes and enzymes conferring resistance to the non-natural toxin bromoacetate. Proc. Nat. Acad. Sci. USA. 2010;107:17958–17973.
- Dassler T, Maier T, Winterhalter C, Bock A. Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol. Microbiol. 2000;36:1101–1112.10.1046/j.1365-2958.2000.01924.x
- Franke I, Resch A, Dassler T, Maier T, Bock A. YfiK from Escherichia coli promotes export of O-acetylserine and cysteine. J. Bacteriol. 2003;185:1161–1166.10.1128/JB.185.4.1161-1166.2003
- Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 2001;183:6384–6393.10.1128/JB.183.21.6384-6393.2001
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2006:0008:1–11.
- Jishage M, Ishihama A. Variation in RNA polymerase sigma subunit composition within different stocks of Escherichia coli W3110. J. Bacteriol. 1997;179:959–963.
- Simons RW, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96.10.1016/0378-1119(87)90095-3
- Huerta AM, Collado-Vides J. Sigma70 promoters in Escherichia coli: specific transcription in dense regions of overlapping promoter-like signals. J. Mol. Biol. 2003;333:261–278.10.1016/j.jmb.2003.07.017
- Vanet A, Plumbridge JA, Alix JH. 1993, Cotranscription of two genes necessary for ribosomal protein L11 methylation (prmA) and pantothenate transport (panF) in Escherichia coli K-12. J. Bacteriol. 1993;175:7178–7188.
- Kurata T, Katayama A, Hiramatsu M, Kiguchi Y, Takeuchi M, Watanabe T, Ogasawara H, Ishihama A, Yamamoto K. Identification of the set of genes, including non-annotated morA, under the direct control of ModE in Escherichia coli. J. Bacteriol. 2013;195:4496–4505.10.1128/JB.00304-13
- Casadaban MJ. Transcription and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J. Mol. Biol. 1976;104:541–555.10.1016/0022-2836(76)90119-4
- Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external zinc. J. Bacteriol. 2005;187:6333–6340.10.1128/JB.187.18.6333-6340.2005
- Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 2005;56:215–227.10.1111/j.1365-2958.2005.04532.x
- Yamanaka Y, Ishihama A, Yamamoto K. Induction of YdeO, a regulator for acid resistance genes, by ultraviolet irradiation in Escherichia coli. Biosci. Biotechnol. Biochem. 2012;76:1236–1238.
- Miller JH. Experiments in molecular genetics. New York (NY): Cold Spring Harbor Laboratory Press; 1972.
- Shinohara K, Kilpatrick M. The stability of cystine in acid solution. J. Biol. Chem. 1934;105:241–251.
- Asquith RS, Hirst L. The photochemical degradation of cystine in aqueous solution in the presence of air. Biochim. Biophys. Acta. 1969;184:345–357.10.1016/0304-4165(69)90037-3
- Jack DL, Yang NM, Saier MH Jr. The drug/metabolite transporter superfamily. Eur. J. Biochem. 2001;268:3620–3639.10.1046/j.1432-1327.2001.02265.x
- Pao SS, Paulsen IT, Saier MH Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998;62:1–34.
- Daley DO, Rapp M, Granseth E, Melén K, Drew D, von Heijne G. Global topology analysis of the Escherichia coli inner membrane proteome. Science. 2005;308:1321–1323.10.1126/science.1109730
- Baptist EW, Kredich NM. Regulation of L-cystine transport in Salmonella typhimurium. J. Bacteriol. 1977;131:111–118.
