Abstract
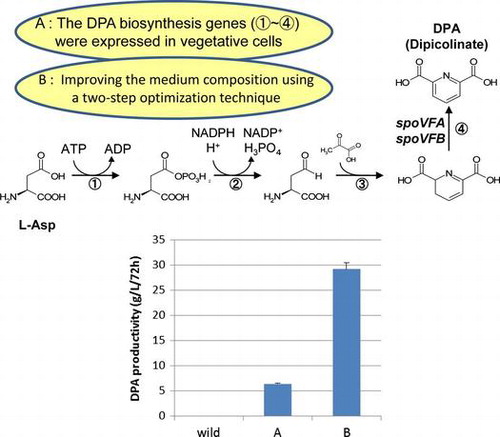
Dipicolinic acid (DPA) is a multi-functional agent for cosmetics, antimicrobial products, detergents, and functional polymers. The aim of this study was to design a new method for producing DPA from renewable material. The Bacillus subtilis spoVF operon encodes enzymes for DPA synthase and the part of lysine biosynthetic pathway. However, DPA is only synthesized in the sporulation phase, so the productivity of DPA is low level. Here, we report that DPA synthase was expressed in vegetative cells, and DPA was produced in the culture medium by replacement of the spoVFA promoter with other highly expressed promoter in B. subtilis vegetative cells, such as spoVG promoter. DPA levels were increased in the culture medium of genetically modified strains. DPA productivity was significantly improved up to 29.14 g/L in 72 h culture by improving the medium composition using a two-step optimization technique with the Taguchi methodology.
Graphical Abstract
Expression of DPA synthase operon in vegetative cells in B. subtilis and the optimization of the medium composition significantly improved DPA productivity.
Spores of various Bacillus species contain high levels (25% of spore core dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA])Citation1) DPA is a multi-functional compound, and its derivatives are expected to use as antimicrobial activity reagents,Citation2) antioxidants,Citation3) biodegradable chelating reagents,Citation4) hair growth-promoting reagents,Citation5) and components of sustainable bio-based polymers.Citation6) Despite the unique features of DPA, there are few reports of DPA production by fermentation.Citation7–10)
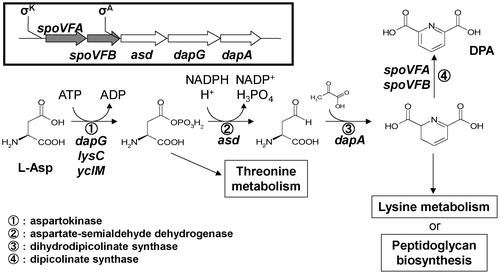
Strains of Bacillus subtilis possess an efficient DPA synthetic pathway, encoded by the spoVF divergon (operons spoVFAB, asd, dapG, and dapA).Citation11) The DPA biosynthesis pathway in B. subtilis is well studied (Fig. ). Aspartate kinase, encoded by dapG, is responsible for the first step of the biosynthesis cascade, producing L-4-aspartyl phosphate from L-aspartate. Aspartate-semialdehyde dehydrogenase, encoded by asd, is responsible for the second step, producing L-aspartate 4-semialdehyde. Dihydrodipicolinate synthase, encoded by dapA, is responsible for the third step, producing L-2,3-dihydrodipicolinate. These steps are also used in lysine biosynthesis. DPA synthase, encoded by spoVFA and spoVFB, is responsible for the final step, producing DPA.Citation12) The promoter of the spoVFA is regulated by the mother cell-specific transcription factor σK in the latter period of sporulation, and thus DPA is made in the sporulation phase and is not observed in vegetative cells. Finally, the uptake of DPA into developing spores is carried out by SpoVAD.Citation13)
Fig. 1. Biosynthetic pathway of dipicolinate in B. subtilis.
Note: Symbols for genes that have been identified are given above the corresponding reaction step. Names of enzymes encoded by the genes described in this paper are also given. Branches of the aspartate pathway that lead to the biosynthesis of threonine, methionine, lysine, and peptidoglycan are shown in the boxes.
To our knowledge, on the other hand, there is no study of DPA production by genetic recombination of B. subtilis. In this study, we examined the forced production of DPA in vegetative cells in expectation of the secretion of DPA. Because forespore, to which synthesized DPA is usually exported, do not exist in this phase. The reason why the spoVFAB operon is not transcribed is sigma K does not exist in vegetative cells. So we tried to replace the promoter of spoVFA with other promoter that expressed in B. subtilis vegetative cells. We used the promoters of a cellulase gene, egl-237Citation14) or early stage sporulation-associated gene, spoVG,Citation15) which are regulated by σA and σH, respectively.
Furthermore, for effective utilization of any microbial system at bioprocess level, it is necessary to optimize various nutritional and environmental requirements for microbial growth and subsequent biochemical production. Statistically planned experiments, such as Taguchi methods,Citation16) are effective for these optimization studies. The Taguchi orthogonal array (OA) experimental design method involves evaluating a given system based on a set of independent variables (factors) over a specific region of interest (levels).Citation16,17) Using this approach, the effects of individual factors can be identified, and the relationship between variables and operational conditions established.Citation18) Analysis of experimental data using analysis of variance (ANOVA) provides the statistical relationship of the output. One statistical design for screening independent variables for medium optimization is to first screen for key factors. The factors selected for study can be either nutritional components or environmental conditions. The second step is to optimize the levels of the key factors identified in the first step.
Here, we aimed to cost-effectively enhance the productivity of DPA by B. subtilis with secretion of DPA into the supernatant and to subsequently optimize the medium component. In this context, we have investigated the optimal nutritional requirements for maximum production of DPA, using genetic recombination B. subtilis by two-stage medium optimization according to the Taguchi method. By optimizing the culture conditions, the DPA was secreted in the broth at levels reaching 29.14 g/L.
Materials and methods
Strains and plasmids
B. subtilis 168 was used as the host.Citation19) pHA237 contains the promoter of egl-237 and Egl-237 cellulase gene from Bacillus sp. KSM-237.Citation14) pC194 contains the chloramphenicol-resistant cassette.Citation20)
Media and cultivation conditions
B. subtilis was grown in Luria–Bertani (LB) medium (1% tryptone [Difco], 0.5% yeast extract [Difco], and 1% sodium chloride). The seed culture was prepared by growing the bacterium in 5 mL of the medium containing 2% tryptone, 1% yeast extract, 0.2% L-tryptophan, 0.3% L-lysine hydrochloride, 1% sodium chloride, 7.5% maltose monohydrate, and 0.00075% MnSO4 5H2O in 50-mL test tubes for 16 h with agitation (250 strokes/min, reciprocal shaker) at 30 °C. Carbon sources and the other ingredients were autoclaved separately for 15 min at 121 °C. When necessary, LB medium and seed medium also contained 0.001% chloramphenicol. Main medium 1 was the same as the seed medium. Main medium 2 contained 0.5% corn steep liquor (Oriental Yeast), 1% yeast extract, 0.2% L-tryptophan, 0.3% L-lysine hydrochloride, 0.05% MgSO4 7H2O, 0.6% urea, 7.5% maltose monohydrate, 0.15% KH2PO4, and 0.35% K2HPO4.Citation21) Main medium 3 contained 3.5% soybean protein powder (Wako), 1% yeast extract, 0.2% L-tryptophan, 0.3% L-lysine hydrochloride, 0.05% MgSO4 7H2O, 0.018% CaCl2·2H2O, 0.0025% FeSO4 7H2O, 0.0022 % MnCl2 4H2O, 7.5% maltose monohydrate, and 0.50% K2HPO4.Citation22) Main medium 4 contained 3.5% monosodium glutamate (Kyowa hakkou), 1% ammonium sulfate, 0.2% L-tryptophan, 0.3% L-lysine hydrochloride, 0.05% MgSO4·7H2O, 0.002% CaCl2·2H2O, 0.005% FeCl3·6H2O, 0.003% MnSO4·5H2O, 0.0005% biotin, 2.0% trisodium citrate dihydrate, 7.5% maltose monohydrate, 0.1% KH2PO4, and 0.1% K2HPO4.Citation23) Main medium 5 contained 2.5% monosodium glutamate, 0.3% yeast extract, 0.2% L-tryptophan, 0.3% L-lysine hydrochloride, 0.05% MgSO4·7H2O, 0.0015% FeSO4·7H2O, 0.00012% CuSO4·6H2O, 0.00006% MnSO4·5H2O, 7.5% maltose monohydrate, and 0.2% K2HPO4.Citation24) These media were used for various secondary metabolite fermentations by some Gram-positive bacteria.
The prepared seed culture was inoculated (2%, v/v) into the main media containing the specified additions (20-mL medium in a 500-mL Erlenmeyer flask) and incubated on a reciprocal shaker (230 strokes/min) at 30 °C or 37 °C.
Table 1. List of primers.
Construction of recombinants
Genetic procedures: standard procedures were used for plasmid preparation, restriction enzyme digestion, ligation, transformation, and agarose gel electrophoresis.Citation25) B. subtilis was transformed by the natural competence method.Citation26) Chromosomal integrations and deletions were confirmed using the appropriate antibiotic marker and polymerase chain reaction (PCR) analysis, and by the examination of the fermentation products.
Chromosomal insertion of the DNA encoding the promoter of egl-237 or spoVG into the spoVFA promoter: Expression of DPA synthase (SpoVFAB) in vegetative cells was achieved by inserting the chloramphenicol-resistant cassette and the egl-237 promoter or the spoVG promoter in the lower region of the spoVFA promoter. Specific primers were designed to amplify the DNA-encoding spoVFA promoter, the upper part of the structural gene of spoVFA, the chloramphenicol-resistant cassette, the egl-237 promoter or the spoVG promoter (Table ). The chloramphenicol-resistant cassette was amplified from vector pC194 using primers 1 and 2; the egl-237 promoter was amplified from vector pHA237 using primers 3 and 4; and the spoVFA promoter, the upper part of the structural gene of spoVFA, and the spoVG promoter were amplified from the genome DNA of B. subtilis 168 using primer sets 5 and 6, 7 and 8, or 9 and 10, respectively. The uniting gene fragment, in order of spoVFA promoter, the chloramphenicol-resistant cassette, the egl-237 promoter or the spoVG promoter, and the upper part of the structural gene of spoVFA, was made by one-step overlap extension PCR using nested primers 11 and 12.Citation27) These constructed DNAs were inserted in the spoVFA promoter region on the B. subtilis 168 genome using the natural competence method. The united products were mixed with competent B. subtilis 168 cells, and the resulting transformants were selected on LB agar plates containing 10 mg/L chloramphenicol. The positive transformants were confirmed by PCR using primers 5 and 8, followed by agarose gel electrophoresis.
Analytical methods
After centrifuging at 15,000 rpm for 3 min to remove the cells and other suspended solids, the supernatant was diluted by double-deionized water. DPA concentrations were determined by reverse-phase high-performance liquid chromatography (HITACHI, Tokyo, Japan) as described previously.Citation28) Glutamate concentrations were determined by enzymatic analysis according to the YAMASA L-Glutamate Assay Kit II (YAMASA, Tokyo, Japan). Total sugar concentrations were determined by colorimetric method.Citation29)
Experimental design and data analysis for optimization of medium composition
Design of experiments: The first phase determined the various factors to be optimized in the culture medium that have a critical effect on DPA yield. Various levels of eight factors (tryptophan, monosodium glutamate, yeast extract, MgSO4·7H2O, metal mix, lysine hydrochloride, maltose monohydrate, and K2HPO4) were selected (Table (A)). The metal mix solution contained 1.5% FeSO4·7H2O, 0.12% CuSO4·5H2O, and 0.06% MnSO4·5H2O. In the next step, a matrix was designed with an L18 orthogonal array (L18 OA, indicating 18 experimental trials). Three levels of each factor were assigned [except tryptophan], with a layout of L18 (21 × 37; Table (B)).
Analysis of experimental data and prediction of performance: The influence of individual factors on DPA production and their performance under optimum conditions were analyzed using the statistical characteristics. Impact of selected factor assigned levels on DPA production was estimated by average analysis (Supplemental data 1). In the Taguchi method, quality is measured based on the deviation of a characteristic from its target value, and the loss function [L(y)] is estimated for the deviation as L(y) = k × (y − m)2, where k denotes the proportionality constant, m represents the target value, and y is the experimental value obtained for each trial.Citation17) For bigger-is-better quality characteristics, the loss function can be written as L(y) = k × (1/y2) and the expected loss function can be represented by E[L(y)] = k × E(1/y2), where E(1/y2) can be estimated from n number of samples as: Σni = 1[1/yi2]/n. In this report, the DPA concentration in the media was defined as the output and sample number (n) was defined as the error factor. The effect of each variable on each response was determined by subtracting the mean response at each level.
Validation: To validate the methodology, a fermentation experiment was performed for DPA production using the optimized culture conditions.
Results
Expression of DPA synthase in B. subtilis vegetative cells
To produce DPA in B. subtilis vegetative cells, we replaced the spoVFA promoter with other promoter that expressed in B. subtilis vegetative cells. We used the egl-237 promoter and the spoVG promoter. Ribosomal binding sites downstream from these promoters were also used. All of the expression cassettes were introduced into the chromosome of B. subtilis 168 in the upper locus of the initiation codon of spoVFA. The strain containing the egl-237 promoter was designated 168E237spoVF, and the strain containing the spoVG promoter was designated 168VGspoVF.
Production of DPA with recombinant B. subtilis
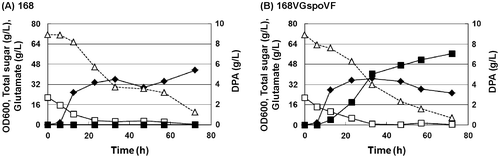
The pre-experiments were performed to investigate the influence of various media on DPA production by B. subtilis 168, 168E237spoVF, and 168VGspoVF. No DPA was produced by B. subtilis 168 in any medium, but 168E237spoVF and 168VGspoVF, which enforced DPA synthase, produced DPA in the medium supernatant (Table ). No significant difference in DPA productivity between genetically modified strains was observed in the medium 1 but higher DPA productivity was detected in the 168VGspoVF culture compared with the 168E237spoVF in medium 2–5. The highest DPA productivity was 6.35 g/L, and it was achieved when 168VGspoVF was cultivated in medium 5. The time-course of DPA productivity of B. subtilis 168 and 168VGspoVF in medium 5 is shown in Fig. . While B. subtilis 168 produced no DPA in any phase, 168VGspoVF produced DPA from the exponential phase to the stationary phase.
Table 2. Effects of various media on DPA production with B. subtilis 168 and genetically modified strains.
Optimization of medium components for DPA production using the two-step Taguchi method
Medium optimization experiments were performed to investigate the influence of the components of medium 5 on DPA production by 168VGspoVF. The L18 design was used to evaluate the effects of the components in medium 5 (Table (A)). Each trial used 168VGspoVF and medium 5 as the basal medium. The experimental and predicted DPA production values varied from 4.49 to 11.00 and 4.22 to 11.01 g/L, respectively (Table (B)).
Table 3. (A) Selected fermentation factors and their levels and (B) experimental layout of fractional design of L18(2 × 37) OA assigned for the first optimization experiment of DPA production by 168VGspoVF.
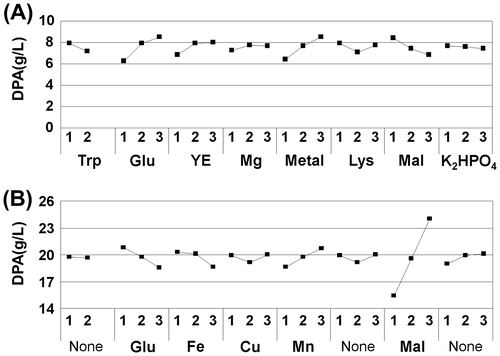
The impact of selected fermentation factors on DPA production varied at the evaluated levels, indicating the influence of individual factor concentrations on DPA production by 168VGspoVF. DPA production progressively increased with an increase in glutamate or the metal mix level, and a decrease in the maltose monohydrate level (Fig. (A), supplemental data 2A).
Fig. 3. Impact of selected factor assigned levels on DPA production by 168VGspoVF (A) in the first and (B) the second L18 optimization experiment.
Note: The “X” axis provides the assigned levels of each selected factor, and the “Y” axis indicates DPA production (g/L). Trp (L-tryptophan), Glu (monosodium glutamate), YE (yeast extract), Mg (MgSO4·7H2O), Metal (metal mix), Lys (L-lysine hydrochrolide), Mal (maltose monohydrate), K2HPO4, Fe (FeSO4·7H2O), Cu (CuSO4·6H2O), Mn (MnSO4·5H2O). Filled squares indicate DPA levels.
The metal mix contained three components, so the medium optimization experiments were performed again. In these experiments, an L18 design was used to evaluate the effects of the monosodium glutamate, FeSO4·7H2O, CuSO4·5H2O, MnSO4·5H2O and maltose monohydrate (Table (A)). The other components were selected based on their most effective levels (Fig. (A)), that is, tryptophan 0.2%, yeast extract 1.5%, MgSO4·7H2O 0.125%, L-lysine hydrochloride 0.1%, and K2HPO4 0.2%.
Table 4. (A) Selected fermentation factors and their levels and (B) experimental layout of fractional design of L18(2 × 37) OA assigned for the second optimization experiment of DPA production by 168VGspoVF.
The experimental and predicted DPA production values varied from 14.59 to 26.46 and 14.20 to 26.23 g/L, respectively (Table (B)), and the variation between these two values ranged from −0.63 to 0.63 g/L, indicating the influence of nutritional and subsequent DPA production. The statistical results based on ANOVA of the medium optimization are shown in supplemental data 2.
DPA production progressively improved with an increase in the maltose and manganese concentrations (Fig. (B)). An increase in the glutamate and iron concentrations decreased DPA production. The data shown in Fig. (B) show the opposite tendency from those shown in Fig. (A). The comparison condition was A1B1C3D3E3F3G3H3 (the experiment No. 3 in Table (B), 8% monosodium glutamate, 0.009% FeSO4·7H2O, 0.00066% CuSO4·5H2O, 0.00033% MnSO4·5H2O, and 10% maltose monohydrate), which was the best condition in the second L18 experiment. As shown in Fig. (B), the theoretical optimized concentration of the medium was A1B1C1D1E3F3G3H3 (8% monosodium glutamate, 0.003% FeSO4·7H2O, 0.00022% CuSO4·5H2O, 0.00033% MnSO4·5H2O, and 10% maltose monohydrate), and the predicted DPA production was 27.77 g/L. Columns A, F, and H were not selected any factor, so same level was selected at comparison condition. Table shows the results of the validation examination. DPA production under the theoretically optimized conditions exceeded the estimate (29.14 g/L, Table ).
Table 5. Validation examination of DPA production by 168VGspoVF.
Discussion
The aim of the present study was to develop an inexpensive DPA production method from natural sources using B. subtilis that highly expressed the spoVF operon. This is the first report of the production of DPA by genetically modified B. subtilis.
Our hypothesis is that the production of DPA in vegetative cells would promote the secretion of DPA by B. subtilis, because B. subtilis forespore do not exist in this phase. We constructed the 168E237spoVF and 168VGspoVF strains that could synthesize DPA before their sporulation phase. Culture of these strains led to the accumulation of DPA in the supernatant (Table ). On the other hand, the growth curves of the parent strain; B. subtilis 168; and 168VGspoVF exhibited no significant differences until 33 h (Fig. ), suggesting that the produced DPA is not leaked by bacteriolysis during growth phase. Thereby, it is likely that DPA is exported to extracellular by a passive or active transporter in B. subtilis. The mechanism of DPA secretion is unclear, and further study is necessary for the elucidation.
There is no general defined medium for DPA production by recombinant B. subtilis because this is the first report. Exploration of all the main nutritional factors and their optimum levels is a complex process. Statistically mediated experimental designs are key to minimizing the need for experimentation.Citation18) In our study, we focused on media used for the production of secondary metabolites among Gram-positive bacteria, including B. subtilis. Statistically based experimental designs proved to be a valuable tool for optimizing the medium for DPA production by recombinant B. subtilis 168VGspoVF.
Statistical optimization of DPA production by 168VGspoVF was investigated under submerged fermentation environments using an L18 OA according to the Taguchi method. Among the eight different bacterial metabolic influential factors, the concentration of monosodium glutamate was the most important factor for DPA production (Fig. (A), supplemental data 2A). The DPA production rate was promoted by an increase in glutamate, suggesting that DPA production might be suppressed by glutamate depletion in culture (Fig. ).
On the other hand, the result of the second optimized experiment was the opposite that of the first experiment with regard to the effect of glutamate. DPA production improved when the glutamate concentration was decreased in the second optimization experiment (Fig. (B)). The reason for this might be the excess nitrogen concentration in the second experiment, because the range of the concentration of glutamate in the second experiment was higher than in the first experiment, and the maltose concentration was lower. By the same reason, the effect of maltose to increase DPA productivity was quite large in the second experiment (Fig. (B)). While the contribution of Fe2+ and Cu2+ was estimated to be very low (Fig. (B), supplemental data 2B), their effects on DPA production were not small. A DPA production improvement of 3.76 g/L was achieved only by adjusting the concentration of the Fe2+ and Cu2+ (Table ). This is one of the problems with the L18 OA analysis; when one factor has an extremely large effect relative to the other factors, the other factors tend to have lower estimated effects.
DPA was obtained at 29.14 g/L under theoretically optimum culture conditions, an amount 4.6-fold higher than that using medium 5. This result is remarkable, because only three experiments were carried out for optimization. To examine all of the components in the medium to optimize DPA production would require at least 11 more experiments. Thus, statistical experimental design provides a practical approach to medium optimization. The medium components evaluated here are inexpensive and readily available, providing adequate amounts of DPA for industrial applications.
Previous reports described several DPA productivity, 334 mg/L by B. subtilis,Citation8) 10.6 g/L by fungus,Citation9) and 10.0 g/L by genetic recombination of Brevibacterium lactofermentum.Citation10) The DPA productivity in this report is greatly high level compared to these prior arts.
On the other hand, the mechanism of high production of DPA by each medium component is still unknown. Due to the highly complex nature of biologic systems, however, DPA production may be more complicated than currently appreciated and requires further investigation.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.978261.
Supplemental Materials
Download Zip (40 KB)Acknowledgments
We thank Dr Yoshinori Takema, and Dr Kazuaki Igarashi of KAO Corporation, Dr Tohru Kobayashi of Japan agency for marine-earth science and technology for helpful comments.
References
- Lundin RE, Sacks LE. High-resolution solid-state 13C nuclear magnetic resonance of bacterial spores: Identification of the α-carbon signal of dipicolinic acid. Appl. Environ. Microbiol. 1988;54:923–928.
- Siddiqi ZA, Khalid M, Kumar S, Shahid M, Noor S. Antimicrobial and SOD activities of novel transition metal complexes of pyridine-2,6-dicarboxylic acid containing 4-picoline as auxiliary ligand. Eur. J. Med. Chem. 2010;45:264–269.10.1016/j.ejmech.2009.10.005
- Murakami K, Yoshino M. Dipicolinic acid as an antioxidant: protection of glutathione reductase from the inactivation by copper. Biomed. Res. 1999;20:321–326.
- Murakami K, Tanemura Y, Yoshino M. Dipicolinic acid prevents the copper-dependent oxidation of low density lipoprotein. J. Nutr. Biochem. 2003;14:99–103.10.1016/S0955-2863(02)00252-8
- Takezawa H, Naito K, Suzuki I, Okura A. Hair growth agent. Japan patent JP1989301612A. 1989.
- Izaki T, Mimizuka T, Takanishi K. Method for producing polyamide resin, polyamide resin and molding comprising the same. Japan patent JP2006137820A. 2006.
- Hodson PH, Darlington WA. Process for preparing dipicolinic acid. US patent US19673334021A. 1967.
- Killinger A, Peschke G. Verfahren zur gewinnung von pyridin-2,6-dicarbonsaure auf fermentativem wege (A method for extraction of pyridin2,6-dicarboxylic acid via fermentation routes). German patent DE19742300056A. 1974.
- Forrester RB, Vipond PW. Process for the production of dipicolinic acid. Great Britain patent GB 19781505654A. 1978.
- Sawai H, Nagao E, Yamada M, Echigo Y. 2,6-Pyridinecarboxylic acid or its salt, method for producing the same and chelating agent. Japan patent JP2002371063A. 2002.
- Chen NY, Jiang SQ, Klein DA, Paulus H. Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase. J. Biol. Chem. 1993;268:9448–9465.
- Daniel RA, Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis. J. Mol. Biol. 1993;232:468–483.10.1006/jmbi.1993.1403
- Li Y, Davis A, Korza G, Zhang P, Li YG, Setlow B, Setlow P, Hao B. Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 2012;194:1875–1884.10.1128/JB.00062-12
- Hakamada Y, Hatada Y, Koike K, Yoshimatsu T, Kawai S, Kobayashi T, Ito S. Deduced amino acid sequence and possible catalytic residues of a thermostable, alkaline cellulase from an alkaliphilic bacillus strain. Biosci. Biotechnol. Biochem. 2000;64:2281–2289.10.1271/bbb.64.2281
- Eymann C, Mittenhuber G, Hecker M. The stringent response, σH -dependent gene expression and sporulation in Bacillus subtilis. Mol. Gen. Genet. 2001;264:913–923.10.1007/s004380000381
- Taguchi G. Introduction to quality engineering. White Plains (MI): UNIPUB/Kraus International; 1986.
- Roy RK. A primer on the Taguchi method. Dearborn (MI): Society of Manufacturing Engineers; 1990.
- Prakasham RS, Rao ChS, Rao RS, Lakshmi GS, Sarma PN. L-asparaginase production by isolated Staphylococcus sp.-6A: design of experiment considering interaction effect for process parameter optimization. J. Appl. Microbiol. 2007;102:1382–1391.10.1111/jam.2007.102.issue-5
- Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Cummings NJ, Daniel RA, Denizot F, Devine KM, Düsterhöft A, Ehrlich SD, Emmerson PT, Entian KD, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim SY, Glaser P, Goffeau A, Golightly EJ, Grandi G, Guiseppi G, Guy BJ, Haga K, Haiech J, Harwood CR, Hénaut A, Hilbert H, Holsappel S, Hosono S, Hullo MF, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee SM, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado RP, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park SH, Parro V, Pohl TM, Portetelle D, Porwollik S, Prescott AM, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror SJ, Serror P, Shin BS, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa H-F, Zumstein E, Yoshikawa H, Danchin A. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256.10.1038/36786
- Gros MF, Riele H, Ehrlich SD. Rolling circle replication of single-stranded DNA plasmid pC194. EMBO J. 1987;6:3863–3869.
- Lee KH, Park YH, Han JK, Park JH, Lee KH, Choi H. Microorganism for producing riboflavin and method for producing riboflavin using the same. WIPO patent WO2004050862 A. 2004.
- Yoneda T, Miyota Y, Furuya K, Tsuzuki T. Production process of surfactin. WIPO patent WO200226961A. 2002.
- Goshima A, Kunioka M. Production of gamma-polyglutamic acid. Japan patent JP5304977A. 1993.
- Hagihara H, Araki H. Method for producing unsaturated fatty acid or derivative of the same. Japan patent JP2005065658A. 2005.
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1982.
- Cutting SM, Vander-Horn PB. Genetic analysis. In: Harwood CR, Cutting SM, editors. Molecular biological methods for Bacillus. Chichester: Wiley; 1990. p. 27–74.
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68.10.1016/0378-1119(89)90359-4
- Paulus H. Determination of dipicolinic acid by high-pressure liquid chromatography. Anal. Biochem. 1981;114:407–410.10.1016/0003-2697(81)90502-9
- DuBois M. Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356.10.1021/ac60111a017