Abstract
A colorimetric method for the reducing monosaccharide determination is optimized for the assay of glucose isomerase, which converts glucose (Glc) to fructose (Fru). Test solution was mixed with 20-fold volume of the 50 mM Na2SiO3, 600 mM Na2MoO4, and 0.95 M HCl aqueous solution (pH 4.5), in which a yellow molybdosilicate species was formed. The mixture was kept at 70 °C for 30 min. Test solution containing 10 mM level Fru gave a remarkable blue reaction mixture, in which the Mo(VI) species was reduced by Fru to form a blue molybdosilicate species. The blueness increased with the Fru concentration. Glc cannot render the reaction mixture blue as strong as Fru. Thus, the colorimetric method can be used advantageously for the determination of 10 mM level Fru in the Glc isomerase reaction mixture, even in the presence of 100 mM level Glc, and has been applied successfully to the microtiter plate assay of the enzyme.
Graphical Abstract
Our colorimetric method for the fructose determination can be advantageously used for the microtiter plate assay of glucose isomerase.
Glucose isomerase (d-xylose ketol-isomerase; EC. 5.3.1.5) converts d-glucose (Glc) and d-xylose to d-fructose (Fru) and d-xylulose, respectively,Citation1), and the enzyme is widely used in industrial production of high-fructose corn syrup.Citation2) Many quantification methods to determine Fru concentration, for example, an enzymatic, chemoselective reduction,Citation3) HPLC, and cysteine–carbazoleCitation4) methods have been developed, and they have been applied to the glucose isomerase assay. However, problems still remain for the assay. Glucose isomerase has a high Km value of several hundred mM for Glc,Citation1) and the assay of the enzyme consequently needs high amount of Glc. The initial increase of the Fru concentration is very laborious to measure accurately and precisely in the presence of excess Glc. The general quantification of Fru by HPLC using refractive index detectorCitation5) or labeling reagentsCitation6) is interfered by the high concentration of Glc. Although the colorimetric cysteine–carbazole method is specific for the determination of a ketose,Citation4) it is subject to time dependency and low reproducibility, which requires skilled experience. Furthermore, these traditional methods for glucose isomerase assay are ineligible for rapid processing of multiple samples.
In a previous study,Citation7) we have presented a colorimetric method for the determination of reducing monosaccharide. The method is based on the reduction of Mo(VI) species directly by the saccharide to form blue molybdosilicate species in a Si(IV)–Mo(VI) organic water-mixed solution, which was initially yellowish due to the formation of yellow molybdosilicate. The method is simple and easy to carry out in comparison with the conventional colorimetric monosaccharide determination using copper(II) reagent.Citation8,9) Since poly- and oligosaccharides would not interfere with the determination of monosaccharide at same concentration level, the colorimetric method can be applied advantageously to the microtiter plate assay of saccharifying enzyme.
The sensitivity of our colorimetric assay depends on the reduction rate, which is different among monosaccharides. Therefore, the method would be utilized in the assay of enzyme, which catalyzes the conversion between monosaccharides. In this study, we found that, in a Si(IV)–Mo(VI) aqueous system, the reduction rate by Fru is much faster than that by Glc, suggesting that Fru would be determined colorimetrically even in the presence of excess Glc. Thus, we examined the applicability of the colorimetric method to the plate assay of glucose isomerase.
Materials and methods
Chemicals. Sodium metasilicate enneahydrate, disodium molybdate(VI) dihydrate, and the 5 M HCl solution for volumetric analysis were obtained from Wako Pure Chemical Industries, Ltd, and were used to prepare the Si(IV)–Mo(VI) solution. d(+)-Fructose, d(+)-glucose, and d(+)-xylose were obtained from Wako Pure Chemical Industries, Ltd. d-xylulose was obtained from Sigma. Glucose isomerase from Streptomyces rubiginosus was obtained from Hampton Research. These were used without further purification. Other chemicals were of reagent grade and were used as received.
The Si(IV)–Mo(VI) solution was prepared as follows: A 6 mL of the 1 M Na2MoO4 aqueous solution was mixed with 2 mL of the 0.25 M Na2SiO3 aqueous solution. A 1.9 mL of 5 M HCl aqueous solution was added slowly with stirring. The mixture was filled up to 10 mL to obtain the 50 mM Na2SiO3, 600 mM Na2MoO4, and 0.95 M HCl solution (pH 4.5).
Spectrophotometric measurements. Test solution was mixed with 20-fold volume of the Si–Mo(VI) solution, which was stored at 70 °C, and the mixture was kept at the temperature for a given time. The absorption spectra and the absorbance at 750 nm vs. time, A750-t, curves of the reaction mixtures were recorded by a spectrophotometer (JASCO V-630) with a temperature controller. The pass length was 1 cm, and distilled water was used as the blank solution.
Microtiter plate assay. The monosaccharide assay with multiwell microtiter plate was performed as follows. A 10 μL aliquot of the test solution was transferred into the wells. Each test solution was mixed with 200 μL of the Si(IV)–Mo(VI) solution, which was stored at room temperature until the reaction started. The microtiter plate was heated at 70 °C for 30 min, using a plate shaker-thermostat (BioSan PST-100HL). Then, the plate was cooled down to room temperature or lower, and the A750-value of the reaction mixture in the well, A′750, was measured with a microplate reader (Bio-Rad iMark).
Glucose Isomerase Assay. Glucose isomerase was added to a 200 mM NaH2PO4-Na2HPO4 buffer (pH 7.3) containing 400 mM Glc and 10 mM MgCl2, and the mixture was incubated at 60 °C for 1 h. Immediately after the incubation, 1/5 volume of 1 M HCl was added to terminate the enzymatic reaction, and the 10 μL aliquot of the mixture was examined by the present microtiter plate assay. The enzyme was assayed also by the method for ketose determination using phenol–acetone–borate reagent.Citation3)
Results and discussion
Formation of blue molybdosilicate by Fru and Glc
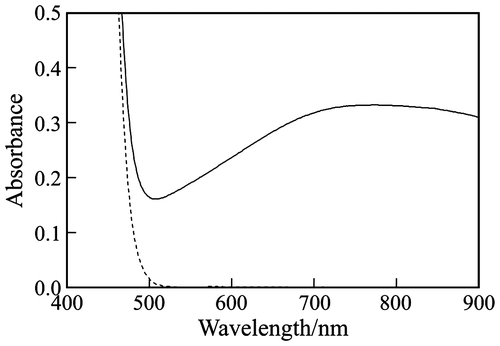
Dashed line in Fig. shows the absorption spectrum of the Si(IV)–Mo(VI) solution, which was yellowish due to the formation of the yellow 12-molybdosilicate ([SiMo12O40]4−) and/or the slightly yellow 11-molybdosilcate ([HnSiMo11O39](8−n)−) anions, according to the reactionsCitation10):(1)
Fig. 1. Absorption spectra of the Si(IV)–Mo(VI) solution (pH 4.5) consisting of 50 mM Na2SiO3, 600 mM Na2MoO4, and 0.95 M HCl (dashed line), and the reaction mixture (70 °C, 30 min) of the 20 mM Fru test solution and 20-fold volume of the Si(IV)–Mo(VI) solution (solid line).
and(2)
The 20 mM Fru test solution gave a bluish reaction mixture, of which the absorption spectrum is shown by solid line in Fig. . An absorption maximum was observed at around 750 nm, indicating the formation of a blue 12-molybdosilicate anionic species.Citation11)
Curve a in Fig. shows the A750-t curve of the reaction mixture at 70 °C. Within the time range tested, the A750-value increased monotonously and did not reach at a constant value, indicating the color reaction did not terminate. Dashed line in the figure shows the regression line at t ≤ 30 min. In the time rage, the A750-value increased linearly, indicating that the decrease in bulk concentration of Fru can be neglected. When t > 30 min, the experimental A750-value became lower than the calculated one by the regression line. Thus, the color reaction was performed for 30 min below.
Fig. 2. Absorbance at 750 nm vs. time, A750-t, curves of the reaction mixtures at 70 °C given by the 20 mM (a) Fru and (b) Glc test solutions.
Note: Dashed line, regression line of curve a when t < 30 min.
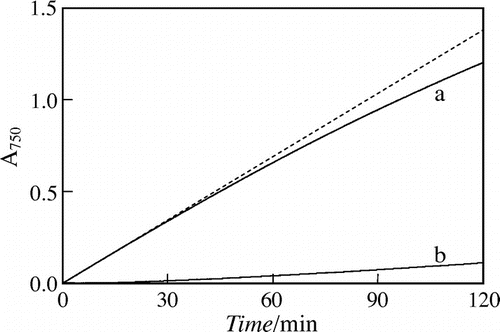
Curve b in Fig. shows the A750-t curve given by the 20 mM Glc test solution. Within the time range tested, the A750-value increased linearly, and the slope was about 1/20 of that by the initial increase of 20 mM Fru, indicating that the blue molybdosilicate formation by Glc was much slower than that by Fru. In the previous study, the 50 mM Na2SiO3, 600 mM Na2MoO1, 1.5 M CH3COOH, and 0 or 30%(v/v) DMSO solutions were used for the reaction media. In the Si(VI)–Mo(VI) aqueous (pH 4.9) and organic-aqueous media, the blue color-development by saccharide was faster than that in the present aqueous (pH 4.5) medium, but the initial slope of A750-t curve by Glc was somewhat larger than 1/20 of that by Fru. Thus, the use of present Si(IV)–Mo(VI) solution is more preferable for the Fru determination in glucose isomerase assay.
Microtiter plate assay of Fru in the presence of Glc
The microtiter plate assay for the monosaccharide quantification was performed with test solutions at different concentrations of Fru, cFru. As shown by Fig. (A), the A′750-value increased linearly with cFru from 0 to 160 mM, indicating that 10 mM level Fru can be determined by the present method. In the figure, the error bar denotes the 95% confidential limit of the mean value of A′750 (n = 5). In the present microtiter plate assay, the color reaction during the mixing operation and the spectrophotometric measurement was negligible, because of much slow reaction rate at room temperature.Citation7) Also, the Si(IV)–Mo(VI) solution is well buffered with 0.95 M HCl. These may be reasons of the high reproducibility.
Fig. 3. Plots of the A750 of the reaction mixture in the well, A′750, against the concentration of (A) Fru and (B) Glc in the test solution, cFru and cGlc, respectively.
Note: Error bar, 95% confidential limit of the mean value (n = 5).
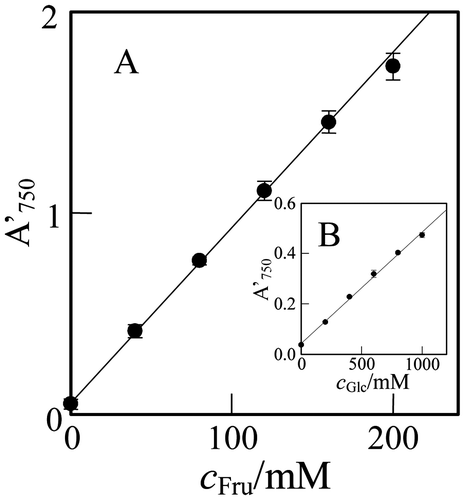
As shown by Fig. (B), the A′750-value increased linearly with the concentration of Glc in test solution, cGlc, from 0 to 1000 mM, indicating that 100 mM level Glc can be determined. However, the Glc assay would become more sensitively by using a Si(IV)–Mo(VI) organic water-mixed solution.Citation7) The slope of the regression line for Glc was 1/20 of that for Fru. The result coincides with the prediction from the A750-t curves in Fig. .
The A′750-values for the 20 mM ketose, such as d-xylulose, d-sorbose, and l-psicose, and aldose, such as d-xylose, d-galactose, d-mannose, and d-arabinose, are listed in Table , together with those for Fru and Glc. The A′750-values for the aldoses were not significantly large in comparison with blank value. On the other hand, except for psicose, ketoses gave larger A′750-value than the aldoses.
Table 1. A′750-values for 20 mM saccharide solutions.
The above results indicate that the method can be utilized to the determination of Fru in the glucose isomerase reaction mixture. In this study, glucose isomerase was examined in the presence of 400 mM Glc, because of the large Km value.Citation1) Thus, test solutions consisting of cFru mM Fru and (400 – cFru) mM Glc were assayed. As shown in Fig. , the A′750-value increased linearly with cFru. The regression line is given as(3)
Fig. 4. Plot of the A′750, against cFru in the test solution containing (400 – cFru) mM Glc.
Note: Error bar, 95% confidential limit of the mean value (n = 5).
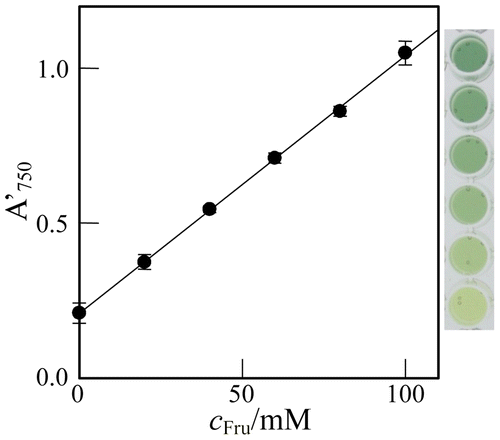
with the mean square of errors, MSE = 6 × 10−5.Citation12) The detection limit was calculated to be 3(MSE)1/2/0.0083 = ~3 mM, indicating again that 10 mM level Fru can be determined in the enzymatic reaction mixture even in the presence of 100 mM Glc. As shown in the figure, the test solution of cFru = 0 and cGlc = 400 mM did not render the reaction mixture blue remarkably, and the blueness of the mixture increased remarkably with cFru. Thus, 10 mM level Fru can be detected also by visual observation.
Glucose isomerase assay
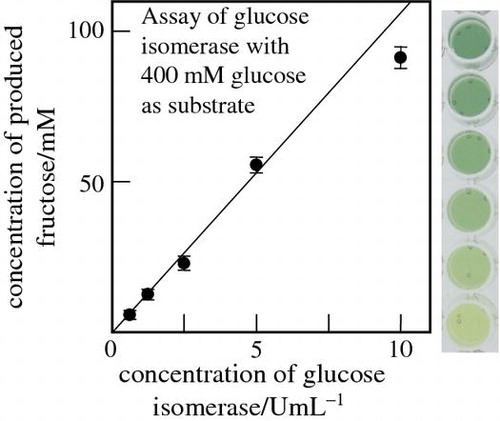
Glucose isomerase was examined with different concentrations, cGI, and the produced amount of Fru was determined by the present method with the calibration curve in Fig. . Fig. (A) shows the plot of the determined cFru-value against cGI. The cFru-value increased with cGI in the range tested. It is noted that, when the Mg2+ ion was absent from the enzymatic reaction mixture, the cFru-value became much lower. Here, before the Fru assay, the enzymatic reaction mixture was terminated by the acidification. We examined also the reaction mixture without the termination. The determined A′750-values were nearly equal to those in Fig. , indicating that the enzymatic reaction was terminated by mixing the Si(IV)–Mo(VI) solution.
Fig. 5. Plots of the cFru in the glucose isomerase reaction mixture (see text) determined by the present method against (A) the enzyme concentration, cGI, and (B) cFru determined by the conventional ketose assay using phenol–acetone–borate reagent.
Note: Error bar, 95% confidential limit of the mean value (n = 5).
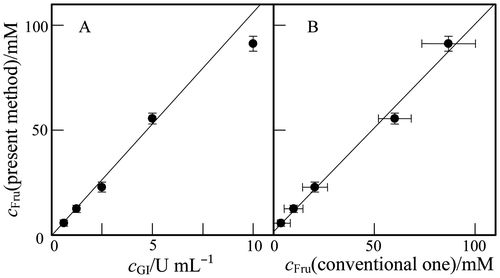
Glucose isomerase has been assayed also by the conventional method for ketose determination.Citation3) In Fig. (B), the cFru-value by the present method was plotted against that determined by the conventional one. These cFru-values are in good agreement with each other, so that the regression line gave the slope of 1.0 ± 0.1 and the intercept of 2 ± 8. Thus, it can be stated that the present method can be applied to the Fru determination in glucose isomerase assay.
As shown in Fig. (B), the experimental error in the present method was much smaller than that in the conventional one. The Si(IV)–Mo(VI) solution can be prepared easily and was stable at room temperature as least 1 month. The color change of the reaction mixture from yellow to blue would be easy to distinguish more than the purplish pink color in the conventional method.
In conclusion, the colorimetric present method can be applied advantageously to the high-throughput microtiter plate assay of glucose isomerase.
Funding
This work was supported by grant-in-aid from Ministry of Education, Science, Sports and Culture of Japan (No. 26410226).
Acknowledgments
This work was supported by grant-in-aid from Ministry of Education, Science, Sports and Culture of Japan (No. 26410226).
References
- Takasaki Y, Kosugi Y, Kanbayashi A. Studies on sugar-isomerizing enzyme purification, crystallization and some properties of glucose isomerase from Streptomyces sp. Agric. Biol. Chem. 1969;33:1527–1534.10.1271/bbb1961.33.1527
- Bhosale SH, Rao MB, Deshpande VV. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996;60:280–300.
- Boratynski J. Colorimetric method for the determination of ketoses using phenol–acetone–boric acid reagent (PABR). Anal. Biochem. 1984;137:528–532.10.1016/0003-2697(84)90122-2
- Dische Z, Borenfreund E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J. Biol. Chem. 1951;192:583–587.
- Itoh T, Mikami B, Hashimoto W, Murata K. Crystal structure of YihS in complex with D-mannose: Structural annotation of Escherichia coli and salmonella enterica YihS-encoded proteins to an aldose–ketose isomerase. J. Mol. Biol. 2008;377:1443–1459.10.1016/j.jmb.2008.01.090
- Honda S, Akao E, Suzuki S, Okuda M, Kakehi K, Nakamura J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem. 1989;180:351–357.10.1016/0003-2697(89)90444-2
- Katano H, Taira S, Uematsu K, Kimoto H. Colorimetric determination of glucosamine and glucose based on the formation of blue molybdosilicate anion and its application to the assay of saccharifying enzyme. Anal. Sci. 2013;29:1021–1025.10.2116/analsci.29.1021
- Tauber H, Kleiner IS. A method for the determination of monosaccharides in the presence of dissacchrides and its application to blood analysis. J. Biol. Chem. 1932;99:249–255.
- Milner Y, Avigad G. A copper reagent for the determination of hexuronic acids and certain ketohexoses. Carbohydr. Res. 1967;4:359–361.10.1016/S0008-6215(00)80191-3
- Katano H, Osakai T, Himeno S, Saito A. A kinetic study of the formation of 12-molybdosilicate and 12-molybdogermanate in aqueous solutions by ion transfer voltammetry with the nitrobenzene–water interface. Electrochim. Acta. 1995;40:2935–2942.10.1016/0013-4686(95)00225-4
- Katano H, Maruyama C, Hamano Y. Detection of biopolymer ε-poly-L-lysine with molybdosilicate anion for screening of synthetic enzymes. Int. J. Polym. Anal. Charact. 2011;16:542–550.10.1080/1023666X.2011.620289
- Montgomery DC, Runger GC, Applied statistics and probability for engineers. (New York): Wiley; 1994. p. 553.
