Abstract
Methyl, propyl and hexyl esters of rosmarinic, caffeic and p-coumaric acids were tested for antiallergic activity, and rosmarinic acid propyl ester exhibited the greatest β-hexosaminidase release suppression (IC50, 23.7 μM). Quadratic correlations between pIC50 and cLogP (r2 = 0.94, 0.98, and 1.00, respectively) were observed in each acid ester series. The antiallergic activity is modulated by hydrophobicity, and alkyl chain bulkiness.
Rosmarinic acid (RA), a major bioactive compound in rosemary, mint, basil, and perilla exerts a variety of biological activities, such as antioxidantCitation1) and anti-inflammatoryCitation2) activities, and reducing liver injury.Citation3) Additionally, RA has been shown to suppress inflammation and to alleviate the symptoms of type I allergic conditions, such as allergic asthmaCitation4) and seasonal allergic rhinoconjunctivitis, in animals and humans.Citation5,6) RA suppresses allergic inflammation by inhibiting IgE response, inflammatory cytokine production, and COX-2 expression.Citation2)
Mast cells, widely distributed throughout the body as a part of the immune system, play an important role in both immediate and late-phase response of type I allergy. The activation of mast cells is initiated by antigen-induced cross-linking of IgE to IgE receptors (FcεRI),Citation7,8) followed by a series of signaling events, leading to phosphorylation of tyrosine kinases, and mobilization of Ca2+ into cytosol.Citation9) Within minutes of activation, mast cells secret several mediators, such as β-hexosaminidase and histamine, that result in acute inflammation and chronic allergic disease.Citation7) The RBL-2H3 mast cell line is considered an excellent model system for the study of mast cell degranulationCitation10) and screening for anti-allergic reagents.Citation10,11) Regarding inhibition of degranulation of RBL 2H3 cells by phenolic compounds, so far there are four possible mechanisms. The first possibility is the inhibition of binding between the FcεRI receptor and an allergen–IgE complex. Apple extract and brown algae phlorotannins inhibit binding between IgE and FcεRI receptor, suppressing mast cell activation.Citation12,13) The second is due to binding inhibition between a cross-linked IgE/FcεRI receptor and the antigen.Citation14) The third is to form insoluble complexes or aggregates with the allergens, inhibit their binding to IgE, and reducing their allergenic capacity.Citation15) The fourth one is the suppression of FcεRI receptor expression in mast cells.Citation16)
We recently isolated a RA-rich extract with high anti-allergic activity from Perilla leaves using a supramolecular separation method.Citation17) In that study, isolation of RA was attained without the use of preparative HPLC, therefore, enabling mass production of RA at a relatively lower cost and widening the possibilities for its use as a functional ingredient in food industries. RA methyl ester, a minor component in Perilla leaf extract, suppressed β-hexosaminidase release 14.3 times greater than RA.Citation12) Therefore, the esterification of such phenylpropanoids is highly expected to enhance antiallergic activity. In this paper, we explored the potential of RA and related compounds as therapeutic agents for type I allergies, and investigated the effects of esterification as an approach to enhance their antiallergic activity. As the assay we have used begins by sensitizing the mast cells with IgE, suppression of degranulation can be attributed to the inhibition of binding of a cross-linked IgE/FcεRI receptor and the antigen.
Esters of RA, caffeic acid (CA), p-coumaric acid (Cou), ferulic acid (FA), and cinnamic acid (Cin) were prepared by reaction with methanolic HCl. Three to four hundred milligrams of the free acids were individually dissolved in 25 mL methanol containing 1% HCl. The reaction was carried out at room temperature and monitored by ultrafast liquid chromatography. The reaction solution was diluted with water (50 mL) and extracted with diethyl ether (50 mL) in a separating funnel. After the diethyl ether layer was collected and washed with water and 5% NaHCO3 aqueous solution, the organic layer was evaporated to dryness. The propyl and hexyl esters were synthesized as described above, using 1-propanol and 1-hexanol in place of methanol.
The antiallergic activity of the free acids and synthesized esters was assessed by their ability to inhibit β-hexosaminidase release from RBL-2H3 cells, using the following method.Citation17,18) In brief, the RBL-2H3 cells were precultured in a 24-well plate (2.5 × 105 cells/well) at 37 °C under a humidified 5% CO2 atmosphere overnight. The cells were washed with PBS and incubated with 500 μL antibody solution (mouse monoclonal anti-dinitrophenyl antibody, 50 ng/mL) for 2 h’ sensitization. A test sample solution of 490 μL was added to each well after washing with modified Tyrode’s buffer (MT buffer). The MT buffer was used as control instead of the sample. Albumin dinitrophenyl (10 μL, final concentration = 50 ng/mL) was added to each well to evoke allergic reactions (degranulation) of the cells for 30 min. The supernatants (50 μL) were transferred into wells (96-well microplate) and incubated with 100 μL substrate (3.3 mM p-nitrophenyl-2-acetamide-2-deoxy-β-d-glucopyranoside) at 37 °C for 25 min. The absorbance (OD) was measured at 405 nm using a microplate reader. The gaining OD reflects β-hexosaminidase release. The calculation was performed using Equations (Equation1(1) ) and (Equation2
(2) ) below. In “blank,” neither antibody solution nor sample was added to cells to account for spontaneous β-hexosaminidase release from cells. In “control,” MT buffer instead of samples was added to cells to account for β-hexosaminidase release from cells in conditions without a sample. In “total,” cells were lysed by 0.1% Triton X-100 in MT buffer to measure the total amount of β-hexosaminidase contained in the cells. In “sample,” both antibody solution and samples were added to cells to measure β-hexosaminidase release from cells in the presence of our test compounds.
(1)
The ratio of β-hexosaminidase release (%) of “control” against “total” (Triton X-100) ranged from 29 to 50%. Relative rate of β-hexosaminidase release of “sample” against “control” was expressed as β-hexosaminidase release (%) as shown below,(2)
The data are presented as mean ± standard derivation (SD) of quadruplicate wells. Statistical differences were assessed using one-way ANOVA, followed by the New–Keuls test. p values below 0.05 were considered statistically significant. Statistical analysis was carried out by GraphPad PRISM 5.
RA, CA, Cou, FA, Cin, and their methyl esters suppressed β-hexosaminidase release in a dose-dependent manner up to the concentration of 2.0 mM, as shown in Fig. . The IC50 values for all methyl esters were below 0.4 mM (RA-me, 0.06 mM; CA-me, 0.12 mM; Cou-me, 0.18 mM; FA-me, 0.14 mM; and Cin-me, 0.33 mM), and thus, smaller than the IC50 values of all free acids (RA, 1.31 mM; CA, 1.46 mM; Cou, 1.04 mM; FA, 0.98 mM; and Cin, 1.50 mM). RA-me exerted the greatest β-hexosaminidase release suppression (IC50, 0.06 mM), followed by CA-me (IC50, 0.12 mM) among all of the methyl esters. CA-me suppressed β-hexosaminidase release similar to RA-me, and then similar suppression activity was observed between RA (1.31 mM) and CA (1.46 mM). It is indicated that β-hexosaminidase release suppression of RA and its methyl ester is mainly due to its CA moiety, which may interact with a cross-linked IgE/FcεRI receptor of RBL-2H3 cells. This is partly supported by the results that 3-(3,4-dihydroxyphenyl)lactic acid (a similar moiety of RA partial structure) related compounds did not suppress β-hexosaminidase release at all.
Fig. 1. Antiallergic activity of RA, its related compounds, and their methyl esters by β-hexosaminidase release suppression.
Note: Each IC50 value represents mean ± SD in quadruplicate. Abbreviation of compounds shown here was followed the abbreviation list.
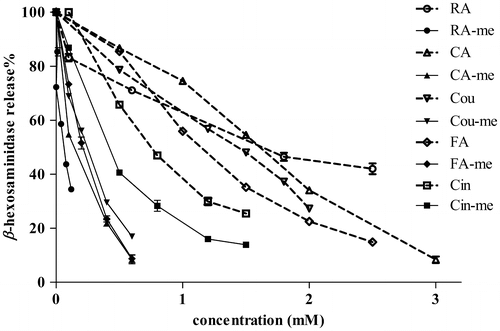
As a greater suppression of β-hexosaminidase release was achieved by methyl esterification of such phenylpropanoids was observed, we investigated the effects of longer alkyl chain esters of RA and its related phenylpropanoids on β-hexosaminidase release suppression. Furthermore, RA-me, RA-pro, and RA-hex suppressed β-hexosaminidase release to a greater extent than the corresponding esters of CA and Cou (Table ). Among all free acids and esters, rosmarinic acid propyl ester (RA-pro, IC50, 23.7 μM) exhibited the greatest suppression on β-hexosaminidase release, followed by rosmarinic acid hexyl ester (RA-hex, IC50, 34.5 μM), caffeic acid propyl ester (CA-pro, IC50, 55.3 μM), and rosmarinic acid methyl ester (RA-me, IC50, 57.3 μM), as shown in Table . The activity of rosmarinic acid propyl ester was only 9.1 times lower than luteolin (IC50, 2.6 μM), a flavonoid with high antiallergic activity that was tested in a parallel experiment of our laboratory,Citation19) but increased 55.4 times compared with the activity of RA.
Table 1. β-Hexosaminidase release suppression and calculated octanol–water partition coefficient of esters of RA, CA, and p-coumaric acid.
In addition, it was recently found that RA can be easily extracted from natural resources in a large scale with a newly established isolation method from Perilla leaves using a supramolecular technique,Citation17) implying that RA, the precursor of RA esters, can be easily supplied from natural resources such as Perilla leaves as well as other Lamiaceae family plants that are rich in RA. Furthermore, for applications in the food industries, we suggest that the ethyl esters might be a good choice as their synthesis can be carried out with ethanol, regarded as a safe ingredient in food manufacturing. Alcoholic beverages or other processed foods containing Perilla leaves may bring great benefit for alleviating allergy symptoms.
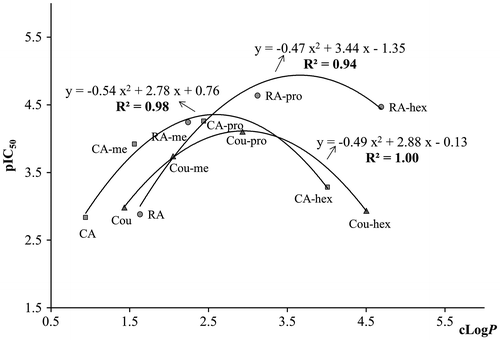
The octanol–water partition coefficient (LogP), a measurement of molecular hydrophobicity, is a vital parameter for membrane permeability, bioavailability, and hydrophobic drug–receptor interactions. Regarding the interaction between polyphenols and receptors, isoflavones are known to bind estrogen receptors and the hydrophobic aglycone binds more than their corresponding glycosides.Citation20) The hydrophobicity of peroxisome proliferators was found to be positively correlated with their binding affinity to mouse peroxisome proliferator-activated receptor (mPPARα).Citation21) Therefore, it is plausible that in our study the hydrophobicity of RA and related compounds may have affected their interaction with a cross-linked IgE/FcεRI receptorCitation8) at the cell surface, inhibiting the signaling events leading to degranulation of RBL-2H3 cells. LogP values were calculated (cLogP in Table ) using the software, Molinspiration Cheminformatics 2014 (http://www.molinspiration.com/cgi-bin/properties), and plotted against pIC50 (−logIC50) values for β-hexosaminidase release (Fig. ). For the RA ester series, as cLogP increased from 1.6 (RA) to 4.7 (RA-hex), β-hexosaminidase release suppression was enhanced from IC50 value of 1313.7 μM (RA) to 23.7 μM (RA-pro), and then decreased to 34.5 μM (RA-hex) (Table ). For CA and Cou ester series, a similar tendency of β-hexosaminidase release suppression was observed to that of RA ester series. Consequently, strong quadratic correlations were observed between cLogP and pIC50 for each of the acid esters series, with correlation coefficients equal to 0.94 (p < 0.05) for the RA series, 0.98 (p < 0.01) for the CA series, and 1.00 (p < 0.01) for the Cou ester series (Fig. ). LogP values for maximal suppression of β-hexosaminidase release were calculated from the individual quadratic equations (Fig. ) as 3.7 for RA esters, 2.6 for CA esters, and 2.9 for Cou esters. The alkyl esters chain lengths deduced from these values would correspond to butyl ester for RA, propyl ester for CA, and propyl ester for Cou (Fig. ). Furthermore, RA butyl ester is expected to have a higher pIC50 value than that was observed for the propyl ester, as deduced from the quadratic equation (y = −0.47 x2 + 3.44 x − 1.35) to be 11.4 μM. The quadratic equations reflect the lowering of antiallergic effects of hexyl esters after the very strong effects of shorter esters. Our results showed that the hydrophobic propyl esters of RA, CA, and Cou strongly suppressed β-hexosaminidase release, thus, it is possible that propyl esters have higher affinity to the cross-linked IgE/FcεRI receptor, while the bulky hexyl esters would bind less efficiently. It has already been reported that steric effects of ligands can interfere with the binding to the cross-linked IgE/FcεRI receptor, influencing mast cell degranulation.Citation22,23) Regarding enzyme reactions, a quadratic correlation between the hydrophobicity of 4-sulfamate derivatives with different alkyl chain length and estrone sulfatase inhibition has been reported.Citation24) Furthermore, Handlogten et al. pointed out that heterobivalent ligands selectively bind to IgE, and competitively inhibit allergen binding,Citation14) similar to enzyme reaction. Taken together, those reports support the quadratic correlation between hydrophobicity and antiallergic effect found in our study, and provide some evidence that hydrophobicity and steric effects play a role in the β-hexosaminidase suppressive effect within each of our phenolic acid esters series.
Fig. 2. The relationship between calculated octanol–water partition coefficient (cLogP) and β-hexosaminidase release suppression (pIC50) of RA, CA, p-coumaric acid, and their esters.
Note: Abbreviation of compounds shown here was followed the abbreviation list.
Another point that should be emphasized in this experiment is the dramatic decrease of antiallergic activity of CA-hex (IC50 523 μM) and Cou-hex (IC50 1163 μM) compared with the mild decrease of activity of the RA-hex (IC50 35 μM) against the propyl ester. The IC50 values of the 5 methyl esters, RA-me, CA-me, Cou-me, FA-me, and Cin-me were 57 μM, 120 μM, 182 μM, 144 μM, and 334 μM, respectively. RA-me, CA-me, and FA-me exhibited greater suppression activity than the other two methyl esters, indicating that the binding affinity of those compounds to binding sites depends on the number of hydroxyl groups and methoxy groups on the backbone aromatic rings. The methyl esterification of these compounds dramatically improved the suppression effect (Fig. ), and that could be due to the increased hydrophobicity as well as the negligible steric hindrance of short alkyl chains when binding to the IgE/FcεRI receptor. We suggest that the increase of bulkiness of alkyl groups could have interfered with the binding affinity between the binding sites and esters with long alkyl chain, such as CA-hex (IC50 523 μM) and Cou-hex (IC50 1163 μM). However, the higher number of hydroxyl groups, as mentioned above, in the RA backbone may imply a stronger affinity (e.g. by hydrogen bonds) for the binding sites, which may be less affected by the steric hindrance caused by the bulky hexyl groups. Molecular docking experiments are necessary to clarify this mechanism, and will be done in the near future.
Furthermore, the binding affinity of RA-me and CA-me, bearing 3,4-dihydroxyphenyl group(s), to the cross-linked IgE/FcεRI receptor may be stronger than those of Cou-me, FA-me, and Cin-me. The 3,4-dihydroxyphenyl moieties of RA- and CA- free/ester series could be essential for the binding of these acids/esters and the cross-linked IgE/FcεRI receptor. The higher inhibitory activity of RA-esters (possessing two 3,4-dihydroxyphenyl moieties) compared with that of CA-esters (possessing only one 3,4-dihydroxyphenyl moiety) support this hypothesis.
We conclude that the esterification of rosmarinic, caffeic, and p-coumaric acids with short alkyl chains influences their antiallergic activity. Propyl esters exhibited the highest antiallergic activity. Hydrophobicity and steric bulkiness of the alkyl chain are likely to be modulating the interaction between phenolic acid/esters, and the binding sites in the IgE/FcεRI receptor. Furthermore, our data indicates that the 3,4-dihydroxyphenyl moieties in phenolic ester backbone could be essential for the inhibitor–binding site interaction. The elucidation of mechanism underlying the interactions between phenolic acids/esters warrants further investigations.
Funding
This study was supported by Monbukagakusho Scholarship (Ministry of Education, Culture, Sports, Science and Technology, Japan).
Notes
Abbreviations: RA, rosmarinic acid; RA-me, rosmarinic acid methyl ester; RA-pro, rosmarinic acid propyl ester; RA-hex, rosmarinic acid hexyl ester; CA, caffeic acid; CA-me, caffeic acid methyl ester; CA-pro, caffeic acid propyl ester; CA-hex, caffeic acid hexyl ester; Cou, p-coumaric acid; Cou-me, p-coumaric acid methyl ester; Cou-pro, p-coumaric acid propyl ester; Cou-hex, p-coumaric acid hexyl ester, FA,ferulic acid; FA-me, ferulic acid methyl ester; Cin, cinnamic acid; Cin-me, cinnamic acid methyl ester; cLogP, calculated logarithmic value of octanol–water partition coefficient; pIC50, −logIC50.
References
- Jun H-I, Kim B-T, Song G-S, Kim Y-S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014;148:367–372.10.1016/j.foodchem.2013.10.028
- Oh H, Park C-S, Ahn HJ, Park YS, Kim HM. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011;236:99–106.10.1258/ebm.2010.010252
- Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radical Biol. Med. 2002;33:798–806.10.1016/S0891-5849(02)00970-X
- Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, Inoue K, Sadakane K, Ichinose T, Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy. 2004;34:971–977.10.1111/cea.2004.34.issue-6
- Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. BioFactors. 2004;21:127–131.10.1002/biof.v21:1/4
- Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E-I, and Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004;229:247–254.
- Abramson J, Licht A, Pecht I. Selective inhibition of the FcɛRI-induced de novo synthesis of mediators by an inhibitory receptor. EMBO J. 2006;25:323–334.10.1038/sj.emboj.7600932
- Abramson J, Pecht I. Regulation of the mast cell response to the type I Fcε receptor. Immunol. Rev. 2007;217:231–254.10.1111/imr.2007.217.issue-1
- Pecht I, Corcia A. Stimulus-secretion coupling mechanisms in mast cells. Biophys. Chem. 1987;26:291–301.10.1016/0301-4622(87)80030-3
- Aketani S, Teshima R, Umezawa Y, Sawada J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol. Lett. 2001;75:185–189.10.1016/S0165-2478(00)00311-4
- Huang F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue K, Takano H, Yoshino S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int. Immunopharmacol. 2008;8:502–507.10.1016/j.intimp.2007.12.009
- Tokura T, Nakano N, Ito T, Matsuda H, Nagasako-Akazome Y, Kanda T, Ikeda M, Okumura K, Ogawa H, Nishiyama N. Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between IgE and FcεRI. J. Agric. Food. Chem. 2008;56:12073–12080.
- Li Y, Lee S-H, Le Q-T, Kim M-M, Kim S-K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food. Chem. 2008;56:12073–12080.10.1021/jf802732n
- Handlogten MW, Kiziltepe T, Moustakas DT, Bilgiçer B. Design of a heterobivalent ligand to inhibit IgE clustering on mast cells. Chem. Biol. 2011;18:1179–1188.10.1016/j.chembiol.2011.06.012
- Chung SY, Champagne ET. Reducing the allergenic capacity of peanut extracts and liquid peanut butter by phenolic compounds. Food Chem. 2009;115:1345–1349.10.1016/j.foodchem.2009.01.052
- Yamashita S, Tsukamoto S, Kumazoe M, Kim Y, Yamada K, Tachibana H. Isoflavones suppress the expression of the FcεRI high-affinity immunoglobulin E receptor independent of the estrogen receptor. J. Agric. Food. Chem. 2012;60:8379–8385.10.1021/jf301759s
- Zhu F, Asada T, Sato A, Koi Y, Nishiwaki H, Tamura H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from perilla leaves. J. Agric. Food. Chem. 2014;62:885–892.10.1021/jf404318j
- Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, Yoshikawa M. Anti type I S of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J. Agric. Food. Chem. 2006;54:2915–2920.10.1021/jf052994o
- Sato A, Shinozaki N, Tamura H. Secoiridoid type of antiallergic substances in olive waste materials of three Japanese varieties of Olea europaea. J. Agric. Food. Chem. 2014;62:7787–7795.10.1021/jf502151b
- Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol. Pharm. Bull. 2001;24:351–356.10.1248/bpb.24.351
- Lewis DFV, Lake BG. Quantitative structure-activity relationship (QSAR) analysis for a series of rodent peroxisome proliferators: interaction with the mouse liver peroxisome proliferator-activated receptor α (mPPARα). Toxicol. In Vitro. 1997;11:99–105.10.1016/S0887-2333(96)00067-7
- Hlavacek WS, Posner RG, Perelson AS. Steric effects on multivalent ligand-receptor binding: exclusion of ligand sites by bound cell surface receptors. Biophys. J. 1999;76:3031–3043.10.1016/S0006-3495(99)77456-4
- Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol. Rev. 2007;217:269–279.10.1111/imr.2007.217.issue-1
- Ahmed S, James K, Owen CP, Patel CK, Patel M. Hydrophobicity, a physicochemical factor in the inhibition of the enzyme estrone sulfatase (ES). Bioorg. Med. Chem. Lett. 2001;11:2525–2528.10.1016/S0960-894X(01)00482-6
