Abstract
Consumption of milk fat globule membrane (MFGM) in combination with habitual exercise suppresses age-associated muscle loss. The effects of high dose MFGM, however, are not known. A double-blind, randomized controlled trial with parallel group design was conducted to evaluate the safety of consuming high dose MFGM tablets. The subjects were 32 healthy adult men and women. Subjects were given 5 times the recommended daily intake of the tablets containing 6.5 g of MFGM or whole milk powder for 4 weeks. Stomach discomfort and diarrhea were observed; however, these symptoms were transitory and slight and were not related to consumption of the test tablets. In addition, there were no clinically significant changes in anthropometric measurements or blood tests. Total degree of safety assessed by the physicians of all subjects was “safe.” These findings suggest that consumption of the tablets containing 6.5 g MFGM for 4 weeks is safe for healthy adults.
Graphical Abstract
The consumption of high dose MFGM tablets for 4 weeks in healthy adults was considered to be safe.
Age-associated muscle loss is called sarcopenia.Citation1) Muscle mass is approximately 40% of body weight in healthy adults, but this percentage decreases by 0.5% per year after age 40 and then decreases at a faster rate after age 65, with a cumulative decline of 30–40% up to age 80.Citation2,3) Muscle loss increases the risk of motor impairment, falls, and fractures and impairs activities of daily living and is therefore a major factor leading elderly people to require nursing care.Citation4–8) Preventing muscle loss and maintaining a healthy status will reduce medical and nursing care costs.Citation9) Milk contains an easily accessible matrix, rich in a large variety of essential nutrients such as minerals, vitamins, and easy digestible proteins with balanced amino acid profiles, and is therefore important to support overall body function.Citation10) Resistance exercise and the consumption of milk enhance gains in muscle mass in healthy adults.Citation11–13)
Milk fat globule membranes (MFGM) are produced from raw milk. The fat-rich cream fraction is separated by centrifuging raw milk, and this fraction is further separated into butter and buttermilk by centrifugation. The buttermilk, a liquid, is concentrated with an ultrafiltration membrane, and the residue is referred to as MFGM.Citation14) MFGM is rich in protein and phospholipids, such as sphingomyelin, which is present in nervous tissue.Citation15) A recent report indicated that the combination of dietary MFGM plus habitual exercise suppresses the age-associated deterioration of muscle mass and strength in senescence-accelerated mice, via development of the neuromuscular junction, which is the synapse of motor units.Citation16) In addition, dietary MFGM combined with regular exercise improves the endurance capacity in mice.Citation17) A recent placebo-controlled clinical trial conducted to evaluate healthy middle-aged adults taking the tablets containing 1.0 g MFGM or whole milk powder (0 g MFGM) plus habitual exercise for 10 weeks indicated that the percentage of change from baseline of a cross-sectional area of the quadriceps muscles was significantly higher in the MFGM group than the placebo group, and there were no safety issues (Ota et al. unpublished results). The recommended daily intake of MFGM tablets based on this result is 1.0 g MFGM per day.
Supplements are considered a simple way to consume nutrients when dietary intake is inadequate.Citation18) The number of people consuming supplements is increasing in Japan and other developed countries, due to the aging society.Citation19) Therefore, there is high interest in the appropriate use of supplements.Citation20) While drugs are monitored for effective and safe use by medical staff, supplements are freely available for consumption based on self-judgment, like general foods.Citation18) General foods have an ordinal taste, flavor, color, volume, and are selected according to feeding preference, but supplements are not.Citation18) Thus, consumers may be at increased risk for consuming high doses of supplements compared to general foods, which are difficult to consume in excess by eating too much of one food. MFGM tablets allow consumers to ingest increased amounts of sphingomyelin without drinking large amounts of milk or buttermilk, but the risk of high dose sphingomyelin consumption might be occurred.
In this study, we evaluated the safety of consuming 5 times the recommended daily intake of MFGM tablets.
Materials and methods
Subjects
Subjects were 32 healthy adult men and women aged 20–64 years (mean age 42.0 ± 11.9 years). Subjects were given an explanation of the study, and informed consent was obtained before initiating the clinical trial.
Test tablets
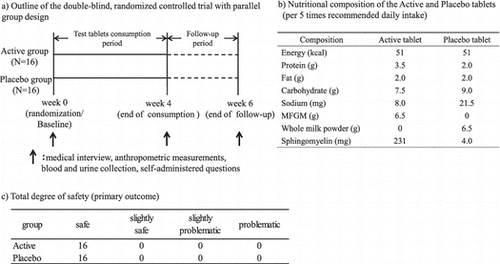
Test tablets were active tablets containing 6.5 g MFGM (231 mg sphingomyelin) per 5 times the recommended daily intake or placebo tablets that contained 6.5 g whole milk powder (4.0 mg sphingomyelin). The nutritional compositions of the active and placebo tablets are shown in Table .
Table 1. Nutritional composition of the active and placebo tablets (per 5 times recommended daily intake).
Protocol
We conducted a double-blind, randomized controlled trial (RCT) with parallel group design. Subjects were randomized into the two groups stratified by age, sex, body mass index (BMI), body weight, body fat ratio, and waist circumference. Subjects were instructed to consume 5 times the recommended daily intake of the active or placebo tablets daily at one or two separate times for 4 weeks. Urine and blood samples were collected at week 0 (randomization/baseline), week 4 (end of consumption period), and week 6 (end of follow-up period). The study was conducted at Yokohama Tsuchida Clinic, Kameido Minamiguchi Clinic, and Yuki Clinic in accordance with the Declaration of Helsinki (2002 revised version). The study protocol was approved by the ethics committee of the Yokohama Tsuchida Clinic.
Adverse events
Physicians examined the results of a medical interview and a written self-record of daily life at weeks 0, 4, and 6; the relationship between those results and the test tablets was determined by the physicians.
Anthropometric measurements
Body height, body weight, body fat ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, and body temperature were measured at weeks 0, 4, and 6, using standard methods. BMI was calculated based on the body height and body weight.
Blood analysis
Blood test parameters included white and red blood cell counts, hemoglobin, hematocrit, and blood platelets. Blood biochemistry parameters included triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, non-esterified fatty acid, glucose, aspartate aminotransferase, alanine transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase (ALP), lactate dehydrogenase (LD), total protein (TP), albumin, uric acid, blood urea nitrogen (BUN), creatinine (CRE), sodium, chloride, potassium, calcium, inorganic phosphorus, magnesium, and serum iron (Fe). Blood analysis was performed at weeks 0, 4, and 6, by SRL, Inc. (Tokyo, Japan) using standard methods.
Urine analysis
Urinalysis parameters included glucose (qualitative), protein (qualitative), urobilinogen, bilirubin, ketone bodies (qualitative), occult blood, pH, specific gravity, and sediment (performed only when the protein test result was positive) at weeks 0, 4, and 6 by SRL, Inc. (Tokyo, Japan) using standard methods.
Self-administered questionnaire regarding physical symptoms
In the questionnaire on physical symptoms, subjects were asked to answer whether or not they experienced “stumbling,” “stiff shoulder,” “backache,” “knee pain,” leg cramp,” “declining leg strength,” and “fatigue” at week 0. Subjects who answered “yes” at week 0 were asked to rate their improvement at week 4 using a 5-point Likert scale (“improved,” “slightly improved,” “no change,” “slightly worse,” and “worse”).
Statistical analysis
Values are expressed as mean ± standard deviation. Statistical analysis was performed based on statistical analysis specifications prepared in advance. For continuous data, statistically significant differences between the active and placebo groups were examined using Student’s t-test, and changes from the baseline within the same groups were analyzed using Dunnett’s multiple comparison. For categorical data, statistically significant differences between the active and placebo groups were examined using the Mann–Whitney U test, and the changes from baseline within the same groups were analyzed using the Wilcoxon signed-rank test. These statistical analyses were performed using IBM SPSS Statistics 22 (IBM Japan). Statistical tests were two-sided, and p < 0.05 was considered statistically significant. The primary outcome was total degree of safety (“safe,” “slightly safe,” “slightly problematic,” or “problematic”) for each subject by the physicians based on the subject’s reporting of the adverse events, anthropometric measurements, blood analysis, and urine analysis.
Results
Subjects
A total of 32 subjects (42.0 ± 11.9 years, 16 men, 16 women) were screened for the study and randomized into two groups. The characteristics of the subjects are shown in Table . None of these characteristics were significantly different between the two groups. All subjects completed the study. No subject deviated from the protocol during the study period, and thus, all 32 subjects were included in the analysis. The percentages of test tablet consumption were 99.8 ± 0.8% and 100 ± 0% in the active and placebo groups, respectively.
Table 2. Subject characteristics.
Adverse events
Four adverse events: diarrhea, stomach discomfort, rhinitis, and cough were reported in the active group (Table ). These symptoms, however, were transitory and slight. All subjects were recovered without discontinuing taking the test tablet or additional treatment. Physicians determined that there was no relationship between the tablet consumption and the adverse events.
Table 3. Adverse events.
Anthropometric measurements
Changes in body weight, BMI, body fat ratio, SBP, DBP, pulse rate, and body temperature are shown in Table . The pulse rate differed significantly between the two groups at week 6 (p < 0.01). The pulse rate was significantly decreased in the active group at week 6 compared with week 0 (p < 0.05). Body weight, BMI, body fat ratio, SBP, DBP, and body temperature did not differ significantly between the two groups or within groups.
Table 4. Anthropometric measurements.
Blood analysis
Changes in blood test parameters are shown in Table . Hematocrit was significantly decreased in the active group at week 6 compared with week 0 (p < 0.05). No significant differences were detected in any other blood test parameters between the two groups or within groups.
Table 5. Blood test parameters.
Changes in blood biochemistry parameters are shown in Table . Serum Fe levels differed significantly between the two groups at week 4 (p < 0.05). ALP, LD, TP, and BUN values were significantly decreased in the active group at week 6 compared with week 0 (p < 0.05, p < 0.01, p < 0.01, and p < 0.05, respectively). The CRE value was significantly increased in the active and placebo groups at week 6 compared with week 0 (p < 0.01 and p < 0.05, respectively). No significant differences were detected in any other blood biochemistry parameters between the two groups or within groups.
Table 6. Blood biochemistry.
Urine analysis and total safety degree
The urinalysis parameters did not differ significantly between the two groups or within groups (data not shown).The total degree of safety, determined by the physicians, of all subjects was “safe.”
Questionnaire regarding physical symptoms
Subjects who reported symptoms of “stumbling,” “stiff shoulder,” “backache,” “knee pain,” “leg cramp,” “declining leg strength,” and “fatigue” were asked to provide ratings regarding any improvement or worsening of physical symptoms at week 4 (Table ). Ratings of stiff shoulder differed significantly between the two groups at week 4 (p < 0.05).
Table 7. Rating of questionnaire of improvement on physical symptoms.
Discussion
The MFGM tablets contain 46.2 mg sphingomyelin per recommended daily intake, which is equivalent to ~600 mL of milk or ~22 g of buttermilk. Many Europeans and Americans regularly consume buttermilk as a beverage or in powder form. Although there are no definitive reports regarding buttermilk consumption, mean daily milk consumption in countries with mass consumption of milk is reported to be 580 mL.Citation21) Thus, MFGM tablets are an easy way to consume sphingomyelin without drinking such a large amount of milk or buttermilk, but there is a risk of consuming an excessively high dose. For example, high dietary intake (>10,000 IU/day) of preformed vitamin A during early pregnancy appears to be teratogenic.Citation22) Also, high dose (>400 IU/day) of vitamin E supplements in patients with chronic disease may increase all-cause mortality.Citation23) Therefore, to investigate the safety of a dose of 5 times the recommended daily intake of MFGM tablets, we conducted a double-blind RCT with parallel group design in 32 healthy adult men and women.
Four adverse events: stomach discomfort, diarrhea, rhinitis, and cough were reported in the active group. Among them, two adverse events, stomach discomfort and diarrhea, were considered clinically important. Lactose intolerance is an extremely common condition worldwide.Citation24) MFGM contains lactose, so it could potentially cause gastrointestinal symptoms such as stomach discomfort and diarrhea. The lactose content of milk is 5%. Lactose consumption in the active group was equivalent to that in 9 mL of milk. The amount of lactose contained in one cup of milk (200 mL) is not a major cause of gastrointestinal symptoms.Citation25) These findings suggest that the lactose in the MFGM tablets is not likely a cause of the stomach discomfort and diarrhea. No other components in the active tablets are considered to cause gastrointestinal symptoms. Stomach discomfort and diarrhea are common in daily life. These findings suggest that the consumption of high dose MFGM tablets is unlikely to cause gastrointestinal symptoms.
Some of the anthropometric measurements, and the blood test and urinalysis results changed significantly during the study. Serum Fe levels at week 4 and pulse rate at week 6 differed significantly between the two groups. Only slight variations were detected at the individual level, and the physicians judged these variations to be clinically unimportant. No significant change was detected in either group from week 0 to week 4. At week 6, however, hematocrit, ALP, LD, TP, and BUN values were significantly lower in the active group (p < 0.05, p < 0.01, p < 0.01, and p < 0.05, respectively), and the CRE value was significantly higher in both the active and placebo groups (p < 0.01 and p < 0.05, respectively). These changes, however, were considered very slight and clinically unimportant.
With regard to the effectiveness of MFGM, the self-reports of a stiff shoulder improved significantly in the active group after consuming MFGM for 4 weeks. Furthermore, stumbling and fatigue also tended to improve in the active group (p = 0.08 and p = 0.10, respectively). In addition, none of the subjects in the active group had worsening of any symptoms. The serum CRE level, which is positively correlated with muscle mass, did not differ significantly within groups.Citation26) Another placebo-controlled trial, comprising the following 4 groups: progressive resistance exercise training, multinutrient supplementation, resistant training plus supplementation, or control (with no intervention) in very elderly people, was conducted over a 10 week period.Citation27) Muscle strength significantly increased in the exercise and multinutrient supplementation plus exercise groups, but the multinutrient supplementation and no exercise group showed no change.Citation27) Thus, the combination of nutrients and exercise is considered necessary for improving muscle strength. In this study, subjects received MFGM tablets without exercise intervention, as the main purpose was safety evaluation. If the subjects had received a combined intervention with MFGM and exercise, muscle mass and strength might have improved.
In conclusion, total degree of safety assessed by the physicians of all subjects was “safe.” These findings suggest that the consumption of high dose MFGM tablets for 4 weeks is safe for healthy adults. Nevertheless, it is important to adhere to the recommended daily intake of MFGM tablets.
Acknowledgments
We are grateful to all of the subjects who participated in this clinical trial; and to Dr Chikama, Dr Yanagisawa, and Mr Ishida of the Kao Corporation, for their support and advice regarding this study.
Notes
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; BUN, blood urea nitrogen; CRE, creatinine; DBP, diastolic blood pressure; Fe, iron; LD, lactate dehydrogenase; MFGM, milk fat globule membrane; RCT, randomized controlled trial; SBP, systolic blood pressure; TP, total protein
References
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J. Lab. Clin. Med. 2001;137:231–243.10.1067/mlc.2001.113504
- Leeuwenburgh C. Role of apoptosis in sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:999–1001.
- Tanimoto Y, Watanabe M, Kono R, Hirota C, Takasaki K, Kono K. Aging changes in muscle mass of Japanese. Nippon Ronen Igakkai Zasshi (in Japanese). 2010;47:52–57.10.3143/geriatrics.47.52
- Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES. Frailty in older men: prevalence, progression, and relationship with mortarity. J. Am. Geriatr. Soc. 2007;55:1216–1223.10.1111/(ISSN)1532-5415
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Iorio AD, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J. Appl. Physiol. 2003;95:1851–1860.
- Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WMC, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12:433–450.10.1007/BF02982704
- Topinkova E. Aging, disability and frailty. Ann. Nutr. Metab. 2008;52:6–11.
- Hartman MJ, Fields DA, Byrne NM, Hunter GR. Resistance training improves metabolic economy during functional tasks in older adults. J. Strength Cond. Res. 2007;21:91–95.10.1519/00124278-200702000-00017
- Livingston G, Manela M, Katona C. Cost of community care for older people. Br. J. Psychiatry. 1997;171:56–59.10.1192/bjp.171.1.56
- Steijns JM. Daily products and healthy: focus on their constituents or on the matrix? Int. Dairy J. 2008;18:425–435.10.1016/j.idairyj.2007.11.008
- Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Med. Sci. Sports Exerc. 2010;42:1122–1130.
- Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007;86:373–381.
- Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012;108:958–962.10.1017/S0007114511006271
- Kanno C, Kim DH. A simple procedure for the preparation of bovine milk fat globule membrane and a comparison of its composition, enzymatic activities, and electrophoretic properties with those prepared by other methods. Agric. Biol. Chem. 1990;54:2845–2854.10.1271/bbb1961.54.2845
- Dewettinck K, Rombaut R, Thienpont N, Le TT, Messens K, Camp JV. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008;18:436–457.10.1016/j.idairyj.2007.10.014
- Haramizu S, Mori T, Yano M, Ota N, Hashizume K, Otsuka A, Hase T, Shimotoyodome A. Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice. SpringerPlus. 2014;3:339–354.10.1186/2193-1801-3-339
- Haramizu S, Ota N, Otuska A, Hashizume K, Sugita S, Hase T, Murase T, Shimotoyodome A. Dietary milk fat globule membrane improves endurance capacity in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:1009–1017.10.1152/ajpregu.00004.2014
- Umegaki K. Actual condition of health foods and their safety and effectiveness. Shokuhin Eiseigaku Zasshi (in Japanese). 2010;6:396–401.10.3358/shokueishi.51.396
- Imai T, Nakamura M, Ando F, Shimokata H. Dietary supplement use by community-living population in Japan: data from the national institute for longevity sciences longitudinal study of aging (nils-lsa). J. Epidemiol. 2006;16:249–260.10.2188/jea.16.249
- Kamohara S. Prospects for the appropriate use of the dietary supplements in geriatric medicine. Nippon Ronen Igakkai Zasshi (in Japanese). 2014;51:141–143.10.3143/geriatrics.51.141
- World Agriculture: towards 2015/2030 An FAO perspective [Internet]. Roma: Food and Agriculture Organization of the United Nations/London, Earthscan; 2003 [cited 2014 Oct 1]. Available from: http://www.fao.org/docrep/005/y4252e/y4252e00.htm
- Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 1995;333:1369–1373.10.1056/NEJM199511233332101
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersms RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46.10.7326/0003-4819-142-1-200501040-00110
- Vesa TH, Marteau P, Korpela R. Lactose intolerance. J. Am. Coll. Nutr. 2000;19:165S–175S.10.1080/07315724.2000.10718086
- Saviano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J. Nutr. 2006;136:1107–1113.
- Moon JS, Lee JE, Yoon JS. Variation in serum creatinine level is correlated to risk of type 2 diabetes. Endocrinol. Metab. 2013;28:207–213.10.3803/EnM.2013.28.3.207
- Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsits LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775.10.1056/NEJM199406233302501
